Integrated 3D Information for Custom-Made Bone Grafts: Focus on Biphasic Calcium Phosphate Bone Substitute Biomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomaterials
- T = 1200 °C@2h: nr.3 samples at peak sintering temperature of 1200 °C for 2 hours;
- T = 1200 °C@4h: nr.3 samples at peak sintering temperature of 1200 °C for 4 hours;
- T = 1200 °C@8h: nr.3 samples at peak sintering temperature of 1200 °C for 8 hours;
- T = 1250 °C@2h: nr.3 samples at peak sintering temperature of 1250 °C for 2 hours;
- T = 1250 °C@4h: nr.3 samples at peak sintering temperature of 1250 °C for 4 hours;
- T = 1250 °C@8h: nr.3 samples at peak sintering temperature of 1250 °C for 8 hours.
2.2. High-Resolution Tomography (microCT)
2.3. Compressive Testing
2.4. X-ray Diffraction
2.5. Statistical Analysis
3. Results
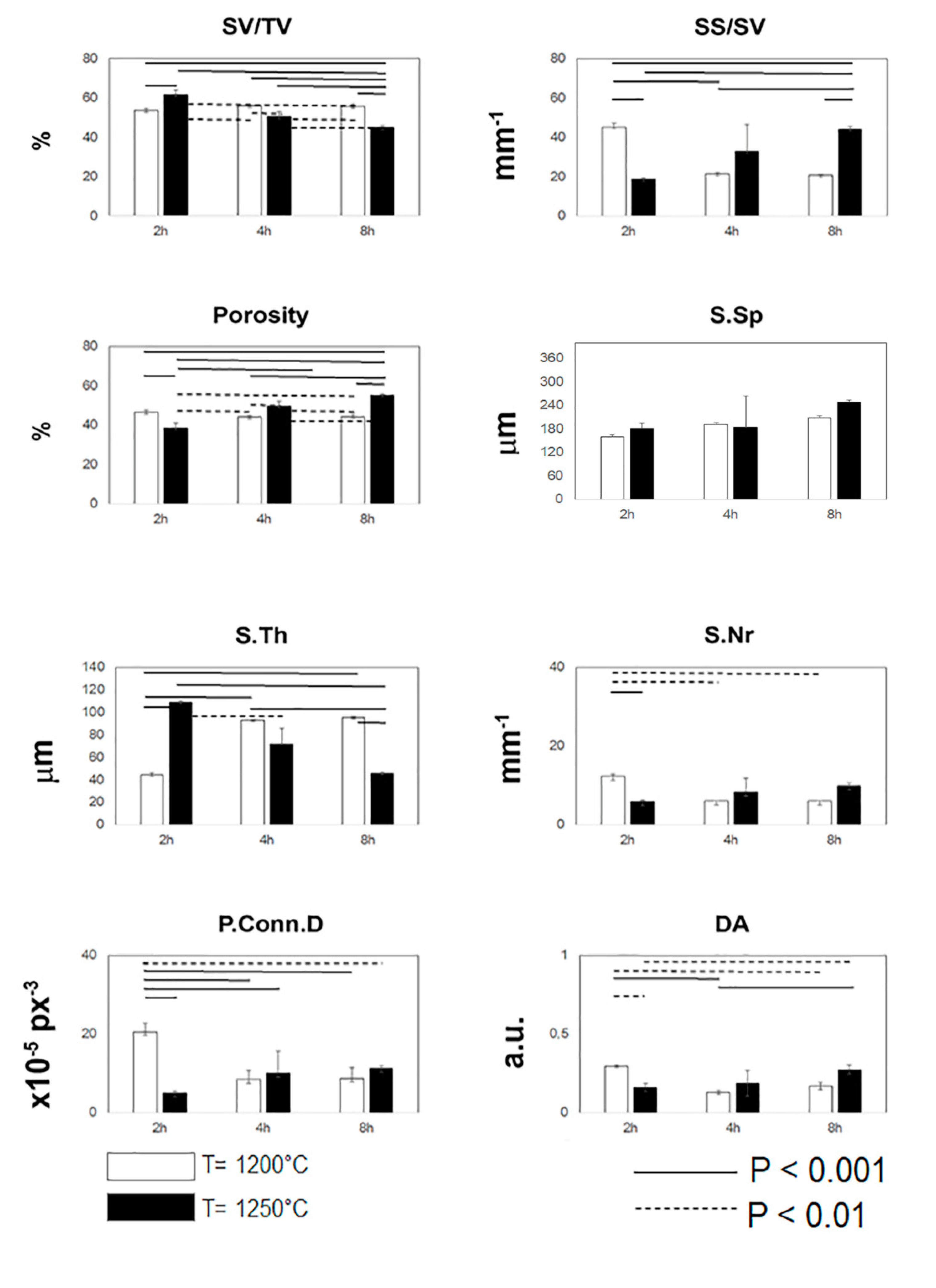
3.1. MicroCT Analysis
3.2. Mechanical Characterization
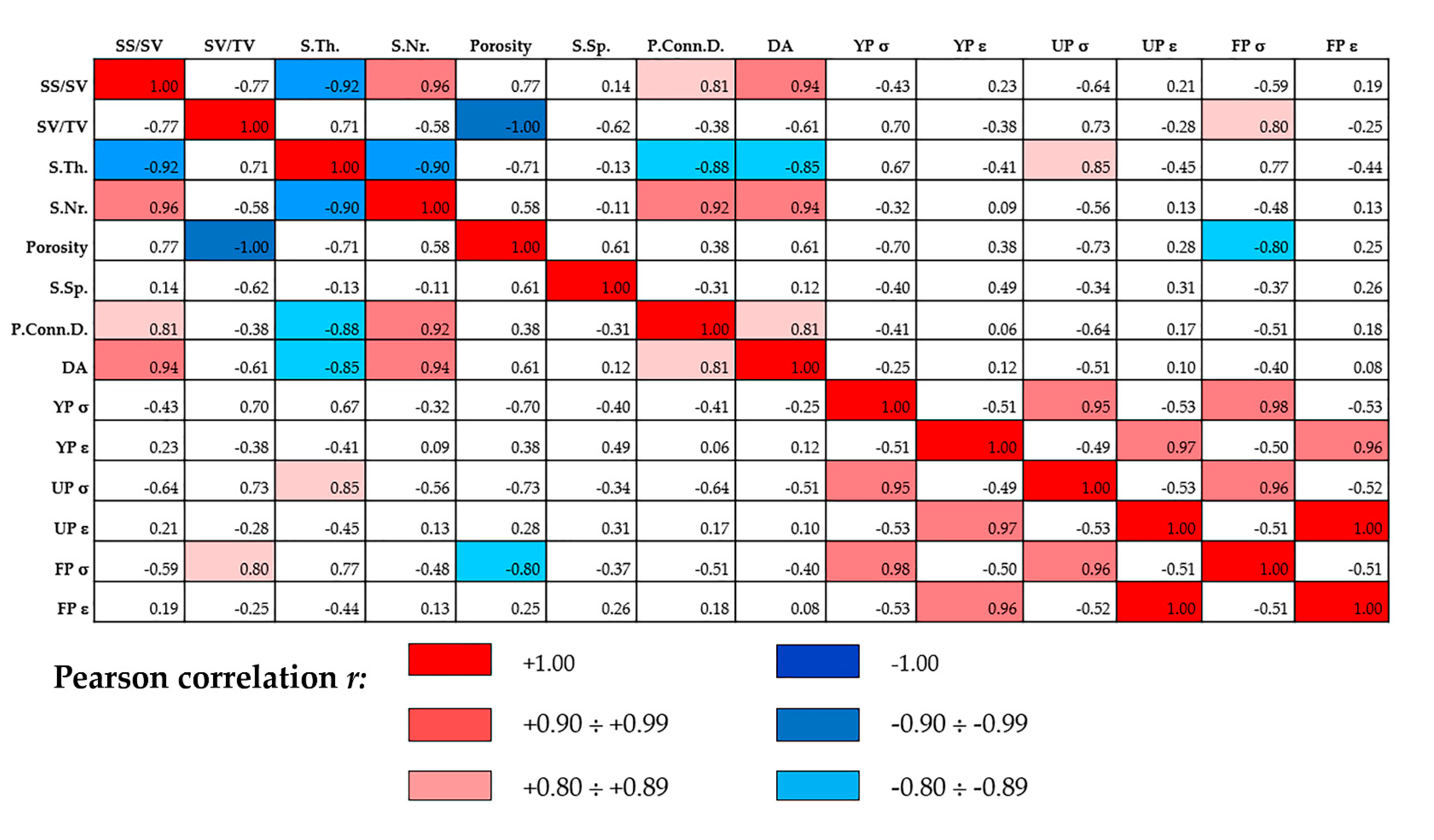
3.3. Statistical Inference
3.4. X-ray Diffraction Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Materials
Abbreviations
| BSB | Bone substitute biomaterial |
| 3D | Three-dimensions |
| BCP | Biphasic calcium phosphate |
| MicroCT | Microtomography |
| PCC | Pearson correlation coefficient |
| XRD | X-ray diffraction |
References
- Mangano, F.; Macchi, A.; Caprioglio, A.; Sammons, R.L.; Piattelli, A.; Mangano, C. Survival and complication rates of fixed restorations supported by locking-taper implants: A prospective study with 1 to 10 years of follow-up. J. Prosthodont. 2014, 23, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Shibli, J.A.; Sammons, R.L.; Veronesi, G.; Piattelli, A.; Mangano, C. Clinical outcome of narrow-diameter (3.3-mm) locking-taper implants: A prospective study with 1 to 10 years of follow-up. Int. J. Oral Maxillofac. Implants 2014, 29, 448–455. [Google Scholar] [CrossRef]
- Tamimi, F.; Torres, J.; Al-Abedalla, K.; López-Cabarcos, E.; Alkhraisat, M.; Bassett, D.C.; Gbureck, U.; Barralet, J. Osseointegration of dental implants in 3D-printed synthetic onlay grafts customized according to bone metabolic activity in recipient site. Biomaterials 2014, 35, 5436–5445. [Google Scholar] [CrossRef]
