Burden of Healthcare Utilization among Chronic Obstructive Pulmonary Disease Patients with and without Cancer Receiving Palliative Care: A Population-Based Study in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Availability of Data and Materials
2.3. Study Design and Population
2.4. Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef]
- Dhamane, A.D.; Moretz, C.; Zhou, Y.; Burslem, K.; Saverno, K.; Jain, G.; Renda, A.; Kaila, S. COPD exacerbation frequency and its association with health care resource utilization and costs. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 2609. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.; Decramer, M.; Celli, B.; Kesten, S.; Liu, D.; Tashkin, D.P. Exacerbation frequency and course of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 653. [Google Scholar] [CrossRef] [PubMed]
- Hoogendoorn, M.; Feenstra, T.L.; Hoogenveen, R.T.; Al, M.; Rutten-van Mölken, M. Association between lung function and exacerbation frequency in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2010, 5, 435. [Google Scholar] [CrossRef] [PubMed]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Beernaert, K.; Van den Block, L.; Morin, L.; Hunt, K.; Miccinesi, G.; Cardenas-Turanzas, M.; Onwuteaka-Philipsen, B.; MacLeod, R.; Ruiz-Ramos, M. Differences in place of death between lung cancer and COPD patients: A 14-country study using death certificate data. NPJ Prim. Care Respir. Med. 2017, 27, 14. [Google Scholar] [CrossRef]
- Ai-Ping, C.; Lee, K.-H.; Lim, T.-K. In-hospital and 5-year mortality of patients treated in the ICU for acute exacerbation of COPD: A retrospective study. Chest 2005, 128, 518–524. [Google Scholar] [CrossRef]
- García-Sanz, M.-T.; Cánive-Gómez, J.-C.; Senín-Rial, L.; Aboal-Viñas, J.; Barreiro-García, A.; López-Val, E.; González-Barcala, F.-J. One-year and long-term mortality in patients hospitalized for chronic obstructive pulmonary disease. J. Thorac. Dis. 2017, 9, 636. [Google Scholar] [CrossRef]
- Brown, H.; Dodic, S.; Goh, S.S.; Green, C.; Wang, W.C.; Kaul, S.; Tiruvoipati, R. Factors associated with hospital mortality in critically ill patients with exacerbation of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2361. [Google Scholar] [CrossRef]
- Lisspers, K.; Larsson, K.; Johansson, G.; Janson, C.; Costa-Scharplatz, M.; Gruenberger, J.-B.; Uhde, M.; Jorgensen, L.; Gutzwiller, F.S.; Ställberg, B. Economic burden of COPD in a Swedish cohort: The ARCTIC study. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 275. [Google Scholar] [CrossRef]
- Mannino, D.M.; Higuchi, K.; Yu, T.-C.; Zhou, H.; Li, Y.; Tian, H.; Suh, K. Economic burden of COPD in the presence of comorbidities. Chest 2015, 148, 138–150. [Google Scholar] [CrossRef]
