The Effect of Bariatric Surgery on Weight Loss and Metabolic Changes in Adults with Obesity
Abstract
1. Introduction
2. Materials and Methods
- Men: IBW = 50 kg + ((height − 150 cm) 0.7 kg/cm),
- Women: IBW = 50 kg + ((height − 150 cm) 0.6 kg/cm).
3. Results
3.1. Characteristics of the Study Group
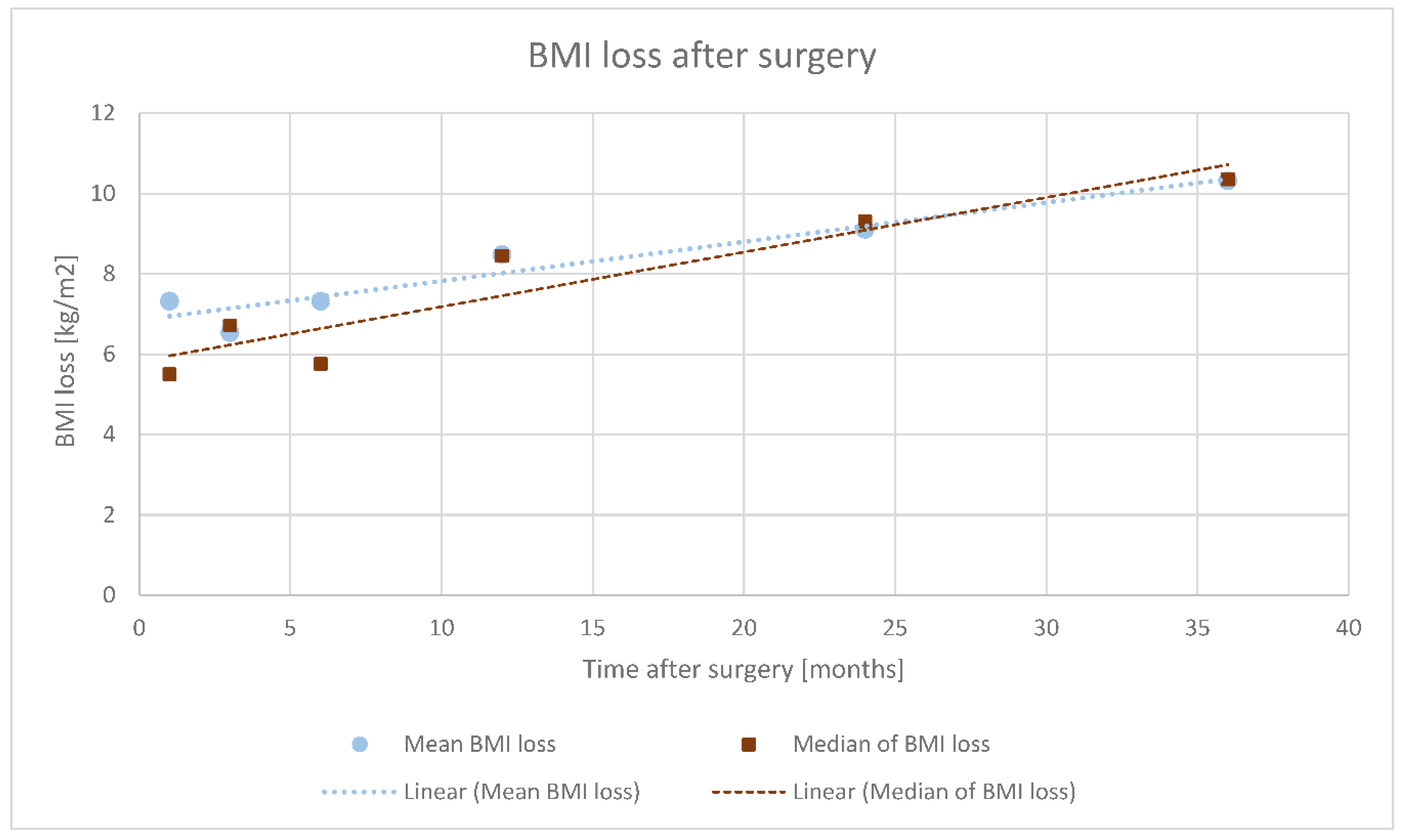
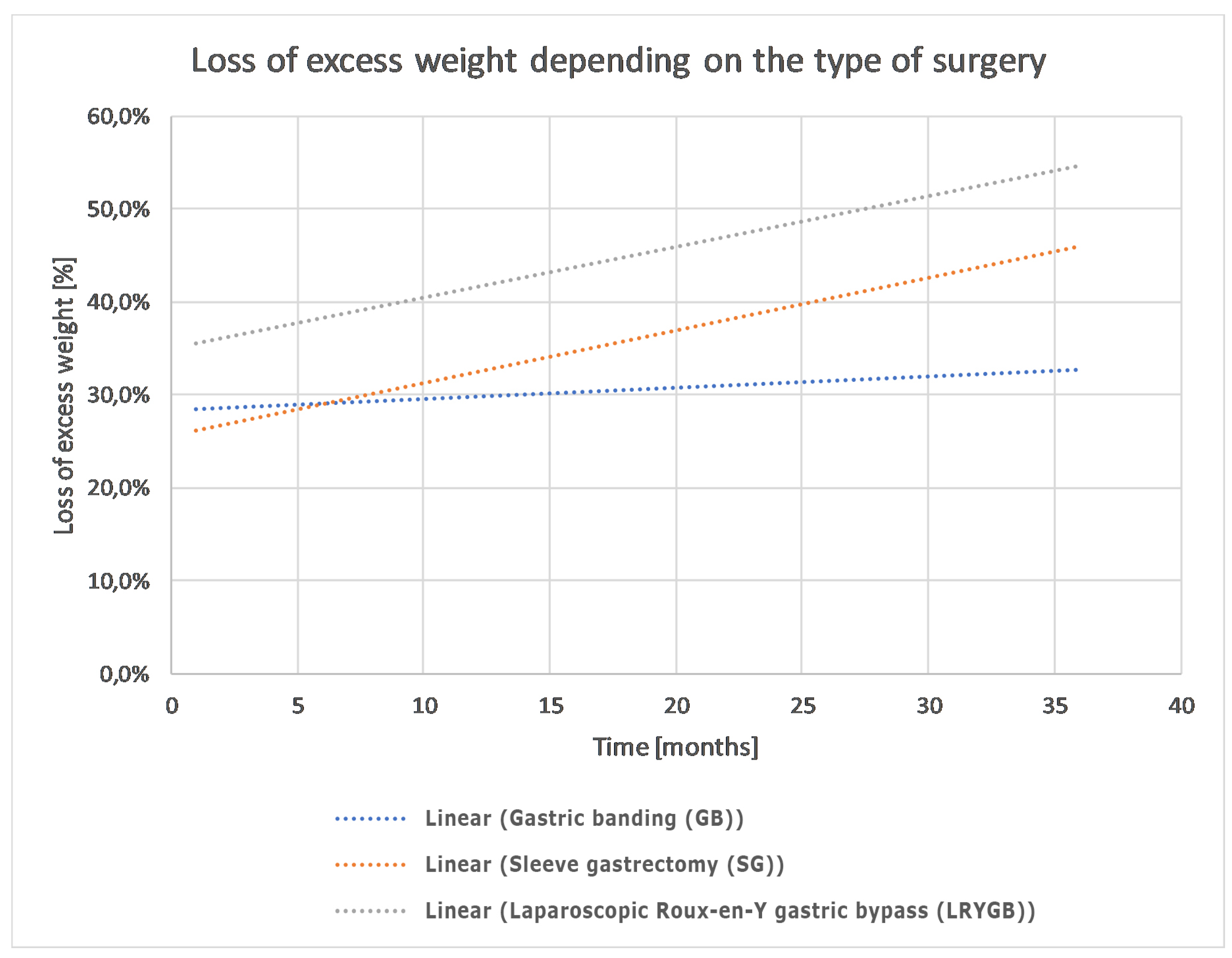
3.2. Analysis of Weight Loss
3.3. Analysis of Metabolic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. World Health Organization. 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 March 2019).
