Hepatitis C Reinfection in People Who Inject Drugs in Resource-Limited Countries: A Systematic Review and Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
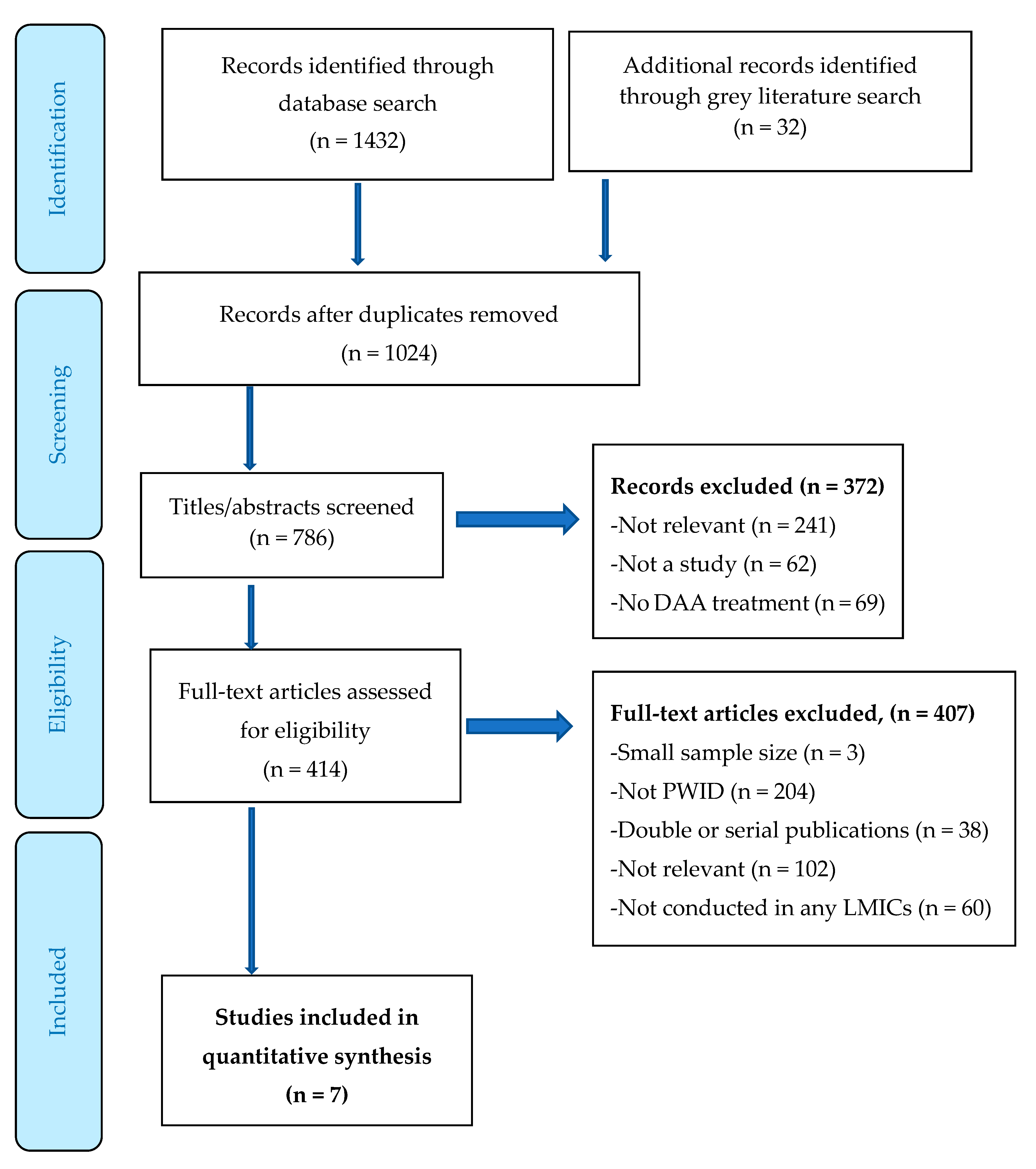
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Asher, A.K.; Portillo, C.J.; Cooper, B.A.; Dawson-Rose, C.; Vlahov, D.; Page, K.A. Clinicians views of hepatitis C virus treatment candidacy with direct-acting antiviral regimens for people who inject drugs. Subst. Use Misuse 2016, 51, 1218–1223. [Google Scholar] [CrossRef]
- Dore, G.J.; Conway, B.; Luo, Y.; Janczewska, E.; Knysz, B.; Liu, Y.; Podsadecki, T. Efficacy and safety of ombitasvir/paritaprevir/r and dasabuvir compared to IFN-containing regimens in genotype 1 HCV patients: The MALACHITE-I/II trials. J. Hepatol. 2016, 64, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Bourliere, M.; Gane, E.J.; Jacobson, I.; Gordon, S.C.; Sulkowski, M.S.; McNabb, B.L.; Reddy, K.R. Long-term follow up of patients with chronic HCV and no or minimal fibrosis shows low risk for liver-related morbidity and mortality after achieving SVR with DAA-based therapy: Results from the Gilead SVR registry. Hepatology 2017, 66 (Suppl. 1), 518A–519A. [Google Scholar]
- Kaberg, M.; Naver, G.; Hammarberg, A.; Weiland, O. Incidence and spontaneous clearance of hepatitis C virus (HCV) in people who inject drugs at the Stockholm Needle Exchange-Importance for HCV elimination. J. Viral Hepat. 2018, 25, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Health Sector Strategy on Viral Hepatitis 2016–2021: Towards Ending Viral Hepatitis; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Martinello, M.; Dore, G.J.; Matthews, G.V.; Grebely, J. Strategies to reduce hepatitis C virus reinfection in people who inject drugs. Infect. Dis. Clin. N. Am. 2018, 32, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.; Bossert, N.; Chen, Y.; Jaanimagi, U.; Markatou, M.; Talal, A.H. Influence of social determinants of health and substance use characteristics on persons who use drugs pursuit of care for hepatitis C virus infection. J. Subst. Abuse Treat. 2019, 102, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rajput, M.K.; Paliwal, D.; Yadav, A.; Chhabra, R.; Singh, S. Genotyping & diagnostic methods for hepatitis C virus: A need of low-resource countries. Indian J. Med. Res. 2018, 147, 445–455. [Google Scholar] [CrossRef]
- Pham, S.T.; Bull, R.A.; Bennett, J.M.; Rawlinson, W.D.; Dore, G.J.; Lloyd, A.R.; White, P.A. Frequent multiple hepatitis C virus infections among injection drug users in a prison setting. Hepatology 2010, 52, 1564–1572. [Google Scholar] [CrossRef]
- Zein, N.N. Clinical significance of hepatitis C virus genotypes. Clin. Microbiol. Rev. 2000, 13, 223–235. [Google Scholar] [CrossRef]
- Graham, C.S.; Swan, T. A path to eradication of hepatitis C in low- and middle-income countries. Antivir. Res. 2015, 119, 89–96. [Google Scholar] [CrossRef]