- Schwab, P.; Dhamane, A.D.; Hopson, S.D.; Moretz, C.; Annavarapu, S.; Burslem, K.; Renda, A.; Kaila, S. Impact of comorbid conditions in COPD patients on health care resource utilization and costs in a predominantly Medicare population. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Simon-Tuval, T.; Scharf, S.M.; Maimon, N.; Bernhard-Scharf, B.J.; Reuveni, H.; Tarasiuk, A. Determinants of elevated healthcare utilization in patients with COPD. Respir. Res. 2011, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, M.; Lovell, N.; Booth, S.; Man, W.D.; Higginson, I.J. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet 2017, 390, 988–1002. [Google Scholar] [CrossRef]
- Nici, L.; ZuWallack, R. An official American Thoracic Society workshop report: The integrated care of the COPD patient. Proc. Am. Thorac. Soc. 2012, 9, 9–18. [Google Scholar] [CrossRef]
- Van Der Plas, A.G.; Oosterveld-Vlug, M.G.; Pasman, H.R.W.; Onwuteaka-Philipsen, B.D. Relating cause of death with place of care and healthcare costs in the last year of life for patients who died from cancer, chronic obstructive pulmonary disease, heart failure and dementia: A descriptive study using registry data. Palliat. Med. 2017, 31, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.S.; Morrison, R.S. Palliative care for the seriously ill. N. Engl. J. Med. 2015, 373, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.S.; Meier, D.E. Palliative care. N. Engl. J. Med. 2004, 350, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Engelberg, R.A.; Nielsen, E.L.; Curtis, J.R. Palliative care for patients dying in the intensive care unit with chronic lung disease compared with metastatic cancer. Ann. Am. Thorac. Soc. 2016, 13, 684–689. [Google Scholar] [CrossRef]
- Chou, W.-C.; Lai, Y.-T.; Hung, Y.-S. Comparing end-of-life care in hospitalized patients with chronic obstructive pulmonary disease with and without palliative care in Taiwan. J. Res. Med. Sci. Off. J. Isfahan Univ. Med Sci. 2013, 18, 594. [Google Scholar]
- Rush, B.; Hertz, P.; Bond, A.; McDermid, R.C.; Celi, L.A. Use of palliative care in patients with end-stage COPD and receiving home oxygen: National trends and barriers to care in the United States. Chest 2017, 151, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F. Early palliative care for patients with metastatic non–small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-M.; Chen, Y.-C.; Cheng, K.-C.; Wang, J.-J.; Ho, C.-H. Trends in intensive care unit admissions of COPD patients from 2003 to 2013 in Taiwan. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2007. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Cantu, R.C.; Nowinski, C.J.; Hedley-Whyte, E.T.; Gavett, B.E.; Budson, A.E.; Santini, V.E.; Lee, H.-S.; Kubilus, C.A.; Stern, R.A. Chronic traumatic encephalopathy in athletes: Progressive tauopathy following repetitive head injury. J. Neuropathol. Exp. Neurol. 2009, 68, 709. [Google Scholar] [CrossRef] [PubMed]