- Jacotti, M.; Barausse, C.; Felice, P. Posterior atrophic mandible rehabilitation with onlay allograft created with CAD-CAM procedure: A case report. Implant Dent. 2014, 23, 22–28. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants—A Cochrane systematic review. Eur. J. Oral Implantol. 2009, 2, 167–184. [Google Scholar]
- LaPrade, R.F.; Botker, J.C. Donor-site morbidity after osteochondral autograft transfer procedures. Arthroscopy 2004, 20, e69–e73. [Google Scholar] [CrossRef]
- Palmer, S.H.; Gibbons, C.L.; Athanasou, N.A. The pathology of bone allograft. J. Bone Jt. Surg. Br. 1999, 81, 333–335. [Google Scholar] [CrossRef]
- Klawitter, J.J.; Hulbert, S.F. Application of porous ceramics for the attachment of load bearing internal orthopedic applications. J. Biomed. Mater. Res. Symp. 1971, 2, 161–229. [Google Scholar] [CrossRef]
- Ayers, R.A.; Wolford, L.M.; Bateman, T.A.; Ferguson, V.L.; Simske, S.J. Qualification of bone ingrowth into porous block hydroxyapatite in humans. J. Biomed. Mater. Res. 1999, 47, 54–59. [Google Scholar] [CrossRef]
- Jin, Q.-M.; Takita, H.; Kohgo, T.; Atsumi, K.; Itoh, H.; Kuboki, Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J. Biomed. Mater. Res. 2000, 51, 491–499. [Google Scholar] [CrossRef]
- Bignon, A.; Chouteau, J.; Chevalier, J.; Fantozzi, G.; Carret, J.-P.; Chavassieux, P.; Boivin, G.; Melin, M.; Hartmann, D. Effect of micro- and macroporosity of bone substitutes on their mechanical properties and cellular response. J. Mater. Sci. Mater. Electron. 2003, 14, 1089–1097. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate-based bioceramics. Materials 2013, 6, 3840–3942. [Google Scholar] [CrossRef]
- Brown, O.; McAfee, M.; Clarke, S.; Buchanan, F. Sintering of biphasic calcium phosphates. J. Mater. Sci. Mater. Med. 2010, 21, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pan, Y.; Shao, C.Y. Preparation of b-TCP with high thermal stability by solid reaction route. J. Mater. Sci. 2003, 38, 1049–1056. [Google Scholar]
- Kasim, S.R.; Muhamad, N.F.; Ramakrishan, S. Effect of sintering temperature on biphasic calcium phosphate (BCP) biocomposite. Adv. Mater. Res. 2015, 1087, 475–478. [Google Scholar] [CrossRef]