- Akiyama, M.J.; Lipsey, D.; Heo, M.; Agyemang, L.; Norton, B.L.; Hidalgo, J.; Lora, K.; Litwin, A.H. Low hepatitis C reinfection following direct-acting antiviral therapy among people who inject drugs on opioid agonist therapy. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, L.; Perez, A.B.; Chueca, N.; Aldamiz-Echevarria, T.; Alados, J.C.; Martinez-Sapina, A.M.; Garcia, F. Relapse or reinfection after failing hepatitis C direct acting antiviral treatment: Unravelled by phylogenetic analysis. PLoS ONE 2018, 13, e0201268. [Google Scholar] [CrossRef] [PubMed]
- Hoornenborg, E.; Coyer, L.N.; Achterbergh, R.C.A.; vanderLoeff, M.F.S.; Bruisten, S.; deVries, H.J.C.; AMste, H.I.V.T.E. High incidence of hepatitis C virus (re-) infections among PrEP users in the Netherlands: Implications for prevention, monitoring and treatment. J. Int. AIDS Soc. 2018, 21, 115. [Google Scholar]
- Simmons, B.; Saleem, J.; Hill, A.; Riley, R.D.; Cooke, G.S. Risk of late relapse or reinfection with hepatitis c virus after achieving a sustained virological response: A systematic review and meta-analysis. Clin. Infect. Dis. 2016, 62, 683–694. [Google Scholar] [CrossRef]
- Hajarizadeh, B.; Cunningham, E.B.; Valerio, H.; Martinello, M.; Law, M.; Janjua, N.Z.; Grebely, J. Hepatitis C reinfection after successful antiviral treatment among people who inject drugs: A meta-analysis. J. Hepatol. 2019. [Google Scholar] [CrossRef]
- Konerman, M.A.; Rogers, M.; Kenney, B.; Singal, A.G.; Tapper, E.; Sharma, P.; Waljee, A. Opioid and benzodiazepine prescription among patients with cirrhosis compared to other forms of chronic disease. BMJ Open Gastroenterol. 2019, 6, e000271. [Google Scholar] [CrossRef]
- Heffernan, A.; Cooke, G.S.; Nayagam, S.; Thursz, M.; Hallett, T.B. Scaling up prevention and treatment towards the elimination of hepatitis C: A global mathematical model. Lancet 2019, 393, 1319–1329. [Google Scholar] [CrossRef]
- WHO. Access to Hepatitis C Testing and Treatment for People Who Inject Drugs and People in Prisons—A Global Perspective; Policy Brief; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Foster, G.R.; Dore, G.J.; Wang, S.; Grebely, J.; Sherman, K.E.; Baumgarten, A.; Conway, B.; Jackson, D.; Asselah, T.; Gschwantler, M.; et al. Glecaprevir/pibrentasvir in patients with chronic HCV and recent drug use: An integrated analysis of 7 phase III studies. Drug Alcohol Depend. 2019, 194, 487–494. [Google Scholar] [CrossRef]
- Huang, M.; Liu, W.; Su, L.; Wu, C.; Wu, P.; Yang, S.; Chang, S. Hepatitis C virus re-infection after viral clearance of HCV among HIV-positive patients with recent HCV infection in Taiwan. J. Int. AIDS Soc. 2018, 21 (Suppl. 8), 157. [Google Scholar] [CrossRef]