- World Health Organization. Nutrition, Physical Activity and Obesity Poland Demographic Data. World Health Organization. Available online: https://www.euro.who.int/__data/assets/pdf_file/0020/243317/Poland-WHO-Country-Profile.pdf (accessed on 10 March 2019).
- Budzyński, A.; Major, P.; Głuszek, S.; Kaseja, K.; Koszutski, T.; Leśniak, S.; Lewandowski, T.; Lipka, M.; Lisik, W.; Makarewicz, W.; et al. Polskie rekomendacje w zakresie chirurgii bariatrycznej i metabolicznej. Med. Prakt. Chir. 2016, 4, 13–25. [Google Scholar]
- Wasiluk, A.; Szczuk, J. Underweight, overweight, and obesity in boys and girls at the age of 7–18 years from eastern Poland in the years 1986–2006. Med. Stud. 2015, 31, 99–105. [Google Scholar] [CrossRef]
- Kabała, M.M.; Wilczyński, J. Obesity and postural stability in women after mastectomy. Med. Stud. 2019, 35, 48–54. [Google Scholar] [CrossRef]
- Rębak, D.; Suliga, E.; Gluszek, S. Metabolic syndrome and professional aptitude. Med. Stud. 2016, 31, 286–294. [Google Scholar]
- Suliga, E.; Koziel, D.; Ciesla, E.; Rebak, D.; Gluszek, S. Coffee consumption and the occurrence and intensity of metabolic syndrome: A cross-sectional study. Int. J. Food Sci. Nutr. 2017, 68, 507–513. [Google Scholar] [CrossRef]
- Suliga, E.; Koziel, D.; Ciesla, E.; Rebak, D.; Gluszek, S. Factors Associated with Adiposity, Lipid Profile Disorders and the Metabolic Syndrome Occurrence in Premenopausal and Postmenopausal Women. PLoS ONE 2016, 11, e0154511. [Google Scholar] [CrossRef] [PubMed]
- Suliga, E.; Koziel, D.; Gluszek, S. Prevalence of metabolic syndrome in normal weight individuals. Ann. Agric. Environ. Med. 2016, 23, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Suliga, E.; Koziel, D.; Ciesla, E.; Gluszek, S. Association between dietary patterns and metabolic syndrome in individuals with normal weight: A cross-sectional study. Nutr. J. 2015, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Kraschnewski, J.L.; Boan, J.; Esposito, J.; Sherwood, N.E.; Lehman, E.B.; Kephart, D.K.; Sciamanna, C.N. Long-term weight loss maintenance in the United States. Int. J. Obes. 2010, 34, 1644–1654. [Google Scholar] [CrossRef]
- Krekora-Wollny, K.; Suliga, E. Changes in body mass during weight loss treatment—A two-year prospective study. Med. Stud. 2017, 33, 290–294. [Google Scholar] [CrossRef]
- Coughlin, J.W.; Brantley, P.J.; Champagne, C.M.; Vollmer, W.M.; Stevens, V.J.; Funk, K.; Dalcin, A.T.; Jerome, G.J.; Myers, V.H.; Tyson, C.; et al. The impact of continued intervention on weight: Five-year results from the weight loss maintenance trial. Obesity 2016, 24, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity with Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2424–2434. [Google Scholar] [CrossRef]
- Correction to Lancet Diabetes Endocrinol 2015, 3, 243–253. Lancet Diabetes Endocrinol. 2015, 3, e4. [CrossRef][Green Version]
- Kozieł, D.; Matykiewicz, J.; Klusek, J.; Wawrzycka, I.; Głuszek, S. Opieka okołooperacyjna nad chorymi na otyłość-doświadczenia własne. Stud. Med. 2011, 24, 4–35. [Google Scholar]