- Safronova, T.; Selezneva, I.I.; Tikhonova, S.A.; Kiselev, A.; Davydova, G.; Shatalova, T.; Larionov, D.; Rau, J.V. Biocompatibility of biphasic α,β-tricalcium phosphate ceramics in vitro. Bioact. Mater. 2020, 5, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Bouler, J.-M.; Royer, J.; Passuti, N.; Daculsi, G.; Trécant, M.; Delécrin, J. Macroporous biphasic calcium phosphate ceramics: Influence of five synthesis parameters on compressive strength. J. Biomed. Mater. Res. 1996, 32, 603–609. [Google Scholar] [CrossRef]
- Yetmez, M. Sintering Behavior and mechanical properties of biphasic calcium phosphate ceramics. Adv. Mater. Sci. Eng. 2014, 2014, 871749. [Google Scholar] [CrossRef]
- Langer, M.; Pacureanu, A.; Suhonen, H.; Grimal, Q.; Cloetens, P.; Peyrin, F. X-Ray Phase nanotomography resolves the 3D human bone ultrastructure. PLoS ONE 2012, 7, e35691. [Google Scholar] [CrossRef]
- Peyrin, F.; Dong, P.; Pacureanu, A.; Langer, M. Micro- and nano-CT for the study of bone ultrastructure. Curr. Osteoporos. Rep. 2014, 12, 465–474. [Google Scholar] [CrossRef]
- Giuliani, A.; Iezzi, G.; Mozzati, M.; Gallesio, G.; Mazzoni, S.; Tromba, G.; Zanini, F.; Piattelli, A.; Mortellaro, C. Bisphosphonate-related osteonecrosis of the human jaw: A combined 3D assessment of bone descriptors by histology and synchrotron radiation-based microtomography. Oral Oncol. 2018, 82, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, G.; Mangano, C.; Barone, A.; Tirone, F.; Baggi, L.; Tromba, G.; Piattelli, A.; Giuliani, A. Jawbone remodeling: A Conceptual study based on Synchrotron High-resolution Tomography. Sci. Rep. 2020, 10, 3777. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Iezzi, G.; Mozzati, M.; Gallesio, G.; Mazzoni, S.; Tromba, G.; Zanini, F.; Piattelli, A.; Mortellaro, C. Three-dimensional microarchitecture and local mineralization of human jaws affected by bisphosphonate-related osteonecrosis. Oral Oncol. 2018, 84, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Tavella, S.; Ruggiu, A.; Giuliani, A.; Brun, F.; Canciani, B.; Manescu, A.; Marozzi, K.; Cilli, M.; Costa, D.; Liu, Y.; et al. Bone turnover in wild type and pleiotrophin-transgenic mice housed for three months in the International Space Station (ISS). PLoS ONE 2012, 7, e33179. [Google Scholar] [CrossRef]