- Latham, N.H.; Doyle, J.S.; Palmer, A.Y.; Vanhommerig, J.W.; Agius, P.; Goutzamanis, S.; Li, Z.; Pedrana, A.; Gottfredsson, M.; Bouscaillou, J.; et al. Staying hepatitis C negative: A systematic review and meta-analysis of cure and reinfection in people who inject drugs. Liver Int. 2019, 39, 2244–2260. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Janjua, N.Z.; Shafiq, T.K.I.; Chowdhury, E.I.; Sarker, M.S.; Khan, S.I.; Reza, M.; Faruque, M.O.; Kabir, A.; Anis, A.H.; et al. Hepatitis C virus treatment in people who inject drugs (PWID) in Bangladesh. Int. J. Drug Policy 2019, 74, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Pol, S.; Thuluvath, P.J.; Kumada, H.; Toyota, J.; Chayama, K.; Levin, J.; Lawitz, E.; Gadano, A.; Ghesquiere, W.; et al. Long-term follow-up of clinical trial patients treated for chronic HCV infection with daclatasvir-based regimens. Liver Int. 2018, 38, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Rockstroh, J.K.; Orkin, C.; Viani, R.M.; Wyles, D.; Luetkemeyer, A.F.; Lazzarin, A.; Lazzarin, A.; Soto-Malave, R.; Nelson, M.R.; Bhagani, S.R.; et al. Safety and efficacy of ombitasvir, paritaprevir with ritonavir ± dasabuvir with or without ribavirin in patients with human immunodeficiency virus-1 and hepatitis C virus genotype 1 or genotype 4 coinfection: TURQUOISE-I Part 2. Open Forum Infect. Dis. 2017, 4, ofx154. [Google Scholar] [CrossRef]
- Sarrazin, C.; Isakov, V.; Svarovskaia, E.S.; Hedskog, C.; Martin, R.; Chodavarapu, K.; Brainard, D.M.; Miller, M.D.; Mo, H.; Molina, J.M.; et al. Late relapse versus hepatitis c virus reinfection in patients with sustained virologic response after sofosbuvir-based therapies. Clin. Infect. Dis. 2017, 64, 44–52. [Google Scholar] [CrossRef]
- Lin, L. Bias caused by sampling error in meta-analysis with small sample sizes. PLoS ONE 2018, 13, e0204056. [Google Scholar] [CrossRef]
- Gountas, I.; Sypsa, V.; Blach, S.; Razavi, H.; Hatzakis, A. HCV elimination among people who inject drugs. Modelling pre- and post–WHO elimination era. PLoS ONE 2018, 13, e0202109. [Google Scholar] [CrossRef]
- Cipriano, L.E.; Goldhaber-Fiebert, J.D. Population health and cost-effectiveness implications of a “Treat All” recommendation for HCV: A review of the model-based evidence. MDM Policy Pract. 2018, 3, 2381468318776634. [Google Scholar] [CrossRef]
- Akiyama, M.C.; Charles, M.; Lizcano, J.; Cherutich, P.K.; Ann, E.; Hepatitis, C. Prevalence, Estimated Incidence, Risk Behaviors, and Genotypic Distribution among People Who Inject Drugs in Kenya. 2018. Available online: https://ssrn.com/abstract=3220107 or http://dx.doi.org/10.2139/ssrn.3220107 (accessed on 10 January 2020). [CrossRef]
- Rossi, C.; Butt, Z.A.; Wong, S.; Buxton, J.; Islam, N.; Yu, A.; Darvishian, M.; Gilbert, M.; Wong, J.; BC Hepatitis Testers Cohort Team. Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. J. Hepatol. 2018, 69, 1007–1014. [Google Scholar] [CrossRef]
- Bethea, E.D.; Chen, Q.; Hur, C.; Chung, R.T.; Chhatwal, J. Reply. Hepatology 2018, 67, 1641–1642. [Google Scholar] [CrossRef]