- Janik, M.R.; Stanowski, E.; Paśnik, K. Present status of bariatric surgery in Poland. Videosurg. Miniinvasive Tech. 2016, 11, 22–25. [Google Scholar] [CrossRef]
- Spivak, H.; Sakran, N.; Dicker, D.; Rubin, M.; Raz, I.; Shohat, T.; Blumenfeld, O. Different effects of bariatric surgical procedures on dyslipidemia: A registry-based analysis. Surg. Obes. Relat. Dis. 2017, 13, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Komorniak, N.; Hoffmann, M.; Walczak, J.; Jaroszek, A.; Kowalewski, B.; Kaseja, K.; Jamiol-Milc, D.; Stachowska, E. Body Weight Reduction and Biochemical Parameters of the Patients After RYGB and SG Bariatric Procedures in 12-Month Observation. Obes. Surg. 2017, 27, 940–947. [Google Scholar] [CrossRef][Green Version]
- Carswell, K.A.; Belgaumkar, A.P.; Amiel, S.A.; Patel, A.G. A Systematic Review and Meta-analysis of the Effect of Gastric Bypass Surgery on Plasma Lipid Levels. Obes. Surg. 2016, 26, 843–855. [Google Scholar] [CrossRef]
- Chang, A.R.; Chen, Y.; Still, C.; Wood, G.C.; Kirchner, H.L.; Lewis, M.; Kramer, H.; Hartle, J.E.; Carey, D.; Appel, L.J.; et al. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016, 90, 164–171. [Google Scholar] [CrossRef]
- Friedman, A.N.; Wahed, A.S.; Wang, J.; Courcoulas, A.P.; Dakin, G.; Hinojosa, M.W.; Kimmel, P.L.; Mitchell, J.E.; Pomp, A.; Pories, W.J.; et al. Effect of Bariatric Surgery on CKD Risk. J. Am. Soc. Nephrol. 2018, 29, 1289–1300. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.A.; Kim, J.H.; Lee, S.Y.; Shin, C.H.; Yang, S.W. Discrepancies between Glycosylated Hemoglobin and Fasting Plasma Glucose for Diagnosing Impaired Fasting Glucose and Diabetes Mellitus in Korean Youth and Young Adults. Diabetes Metab. J. 2019, 43, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Spitznagel, M.B.; Strain, G.; Devlin, M.; Cohen, R.; Crosby, R.D.; Mitchell, J.E.; Gunstad, J. Improved serum leptin and ghrelin following bariatric surgery predict better postoperative cognitive function. J. Clin. Neurol. 2015, 11, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Diabetes Prevention Program Research, G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Kuna, S.T.; Reboussin, D.M.; Borradaile, K.E.; Sanders, M.H.; Millman, R.P.; Zammit, G.; Newman, A.B.; Wadden, T.A.; Jakicic, J.M.; Wing, R.R.; et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep 2013, 36, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Mathus-Vliegen, E.M. Obesity and the elderly. J. Clin. Gastroenterol. 2012, 46, 533–544. [Google Scholar] [CrossRef]
- Wojciak, P.A.; Pawłuszewicz, P.; Diemieszczyk, I.; Komorowska-Wojtunik, E.; Czerniawski, M.; Krętowski, A.; Błachnio-Zabielska, A.; Dadan, J.; Ładny, J.; Hady, H. Laparoscopic sleeve gastrectomy: A study of efficiency in treatment of metabolic syndrome components, comorbidities and influence on certain biochemical markers. Videosurg. Miniinvasive Tech. 2020, 15, 136–147. [Google Scholar] [CrossRef]