- Bloom, C.I.; Slaich, B.; Morales, D.R.; Smeeth, L.; Stone, P.; Quint, J.K. Low uptake of palliative care for COPD patients within primary care in the UK. Eur. Respir. J. 2018, 51, 1701879. [Google Scholar] [CrossRef]
- Meffert, C.; Hatami, I.; Xander, C.; Becker, G. Palliative care needs in COPD patients with or without cancer: An epidemiological study. Eur. Respir. J. 2015, 46, 663–670. [Google Scholar] [CrossRef]
- Faes, K.; Cohen, J.; Annemans, L. Resource use during the last six months of life among COPD patients: A population-level study. J. Pain Symptom Manag. 2018, 56, 318–326.e7. [Google Scholar] [CrossRef]
- Kuo, L.-C.; Chen, J.-H.; Lee, C.-H.; Tsai, C.-W.; Lin, C.-C. End-of-Life health care utilization between chronic obstructive pulmonary disease and lung cancer patients. J. Pain Symptom Manag. 2019, 57, 933–943. [Google Scholar] [CrossRef]
- Teno, J.M.; Gozalo, P.L.; Bynum, J.P.; Leland, N.E.; Miller, S.C.; Morden, N.E.; Scupp, T.; Goodman, D.C.; Mor, V. Change in end-of-life care for Medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA 2013, 309, 470–477. [Google Scholar] [CrossRef]
- Angus, D.C.; Truog, R.D. Toward better ICU use at the end of life. JAMA 2016, 315, 255–256. [Google Scholar] [CrossRef]
- Carlucci, A.; Vitacca, M.; Malovini, A.; Pierucci, P.; Guerrieri, A.; Barbano, L.; Ceriana, P.; Balestrino, A.; Santoro, C.; Pisani, L. End-of-life discussion, patient understanding and determinants of preferences in very severe COPD patients: A multicentric study. COPD J. Chronic Obstr. Pulm. Dis. 2016, 13, 632–638. [Google Scholar] [CrossRef]
- Higginson, I.J.; Reilly, C.C.; Bajwah, S.; Maddocks, M.; Costantini, M.; Gao, W.; GUIDE_Care project. Which patients with advanced respiratory disease die in hospital? A 14-year population-based study of trends and associated factors. BMC Med. 2017, 15, 19. [Google Scholar] [CrossRef]
- Luk, E.; Sneyers, B.; Rose, L.; Perreault, M.M.; Williamson, D.R.; Mehta, S.; Cook, D.J.; Lapinsky, S.C.; Burry, L. Predictors of physical restraint use in Canadian intensive care units. Crit. Care 2014, 18, R46. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooi, A.W.; Peelen, L.M.; Raijmakers, R.J.; Vroegop, R.L.; Bakker, D.F.; Tekatli, H.; Van den Boogaard, M.; Slooter, A.J. Use of physical restraints in Dutch intensive care units: A prospective multicenter study. Am. J. Crit. Care 2015, 24, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Benbenbishty, J.; Adam, S.; Endacott, R. Physical restraint use in intensive care units across Europe: The PRICE study. Intensive Crit. Care Nurs. 2010, 26, 241–245. [Google Scholar] [CrossRef]