- Costa, D.; Lazzarini, E.; Canciani, B.; Giuliani, A.; Spano’, R.; Marozzi, K.; Manescu, A.; Cancedda, R.; Tavella, S. Altered bone development and turnover in transgenic mice over-expressing Lipocalin-2 in bone. J. Cell. Physiol. 2013, 228, 2210–2221. [Google Scholar] [CrossRef]
- Canciani, B.; Ruggiu, A.; Giuliani, A.; Panetta, D.; Marozzi, K.; Tripodi, M.; Salvadori, P.A.; Cilli, M.; Ohira, Y.; Cancedda, R.; et al. Effects of long time exposure to simulated micro- and hypergravity on skeletal architecture. J. Mech. Behav. Biomed. Mater. 2015, 51, 1–12. [Google Scholar] [CrossRef]
- Giuliani, A.; Mazzoni, S.; Ruggiu, A.; Canciani, B.; Cancedda, R.; Tavella, S. High-resolution X-ray tomography: A 3D exploration into the skeletal architecture in mouse models submitted to microgravity constraints. Front. Physiol. 2018, 9, 181. [Google Scholar] [CrossRef]
- Olubamiji, A.D.; Izadifar, Z.; Chen, D.X. Synchrotron Imaging Techniques for bone and cartilage tissue engineering: Potential, current trends, and future directions. Tissue Eng. Part B: Rev. 2014, 20, 503–522. [Google Scholar] [CrossRef]
- Mangano, F.; Mangano, F.; Gobbi, L.; Admakin, O.; Iketani, S.; Giuliani, A. comparative study between laser light stereo-lithography 3D-printed and traditionally sintered biphasic calcium phosphate scaffolds by an integrated morphological, morphometric and mechanical analysis. Int. J. Mol. Sci. 2019, 20, 3118. [Google Scholar] [CrossRef] [PubMed]
- Luca, R.E.; Giuliani, A.; Mănescu, A.; Heredea, R.; Hoinoiu, B.; Constantin, G.D.; Duma, V.-F.; Todea, C.D. Osteogenic potential of bovine bone graft in combination with laser photobiomodulation: An ex vivo demonstrative study in wistar rats by cross-linked studies based on synchrotron microtomography and histology. Int. J. Mol. Sci. 2020, 21, 778. [Google Scholar] [CrossRef]