- Cousien, A.; Leclerc, P.; Morissette, C.; Bruneau, J.; Roy, E.; Tran, V.C.; Cox, J. The need for treatment scale-up to impact HCV transmission in people who inject drugs in Montreal, Canada: A modelling study. BMC Infect. Dis. 2017, 17, 162. [Google Scholar] [CrossRef]
- Eckman, M.H.; Ward, J.W.; Sherman, K.E. Cost effectiveness of universal screening for hepatitis C virus infection in the era of direct-acting, pangenotypic treatment regimens. Clin. Gastroenterol. Hepatol. 2019, 17, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Hajarizadeh, B.; Grebely, J.; Dore, G.J.; Matthews, G.V. HCV cure and reinfection among people with HIV/HCV coinfection and people who inject drugs. Curr. HIV/AIDS Rep. 2017, 14, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Bartlett, S.; Dore, G.; Bopage, R.; Finlayson, R.; Baker, D.; Matthews, G. Universal access to DAA therapy paves theway for HCV control and elimination among people living with HIV in Australia. J. Hepatol. 2018, 68 (Suppl. 1), S312–S313. [Google Scholar] [CrossRef]
- Grebely, J.; Hajarizadeh, B.; Lazarus, J.V.; Bruneau, J.; Treloar, C.; International Network on Hepatitis in Substance Users. Elimination of hepatitis C virus infection among people who use drugs: Ensuring equitable access to prevention, treatment, and care for all. Int. J. Drug Policy 2019, 72, 1–10. [Google Scholar] [CrossRef]
- Falade-Nwulia, O.; Sulkowski, M.S.; Merkow, A.; Latkin, C.; Mehta, S.H. Understanding and addressing hepatitis C reinfection in the oral direct-acting antiviral era. J. Viral Hepat. 2018, 25, 220–227. [Google Scholar] [CrossRef]
- Kelly, D. Hepatitis C viraemia after apparent spontaneous clearance. Lancet 2016, 387, 1968. [Google Scholar] [CrossRef]
- Kerkerian, G.; Alimohammadi, A.; Raycraft, T.; Conway, B. Repeated spontaneous clearance of hepatitis C virus infection in the setting of long-term non-progression of HIV infection. Infect. Dis. Rep. 2017, 9, 91–93. [Google Scholar] [CrossRef]
- Quinones-Mateu, M.E.; Avila, S.; Reyes-Teran, G.; Martinez, M.A. Deep sequencing: Becoming a critical tool in clinical virology. J. Clin. Virol. 2014, 61, 9–19. [Google Scholar] [CrossRef]
- Vickerman, P.; Grebely, J.; Dore, G.J.; Sacks-Davis, R.; Page, K.; Thomas, D. The more you look, the more you find: Effects of hepatitis C virus testing interval on reinfection incidence and clearance and implications for future vaccine study design. J. Infect. Dis. 2012, 205, 1342–1350. [Google Scholar] [CrossRef]
| Author, Year | Study Design | Setting (LMICs in Multi-Site Studies, SES and Median Income of Relevant Countries) | Study Population (N) % Male Initial HCV Prevalence | Testing Interval | Reinfection Rate (per 100 PY) (Available Rates among Relevant Special Populations Noted) | Measurement of Reinfection | Follow-Up Time (Participant Range if Given) | Attrition Rate (Loss to Follow-Up) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Foster et al., 2019 [20] | Integrated analysis of clinical trials | Multi-country Upper middle-income (Mexico = $9673 Romania = $12,306 Russia = $11,288) | N = 1819 Male 57.0% Prevalence = 50.0% * | Followed for 24 weeks, reinfection detected at week 12 | 0.6 | Deep gene sequencing | 24 weeks | 0.01 |