- Kowalewski, P.K.; Olszewski, R.; Walędziak, M.S.; Janik, M.R.; Kwiatkowski, A.; Gałązka-Świderek, N.; Cichoń, K.; Brągoszewski, J.; Paśnik, K. Long-Term Outcomes of Laparoscopic Sleeve Gastrectomy—A Single-Center, Retrospective Study. Obes. Surg. 2018, 28, 130–134. [Google Scholar] [CrossRef]
- Khosravi-Largani, M.; Nojomi, M.; Aghili, R.; Otaghvar, H.A.; Tanha, K.; Seyedi, S.H.S.; Mottaghi, A. Evaluation of all Types of Metabolic Bariatric Surgery and its Consequences: A Systematic Review and Meta-Analysis. Obes. Surg. 2019, 29, 651–690. [Google Scholar] [CrossRef]
- Hajifathalian, K.; Ueda, P.; Lu, Y.; Woodward, M.; Ahmadvand, A.; Aguilar-Salinas, C.A.; Azizi, F.; Cifkova, R.; Di Cesare, M.; Eriksen, L.; et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): A pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol. 2015, 3, 339–355, Erratum in 2016, 4, e6. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Horvath, K.; Jeitler, K.; Siering, U.; Stich, A.K.; Skipka, G.; Gratzer, T.W.; Siebenhofer, A. Long-term effects of weight-reducing interventions in hypertensive patients: Systematic review and meta-analysis. Arch. Intern. Med. 2008, 168, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Inge, T.H.; Courcoulas, A.P.; Jenkins, T.M.; Michalsky, M.P.; Brandt, M.L.; Xanthakos, S.A.; Dixon, J.B.; Harmon, C.M.; Chen, M.K.; Xie, C.; et al. Five-Year Outcomes of Gastric Bypass in Adolescents as Compared with Adults. N. Engl. J. Med. 2019, 380, 2136–2145. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, D.E.; Eid, G.; Maciejewski, M.L. Long-term survival following bariatric surgery in the VA health system-reply. JAMA 2015, 313, 1474–1475. [Google Scholar] [CrossRef] [PubMed]
- Williamson, K.; Nimegeer, A.; Lean, M. Rising prevalence of BMI ≥40 kg/m2: A high-demand epidemic needing better documentation. Obes. Rev. 2020, 21, e12986. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Total (n = 163) | Women (n = 136) X ± SD | Men (n = 27) X ± SD | p-Value | |
|---|---|---|---|---|---|
| X ± SD | Me (Q1–Q3) | ||||
| Age | 39.6 ± 10.6 | 40.0 (31.0–46.0) | 38.4 ± 10.0 | 43.4 ± 11.5 | 0.0071 |
| Height (cm) | 168.2 ± 8.5 | 167.0 (164–172) | 166.3 ± 8.4 | 175.4 ± 9.5 | <0.0001 |
| Initial weight (kg) | 123.6 ± 21.5 | 120.0 (109–134) | 120.9 ± 19.4 | 148.1 ± 25.0 | <0.0001 |
| Initial BMI (kg/m2) | 44.5 ± 6.8 | 43.4 (40.2–46.3) | 44.0 ± 9.2 | 48.1 ± 8.3 | 0.0003 |
| Initial ideal body weight (kg) | 61.3 ± 5.8 | 60.2 (58.4–64.3) | 59.8 ± 5.0 | 67.8 ± 6.7 | <0.0001 |
| Initial excess weight (kg) | 62.3 ± 18.5 | 59.8 (49.8–72.0) | 61.2 ± 18.6 | 80.15 ± 23.4 | <0.0001 |
| Time after Procedure (Months) | N | Excess Weight Loss | |||
|---|---|---|---|---|---|
| X ± SD (kg) | X (%) | Me (kg) | Me (%) | ||
| 1 | 37 | 18.5 ± 16.3 | 27.9 | 16.5 | 24.9 |
| 3 | 17 | 18.3 ± 10.4 | 28.4 | 17.5 | 26.8 |
| 6 | 42 | 19.4 ± 13.4 | 31.4 | 17.0 | 28.2 |
| 12 | 47 | 23.9 ± 11.0 | 40.4 | 23.0 | 39.0 |
| 24 | 39 | 25.1 ± 13.7 | 44.4 | 24.5 | 42.8 |