- Giuliani, A.; Manescu, A.; Mohammadi, S.; Mazzoni, S.; Piattelli, A.; Mangano, C.; Iezzi, G.; Mangano, C. Quantitative kinetics evaluation of blocks versus granules of biphasic calcium phosphate scaffolds (HA/β-TCP 30/70) by synchrotron radiation X-ray microtomography. Implant. Dent. 2016, 25, 6–15. [Google Scholar] [CrossRef]
- Giuliani, A.; Manescu, A.; Larsson, E.; Tromba, G.; Luongo, G.; Piattelli, A.; Mangano, C.; Iezzi, G.; Mangano, C. In vivo regenerative properties of coralline-derived (Biocoral) scaffold grafts in human maxillary defects: Demonstrative and comparative study with beta-tricalcium phosphate and biphasic calcium phosphate by synchrotron radiation X-ray microtomography. Clin. Implant. Dent. Relat. Res. 2013, 16, 736–750. [Google Scholar] [CrossRef]
- Manescu, A.; Giuliani, A.; Mohammadi, S.; Tromba, G.; Mazzoni, S.; Diomede, F.; Zini, N.; Piattelli, A.; Trubiani, O. Osteogenic potential of dual-blocks cultured with periodontal ligament stem cells: In-vitro and synchrotron microtomography study. J. Periodontal.Res. 2016, 51, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, S.; Mohammadi, S.; Tromba, G.; Diomede, F.; Trubiani, O.; Giuliani, A. role of cortico-cancellous heterologous bone in human periodontal ligament stem cell xeno—Free culture studied by synchrotron radiation phase-contrast microtomography. Int. J. Mol. Sci. 2017, 18, 364. [Google Scholar] [CrossRef]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature. symbols. and units. Report of the ASBMR histomorphometry nomenclature committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Degidi, M.; Iezzi, G.; Pecora, G.; Piattelli, M.; Orsini, G.; Caputi, S.; Perrotti, V.; Mangano, C.; Piattelli, A. maxillary sinus augmentation with different biomaterials: A comparative histologic and histomorphometric study. Man. Implant. Dent. 2006, 15, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, G.; Piattelli, A.; Giuliani, A.; Mangano, C.; Barone, A.; Manzon, L.; Degidi, M.; Scarano, A.; Filippone, A.; Perrotti, V. Molecular, cellular and pharmaceutical aspects of bone grafting materials and membranes during maxillary sinus-lift procedures. Part 2: Detailed characteristics of the materials. Curr. Pharm. Biotechnol. 