- Minnick, A.F.; Mion, L.C.; Johnson, M.E.; Catrambone, C.; Leipzig, R. Prevalence and variation of physical restraint use in acute care settings in the US. J. Nurs. Scholarsh. 2007, 39, 30–37. [Google Scholar] [CrossRef]
- Kvale, E.; Dionne-Odom, J.N.; Redden, D.T.; Bailey, F.A.; Bakitas, M.; Goode, P.S.; Williams, B.R.; Haddock, K.S.; Burgio, K.L. Predictors of physical restraint use in hospitalized veterans at end of life: An analysis of data from the BEACON Trial. J. Palliat. Med. 2015, 18, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Miravitlles, M.; Hurst, J.R.; Calverley, P.M.; Albert, R.K.; Anzueto, A.; Criner, G.J.; Papi, A.; Rabe, K.F.; Rigau, D. Management of COPD exacerbations: A European respiratory society/American thoracic society guideline. Eur. Respir. J. 2017, 49, 1600791. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.S.; Curtis, J.R.; Meier, D.E. Proactive Integration of Geriatrics and Palliative Care Principles into Practice for Chronic Obstructive Pulmonary Disease. JAMA Intern. Med. 2020, 180, 815–816. [Google Scholar] [CrossRef] [PubMed]
- Scheerens, C.; Beernaert, K.; Pype, P.; Cohen, J.; Deliens, L.; Chambaere, K. Comparing the use and timing of palliative care services in COPD and lung cancer: A population-based survey. Eur. Respir. J. 2018, 51, 1702405. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, N.; Thompson, M.; Warrender-Sparkes, M.; Eastman, P.; Le, B.; Irving, L.; Philip, J. Integrated respiratory and palliative care may improve outcomes in advanced lung disease. ERJ Open Res. 2018, 4, 00102–02017. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-W.; Ruan, S.-Y.; Huang, C.-T.; Tsai, Y.-J.; Lai, F.; Yu, C.-J. Validity of ICD9-CM codes to diagnose chronic obstructive pulmonary disease from National Health Insurance claim data in Taiwan. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3055. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-Y.; Su, C.-C.; Shao, S.-C.; Sung, S.-F.; Lin, S.-J.; Yang, Y.-H.K.; Lai, E.C.-C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349. [Google Scholar] [CrossRef] [PubMed]

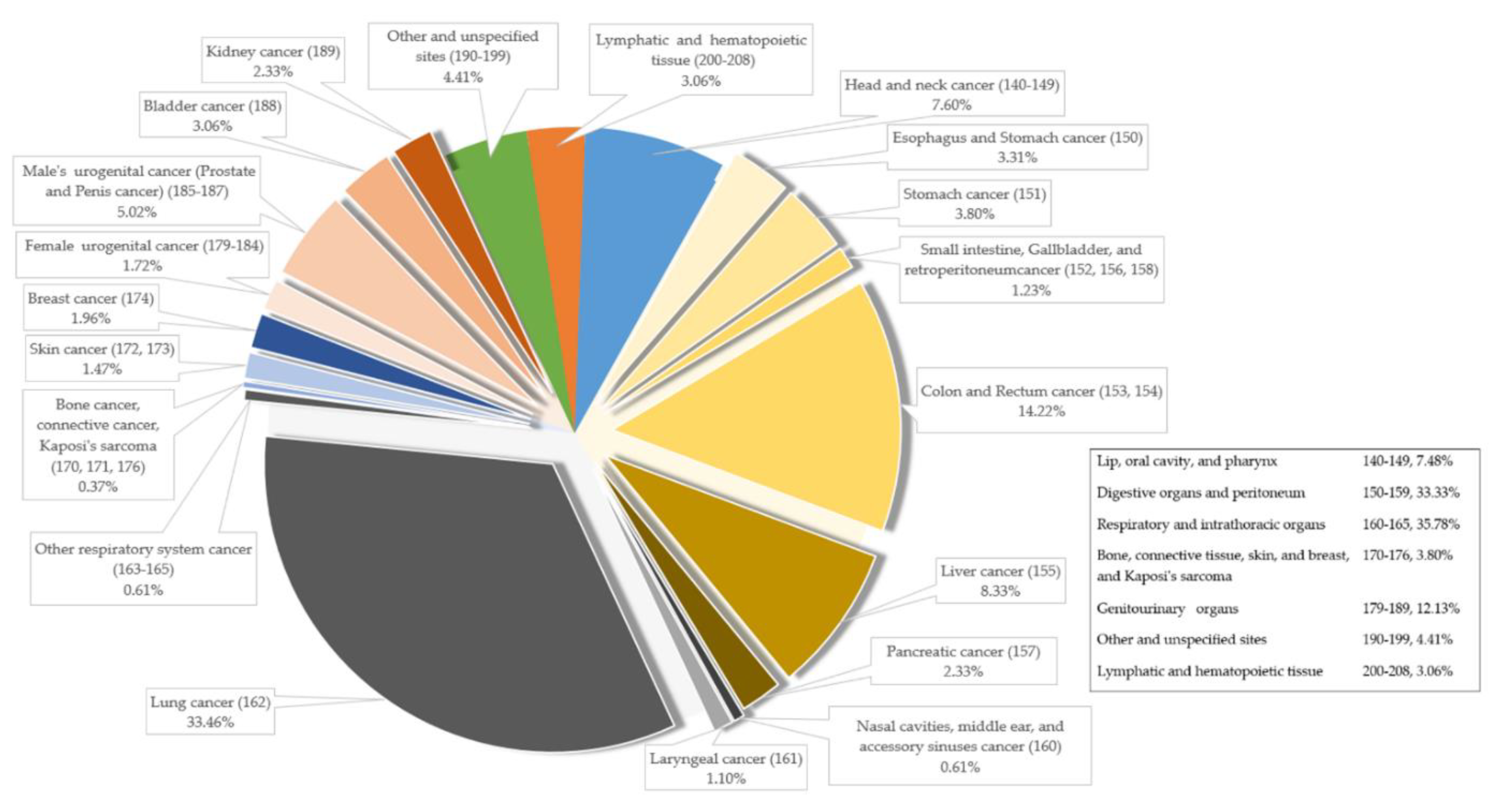
| Variable | Cancer Patients (N = 816) | Non-Cancer (N = 399) | p-Value |
|---|---|---|---|
| Age, mean ± SD | 75.63 ± 9.60 | 80.90 ± 8.53 | <0.0001 |