| 2 | Huang et al., 2019 [21] | Cohort study | Taiwan = $14,273 Upper middle-income | N = 219 Male 100.0% Prevalence = 33.7% | Varied: either 12 months (majority), following abnormal labs (minority) | 10.5 14.1 (for DAA treatment recipients) | No differentiation between reinfection and relapse | 2.1–6.6 years | 0.04 |
| 3 | Latham et al., 2019 [22] | Systematic review and meta-analysis | Multi-country Upper middle-income (Georgia = $4722) | N = 827 Sex not delineated Prevalence not delineated | Varied among studies | 1.9 (for recent PWID) 0.6 (for OST recipients) | Genotyping, deep gene sequencing or none | 24 weeks–3 years | 0.02 |
| 4 | Rahman et al., 2019 [23] | Prospective cohort | Bangladesh = $1698 Lower middle-income | N = 200 Sex not delineated Prevalence = 42% | Once 12 weeks after SVR | 4.2 | Genotyping | 12 weeks | 0.05 |
| 5 | Reddy et al., 2018 [24] | Cohort study | Multi-Country Upper middle-income (Brazil = $9001, Argentina = $11,683, Mexico = $9673, Taiwan = $14,273) | N = 1503 Male 60% Prevalence = 56.8% * | Day 1, week 24, 48, 96 and 144 | 0.02 | Reflex genotyping | 144 weeks | 0.01 |
| 6 | Rockstroh et al., 2017 [25] | Clinical trial | Multi-Country Upper middle-income (Russia = $11,288) | N = 228 Male 80%Prevalence = 50% * | Not delineated | 1.9 | Deep gene sequencing | 12 weeks | 0.02 |
| 7 | Sarrazin et al., 2017 [26] | Cohort Study | Multi-Country Upper middle-income (Russia = $11,288) | N = 3004 Sex not delineated Prevalence = 50% * | Once at 24 weeks | 0.5 | Deep gene sequencing | 24 weeks | Not delineated |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muller, A.; Vlahov, D.; Akiyama, M.J.; Kurth, A. Hepatitis C Reinfection in People Who Inject Drugs in Resource-Limited Countries: A Systematic Review and Analysis. Int. J. Environ. Res. Public Health 2020, 17, 4951. https://doi.org/10.3390/ijerph17144951
Muller A, Vlahov D, Akiyama MJ, Kurth A. Hepatitis C Reinfection in People Who Inject Drugs in Resource-Limited Countries: A Systematic Review and Analysis. International Journal of Environmental Research and Public Health. 2020; 17(14):4951. https://doi.org/10.3390/ijerph17144951
Chicago/Turabian StyleMuller, Abbe, David Vlahov, Matthew J. Akiyama, and Ann Kurth. 2020. "Hepatitis C Reinfection in People Who Inject Drugs in Resource-Limited Countries: A Systematic Review and Analysis" International Journal of Environmental Research and Public Health 17, no. 14: 4951. https://doi.org/10.3390/ijerph17144951
APA StyleMuller, A., Vlahov, D., Akiyama, M. J., & Kurth, A. (2020). Hepatitis C Reinfection in People Who Inject Drugs in Resource-Limited Countries: A Systematic Review and Analysis. International Journal of Environmental Research and Public Health, 17(14), 4951. https://doi.org/10.3390/ijerph17144951