2017, 18, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Perrotti, V.; Shibli, J.A.; Mangano, C.; Ricci, L.; Piattelli, A.; Iezzi, G. Maxillary sinus grafting with biphasic calcium phosphate ceramics: Clinical and histologic evaluation in man. Int. J. Oral Maxillofac. Implant. 2013, 28, 51–56. [Google Scholar] [CrossRef]
- Piattelli, A.; Scarano, A.; Mangano, C. Clinical and histologic aspects of biphasic calcium phosphate ceramic (BCP) used in connection with implant placement. Biomaterials 1996, 17, 1767–1770. [Google Scholar] [CrossRef]
- Mangano, C.; Barboni, B.; Valbonetti, L.; Berardinelli, P.; Martelli, A.; Muttini, A.; Bedini, R.; Tetè, S.; Piattelli, A.; Mattioli, M. In vivo behavior of a custom-made 3D synthetic bone substitute in sinus augmentation procedures in sheep. J. Oral Implant. 2015, 41, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Mangano, C.; Valbonetti, L.; Marruchella, G.; Berardinelli, P.; Martelli, A.; Muttini, A.; Mauro, A.; Bedini, R.; Turriani, M.; et al. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS ONE 2013, 8, e63256. [Google Scholar] [CrossRef] [PubMed]
- Annibali, S.; Iezzi, G.; Sfasciotti, G.L.; Cristalli, M.P.; Vozza, I.; Mangano, C.; La Monaca, G.; Polimeni, A. histological and histomorphometric human results of HA-Beta-TCP 30/70 compared to three different biomaterials in maxillary sinus augmentation at 6 months: A preliminary report. BioMed Res. Int. 2015, 2015, 156850. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Zecca, P.A.; Van Noort, R.; Apresyan, S.; Iezzi, G.; Piattelli, A.; Macchi, A.; Mangano, C. Custom-made computer-aided-design/computer-aided-manufacturing biphasic calcium-phosphate scaffold for augmentation of an atrophic mandibular anterior ridge. case reports. Dentistry 2015, 2015, 941265. [Google Scholar] [CrossRef]
- Mangano, C.; Sinjari, B.; Shibli, J.A.; Mangano, C.; Hamisch, S.; Piattelli, A.; Perrotti, V.; Iezzi, G. A human clinical, histological, histomorphometrical, and radiographical study on biphasic HA-Beta-TCP 30/70 in maxillary sinus augmentation. Clin. Implant. Dent. Relat. Res. 2013, 17, 610–618. [Google Scholar] [CrossRef]