| Age Group, n(%) | |||
| 55–64 | 138 (16.91) | 24 (6.02) | <0.0001 |
| 65–74 | 228 (27.94) | 72 (18.05) | |
| 75–84 | 315 (38.60) | 170 (42.61) | |
| ≧85 | 135 (16.54) | 133 (33.33) | |
| Gender, n(%) | |||
| Male | 632 (77.45) | 269 (67.42) | 0.0002 |
| Female | 184 (22.55) | 130 (32.58) | |
| Comorbidities, n(%) | |||
| Dementia | 56 (6.86) | 93 (23.31) | <0.0001 |
| Diabetes | 240 (29.41) | 135 (33.83) | 0.1283 |
| Heart failure | 102 (12.50) | 106 (26.57) | <0.0001 |
| Liver cirrhosis | 78 (9.56) | 29 (7.27) | 0.1974 |
| Renal failure | 76 (9.31) | 70 (17.54) | <0.0001 |
| IHD | 182 (22.30) | 113 (28.32) | 0.0228 |
| Stroke | 43 (5.27) | 29 (7.27) | 0.1953 |
| Atrial fibrillation | 54 (6.62) | 43 (10.78) | 0.0175 |
| Hypertension | 379 (46.45) | 173 (43.36) | 0.3265 |
| Anxiety and Depression | 45 (5.51) | 15 (3.76) | 0.2063 |
| Pneumonia | 369 (45.22) | 187 (46.87) | 0.6238 |
| Pulmonary tuberculosis | 33 (4.04) | 15 (3.76) | 0.8764 |
| Osteoporosis | 23 (2.82) | 26 (6.52) | 0.0030 |
| Death, n(%) | |||
| Yes | 645 (79.04) | 335 (83.96) | 0.0444 |
| No | 171 (20.96) | 64 (16.04) | |
| Time to Death, month | |||
| Median (IQR) | 0.96 (0.36–2.04) | 1.20 (0.60–2.40) | 0.0232 |
| Variable | Cancer Patients (N = 816) | Non-Cancer (N = 399) | p-Value | Beta-Coefficient * (95% CI) |
|---|---|---|---|---|
| Patients ever visited ED in one year | ||||
| Frequency of whether had ED visits, n(%) | 719 (88.11) | 357 (89.47) | 0.5035 | 0.01 (−0.03–0.05) |
| Whether had ED visits, mean ± SD | 3.73 ± 3.65 | 3.58 ± 3.59 | 0.5163 | −0.05 (−0.12–0.01) |
| Patients ever hospitalized in one year | ||||
| The number of hospitalizations, mean ± SD | 5.17 ± 3.36 | 5.19 ± 4.29 | 0.9468 | −0.03 (−0.09–0.03) |
| The cumulative length of stay (days), median (IQR) | 57 (35–87) | 60 (35–99) | 0.2555 | 0.01 (−0.05–0.07) |
| ICU ever admitted in one year | ||||
| The number of ICU admissions, mean ± SD | 5.87 ± 6.64 | 10.65 ± 15.43 | <0.0001 | 0.16 (0.11–0.22) # |
| The cumulative length of ICU stay (days), median (IQR) | 15 (6–36) | 34 (15–90) | <0.0001 | 0.22 (0.16–0.28) # |
| Variables | Cancer Patients (N = 816) | Non-Cancer (N = 399) | p-Value | Crude OR (95% CI) | Adjusted OR * (95% CI) |
|---|---|---|---|---|---|
| Invasive life support | |||||
| Blood transfusion (whole blood or red blood cells) | 125 (15.32) | 40 (10.03) | 0.0124 | 1.62 (1.11–2.37) # | 1.66 (1.11–2.49) # |
| Enteral tube insertion | 166 (20.34) | 83 (20.80) | 0.8798 | 0.97 (0.72–1.31) | 1.05 (0.76–1.45) |
| Tube feeding | 186 (22.79) | 80 (20.05) | 0.3013 | 1.18 (0.88–1.58) | 1.22 (0.88–1.68) |
| Invasive mechanical ventilation | 50 (6.13) | 31 (7.77) | 0.3271 | 0.78 (0.49–1.23) | 0.64 (0.39–1.06) |