| >24 | 19 | 30.3 ± 19.2 | 50.5 | 31.0 | 56.5 |
| Biochemical Parameters | Time (Months) After Surgery | |||
|---|---|---|---|---|
| 1 | 6 | 12 | >12 | |
| Glucose | 4 | 9 | 8 | 8 |
| Triglyceride | 23 | 15 | 14 | 10 |
| Total cholesterol | 28 | 23 | 19 | 12 |
| LDL | 18 | 5 | 6 | 6 |
| HDL | 17 | 6 | 5 | 8 |
| Biochemical Parameters (mg/dL) | Before Surgery (X ± SD) | Time (Months) After Surgery | p-Value | |||
|---|---|---|---|---|---|---|
| 1 (X ± SD) | 6 (X ± SD) | 12 (X ± SD) | >12 (X ± SD) | |||
| Glucose | 109.6 ± 48.0 | 104 ± 8.1 | 91.3 ± 14.3 | 89.9 ± 18.3 | 86.6 ± 7.9 | 0.003 |
| Triglyceride | 156.9 ± 79.6 | 137.7 ± 55.2 | 126.5 ± 34.3 | 111.6 ± 43.7 | 112.7 ± 44.3 | 0.043 |
| Total cholesterol | 198.4 ± 47.8 | 188.7 ± 36.7 | 165.2 ± 82.5 | 186.9 ± 35.3 | 191.7 ± 42.7 | 0.180 |
| LDL | - | 122.7 ± 40.8 | 137 ± 32.6 | 112 ± 18.8 | 131 ± 36.6 | 0.261 |
| HDL | - | 41.2 ± 12.0 | 41.7 ± 7.8 | 42.6 ± 6.8 | 61.9 ± 23.9 | 0.084 |
| Biochemical Parameters | Concentration before Surgery | Concentration 12 Months after Surgery | p-Value | ||
|---|---|---|---|---|---|
| X ± SD | Me | X ± SD | Me | ||
| Creatinine (mg/dL) | 4.87 ± 18.3 | 0.88 | 2.93 ± 13.7 | 0.86 | 0.3912 |
| CK-MB (mg/dL) | 15.61 ± 15.8 | 14.0 | 13.97 ± 3.6 | 14.0 | 0.6056 |
| CPK (mg/dL) | 156.1 ± 134.5 | 113.0 | 192.24 ± 112.1 | 155.0 | 0.1240 |
| Leptin (pg/mL) | 197.50 ± 257.3 | 50.0 | 75.98 ± 117.7 | 39.8 | 0.0116 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głuszek, S.; Bociek, A.; Suliga, E.; Matykiewicz, J.; Kołomańska, M.; Bryk, P.; Znamirowski, P.; Nawacki, Ł.; Głuszek-Osuch, M.; Wawrzycka, I.; et al. The Effect of Bariatric Surgery on Weight Loss and Metabolic Changes in Adults with Obesity. Int. J. Environ. Res. Public Health 2020, 17, 5342. https://doi.org/10.3390/ijerph17155342
Głuszek S, Bociek A, Suliga E, Matykiewicz J, Kołomańska M, Bryk P, Znamirowski P, Nawacki Ł, Głuszek-Osuch M, Wawrzycka I, et al. The Effect of Bariatric Surgery on Weight Loss and Metabolic Changes in Adults with Obesity. International Journal of Environmental Research and Public Health. 2020; 17(15):5342. https://doi.org/10.3390/ijerph17155342
Chicago/Turabian StyleGłuszek, Stanisław, Arkadiusz Bociek, Edyta Suliga, Jarosław Matykiewicz, Magdalena Kołomańska, Piotr Bryk, Przemysław Znamirowski, Łukasz Nawacki, Martyna Głuszek-Osuch, Iwona Wawrzycka, and et al. 2020. "The Effect of Bariatric Surgery on Weight Loss and Metabolic Changes in Adults with Obesity" International Journal of Environmental Research and Public Health 17, no. 15: 5342. https://doi.org/10.3390/ijerph17155342
APA StyleGłuszek, S., Bociek, A., Suliga, E., Matykiewicz, J., Kołomańska, M., Bryk, P., Znamirowski, P., Nawacki, Ł., Głuszek-Osuch, M., Wawrzycka, I., & Kozieł, D. (2020). The Effect of Bariatric Surgery on Weight Loss and Metabolic Changes in Adults with Obesity. International Journal of Environmental Research and Public Health, 17(15), 5342. https://doi.org/10.3390/ijerph17155342