- Carrel, J.-P.; Wiskott, A.; Moussa, M.; Rieder, P.; Scherrer, S.; Durual, S. A 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation. Clin. Oral Implant. Res. 2014, 27, 55–62. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3-D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Renghini, C.; Giuliani, A.; Mazzoni, S.; Brun, F.; Larsson, E.; Baino, F.; Vitale-Brovarone, C. Microstructural characterization and in vitro bioactivity of porous glass-ceramic scaffolds for bone regeneration by synchrotron radiation X-ray microtomography. J. Eur. Ceram. Soc. 2013, 33, 1553–1565. [Google Scholar] [CrossRef]
- Pathria, M.N.; Chung, C.B.; Resnick, D.L. Acute and stress-related injuries of bone and cartilage: Pertinent anatomy, basic biomechanics, and imaging Perspective. Radiology 2016, 280, 21–38. [Google Scholar] [CrossRef]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues; Springer: New York, NY, USA, 1993. [Google Scholar]
- Rho, J.Y.; Hobatho, M.C.; Ashman, R.B. Relations of density and CT numbers to mechanical properties for human cortical and cancellous bone. Med. Eng. Phys. 1995, 17, 347–355. [Google Scholar] [CrossRef]
- Brazete, D.; Torres, P.M.C.; Abrantes, J.C.C.; Ferreira, J.M.F. Influence of the Ca/P ratio and cooling rate on the allotropic α↔β-tricalcium phosphate phase transformations. Ceram. Int. 2018, 44, 8249–8256. [Google Scholar] [CrossRef]
- Kreidler, E.R.; Hummel, F.A. Phase relations in the system SrO-P2O5 and the influence of water vapor on the formation of Sr4P2O9. Inorg. Chem. 1967, 6, 884–891. [Google Scholar] [CrossRef]
- Elliot, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Kamitakahara, M.; Ohtsuki, C.; Miyazaki, T. Behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J. Biomater. Appl. 2008, 23, 197–212. [Google Scholar] [CrossRef] [PubMed]
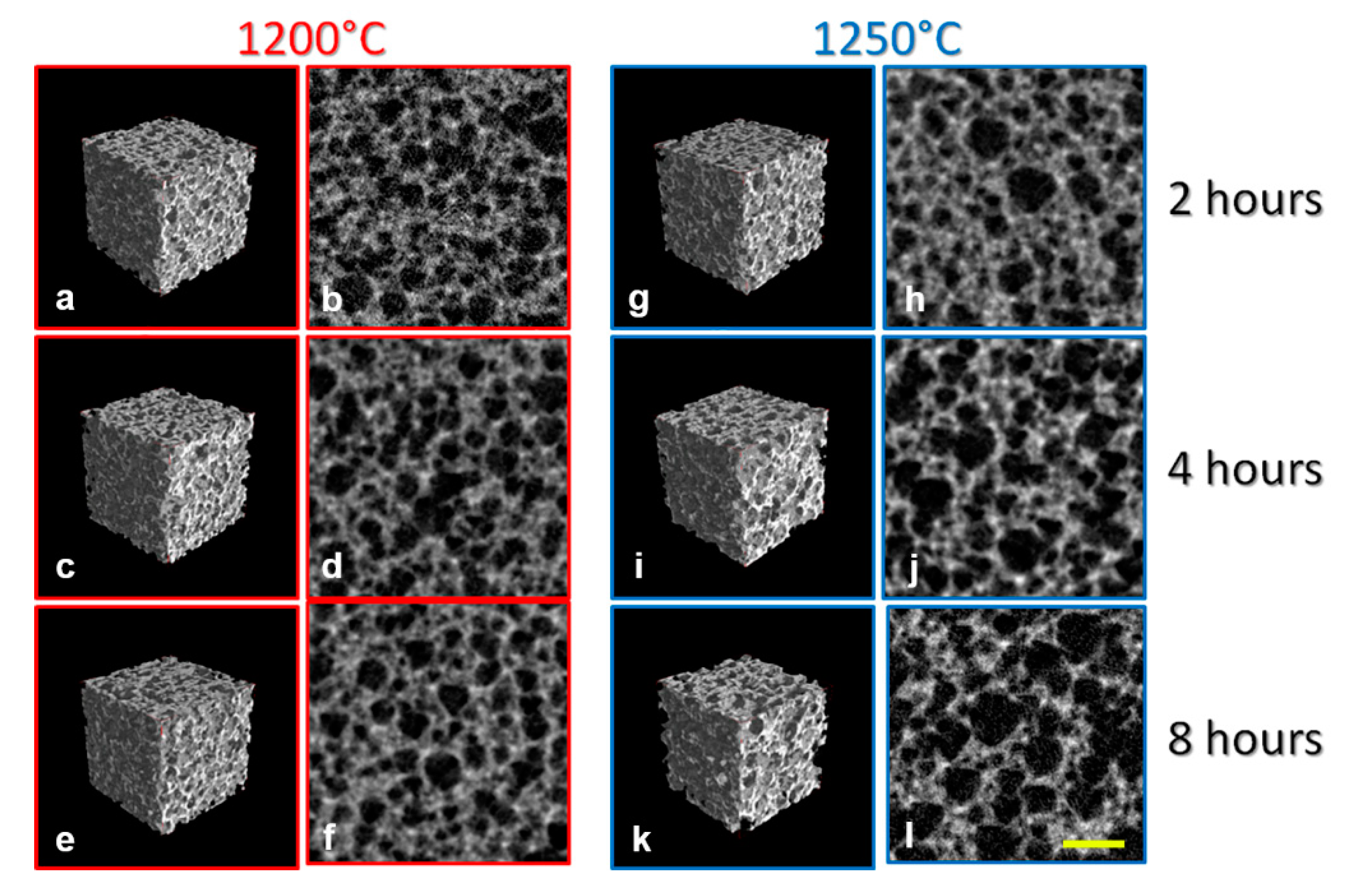
| Group | Yield Point (YP) | Ultimate Point (UP) | Fracture Point (FP) | |||
|---|---|---|---|---|---|---|
| Stress (MPa) | Strain (%) | Stress (MPa) | Strain (%) | Stress (MPa) | Strain (%) | |