| Non-invasive mechanical ventilation | 29 (3.55) | 22 (5.51) | 0.1273 | 0.63 (0.36–1.11) | 0.79 (0.42–1.47) |
| Endotracheal intubation | 27 (3.31) | 15 (3.76) | 0.7385 | 0.88 (0.46–1.67) | 0.85 (0.42–1.70) |
| Tracheostomy | 2 (0.25) | 3 (0.75) | 0.3383 | 0.32 (0.05–1.95) | 0.41 (0.06–2.85) |
| Hemodialysis | 4 (0.49) | 3 (0.75) | 0.6898 | 0.65 (0.15–2.92) | 1.13 (0.20–6.39) |
| Echo-guided intervention | 14 (1.72) | 4 (1.00) | 0.4511 | 1.72 (0.56–5.27) | 1.45 (0.44–4.81) |
| Bronchoscope | 4 (0.49) | 7 (1.75) | 0.0472 | 0.27 (0.08–0.95) # | 0.26 (0.07–0.97) # |
| Cardiopulmonary resuscitation (per 10 min) | 17 (2.08) | 9 (2.26) | 0.8351 | 0.92 (0.41–2.09) | 0.69 (0.28–1.65) |
| Invasive examination | |||||
| Abdominal echo | 45 (5.51) | 16 (4.01) | 0.3273 | 1.40 (0.78–2.50) | 1.59 (0.85–2.97) |
| Computed tomography | 74 (9.07) | 21 (5.26) | 0.0225 | 1.80 (1.09–2.96) # | 1.88 (1.10–3.22) # |
| Invasive healthcare | |||||
| Inpatient physical restraints | 6 (0.74) | 10 (2.51) | 0.0153 | 0.29 (0.10–0.80) # | 0.24 (0.08–0.72) # |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, L.-T.; Cheng, K.-C.; Chen, C.-M.; Ko, S.-C.; Chen, P.-J.; Liao, K.-M.; Ho, C.-H. Burden of Healthcare Utilization among Chronic Obstructive Pulmonary Disease Patients with and without Cancer Receiving Palliative Care: A Population-Based Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 4980. https://doi.org/10.3390/ijerph17144980
Kao L-T, Cheng K-C, Chen C-M, Ko S-C, Chen P-J, Liao K-M, Ho C-H. Burden of Healthcare Utilization among Chronic Obstructive Pulmonary Disease Patients with and without Cancer Receiving Palliative Care: A Population-Based Study in Taiwan. International Journal of Environmental Research and Public Health. 2020; 17(14):4980. https://doi.org/10.3390/ijerph17144980
Chicago/Turabian StyleKao, Li-Ting, Kuo-Chen Cheng, Chin-Ming Chen, Shian-Chin Ko, Ping-Jen Chen, Kuang-Ming Liao, and Chung-Han Ho. 2020. "Burden of Healthcare Utilization among Chronic Obstructive Pulmonary Disease Patients with and without Cancer Receiving Palliative Care: A Population-Based Study in Taiwan" International Journal of Environmental Research and Public Health 17, no. 14: 4980. https://doi.org/10.3390/ijerph17144980
APA StyleKao, L.-T., Cheng, K.-C., Chen, C.-M., Ko, S.-C., Chen, P.-J., Liao, K.-M., & Ho, C.-H. (2020). Burden of Healthcare Utilization among Chronic Obstructive Pulmonary Disease Patients with and without Cancer Receiving Palliative Care: A Population-Based Study in Taiwan. International Journal of Environmental Research and Public Health, 17(14), 4980. https://doi.org/10.3390/ijerph17144980