| T = 1200 °C 2 hours | 2.0 (0.6) | 2.7 (1.9) | 2.7 (0.1) | 4.6 (2.6) | 2.6 (0.8) | 5.0 (2.8) |
| T = 1200 °C 4 hours | 1.7 (0.1) | 4.1 (1.3) | 2.9 (0.4) | 6.1 (1.3) | 2.5 (0.8) | 6.6 (1.6) |
| T = 1200 °C 8 hours | 1.9 (0.2) | 2.2 (0.4) | 3.0 (0.4) | 3.7 (0.4) | 2.8 (0.2) | 4.0 (0.3) |
| T = 1250 °C 2 hours | 3.2 (0.8) | 2.3 (0.2) | 4.4 (1.0) | 3.7 (0.7) | 4.0 (1.3) | 4.0 (0.7) |
| T = 1250 °C 4 hours | 2.0 (0.8) | 2.0 (0.3) | 3.2 (0.6) | 3.4 (1.1) | 2.6 (0.3) | 3.7 (1.2) |
| T = 1250 °C 8 hours | 1.7 (0.5) | 4.0 (1.0) | 2.6 (0.1) | 5.4 (1.4) | 2.3 (0.1) | 5.7 (1.3) |
| Sample | HA | β-TCP | CaO | α-TCP | TTCP |
|---|---|---|---|---|---|
| T = 1200 °C@2hours | 28.27 | 71.15 | >0.04 and < 0.06 | < DL | < DL |
| T = 1250 °C@2hours | 28.51 | 70.77 | >0.04 and < 0.06 | < DL | < DL |
| T = 1250 °C@4hours | 31.00 | 49.79 | < DL | 19.20 | < DL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliani, A.; Gatto, M.L.; Gobbi, L.; Mangano, F.G.; Mangano, C. Integrated 3D Information for Custom-Made Bone Grafts: Focus on Biphasic Calcium Phosphate Bone Substitute Biomaterials. Int. J. Environ. Res. Public Health 2020, 17, 4931. https://doi.org/10.3390/ijerph17144931
Giuliani A, Gatto ML, Gobbi L, Mangano FG, Mangano C. Integrated 3D Information for Custom-Made Bone Grafts: Focus on Biphasic Calcium Phosphate Bone Substitute Biomaterials. International Journal of Environmental Research and Public Health. 2020; 17(14):4931. https://doi.org/10.3390/ijerph17144931
Chicago/Turabian StyleGiuliani, Alessandra, Maria Laura Gatto, Luigi Gobbi, Francesco Guido Mangano, and Carlo Mangano. 2020. "Integrated 3D Information for Custom-Made Bone Grafts: Focus on Biphasic Calcium Phosphate Bone Substitute Biomaterials" International Journal of Environmental Research and Public Health 17, no. 14: 4931. https://doi.org/10.3390/ijerph17144931
APA StyleGiuliani, A., Gatto, M. L., Gobbi, L., Mangano, F. G., & Mangano, C. (2020). Integrated 3D Information for Custom-Made Bone Grafts: Focus on Biphasic Calcium Phosphate Bone Substitute Biomaterials. International Journal of Environmental Research and Public Health, 17(14), 4931. https://doi.org/10.3390/ijerph17144931








