Phylloseptin-1 is Leishmanicidal for Amastigotes of Leishmania amazonensis Inside Infected Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Phylloseptin-1 Synthesis and Isolation
2.2. Leishmania amazonensis
2.3. Animals
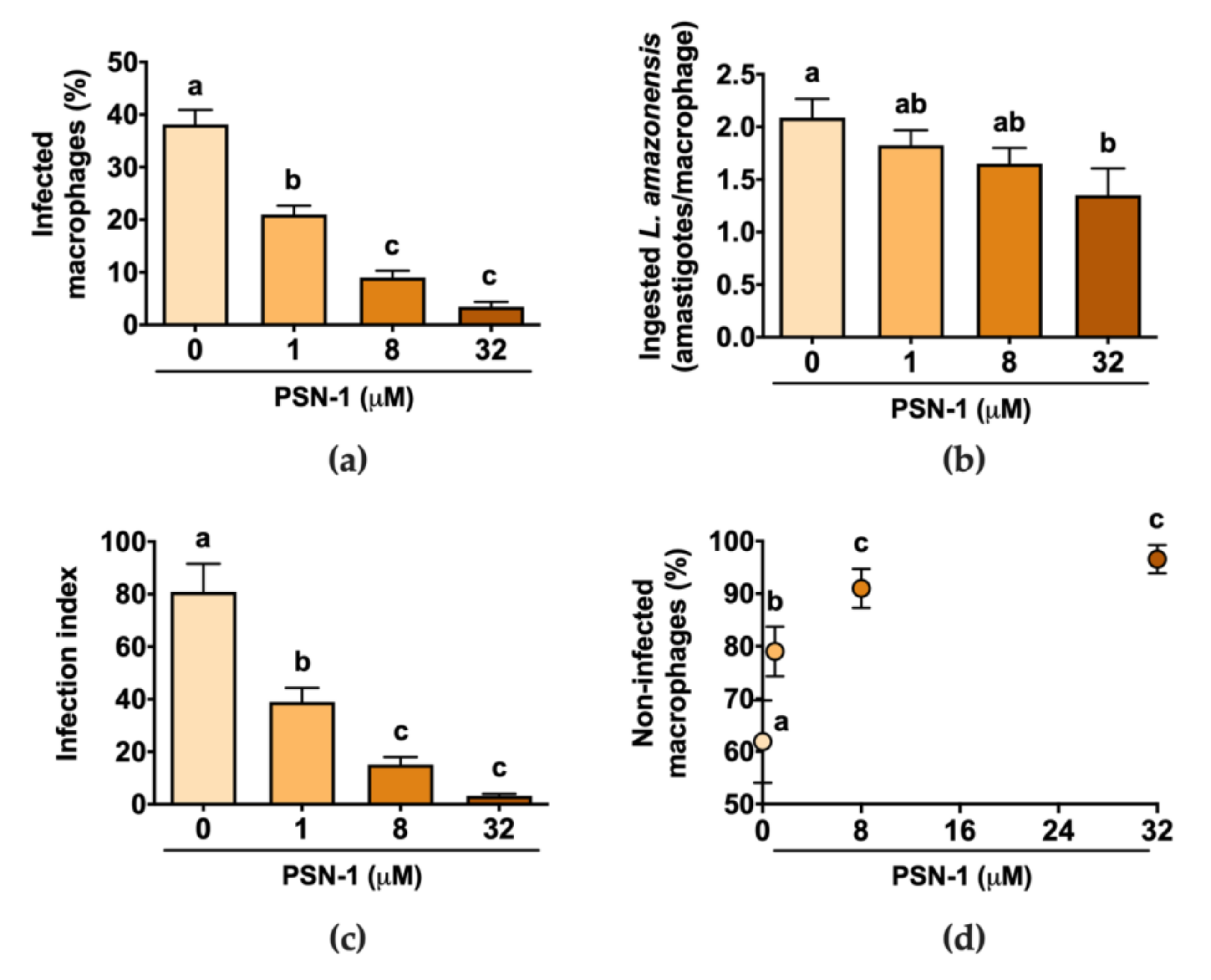
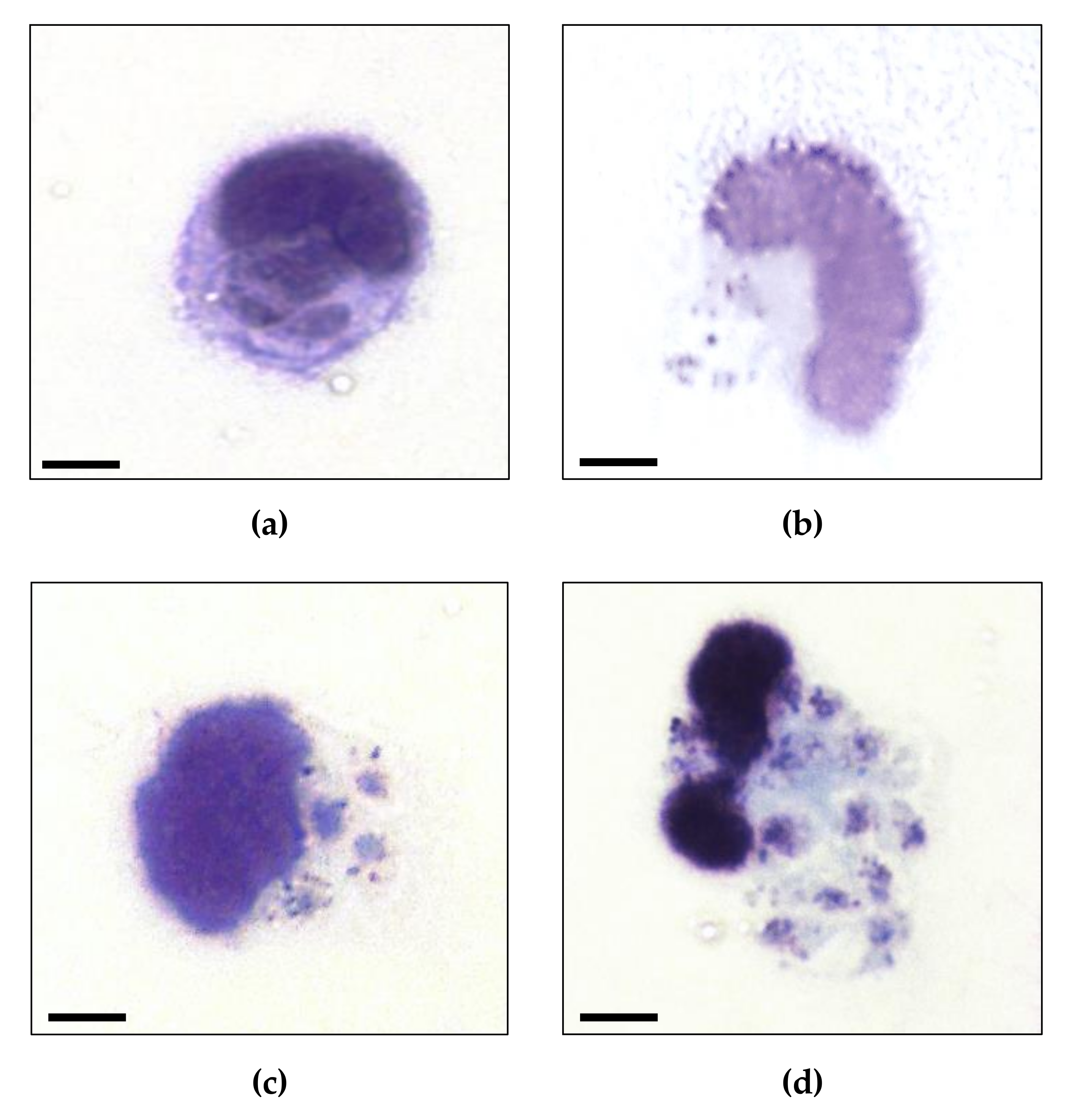
2.4. Macrophage Infection
2.5. Nitric Oxide Production as Estimated by Nitrite Measurement
2.6. Hydrogen Peroxide Production
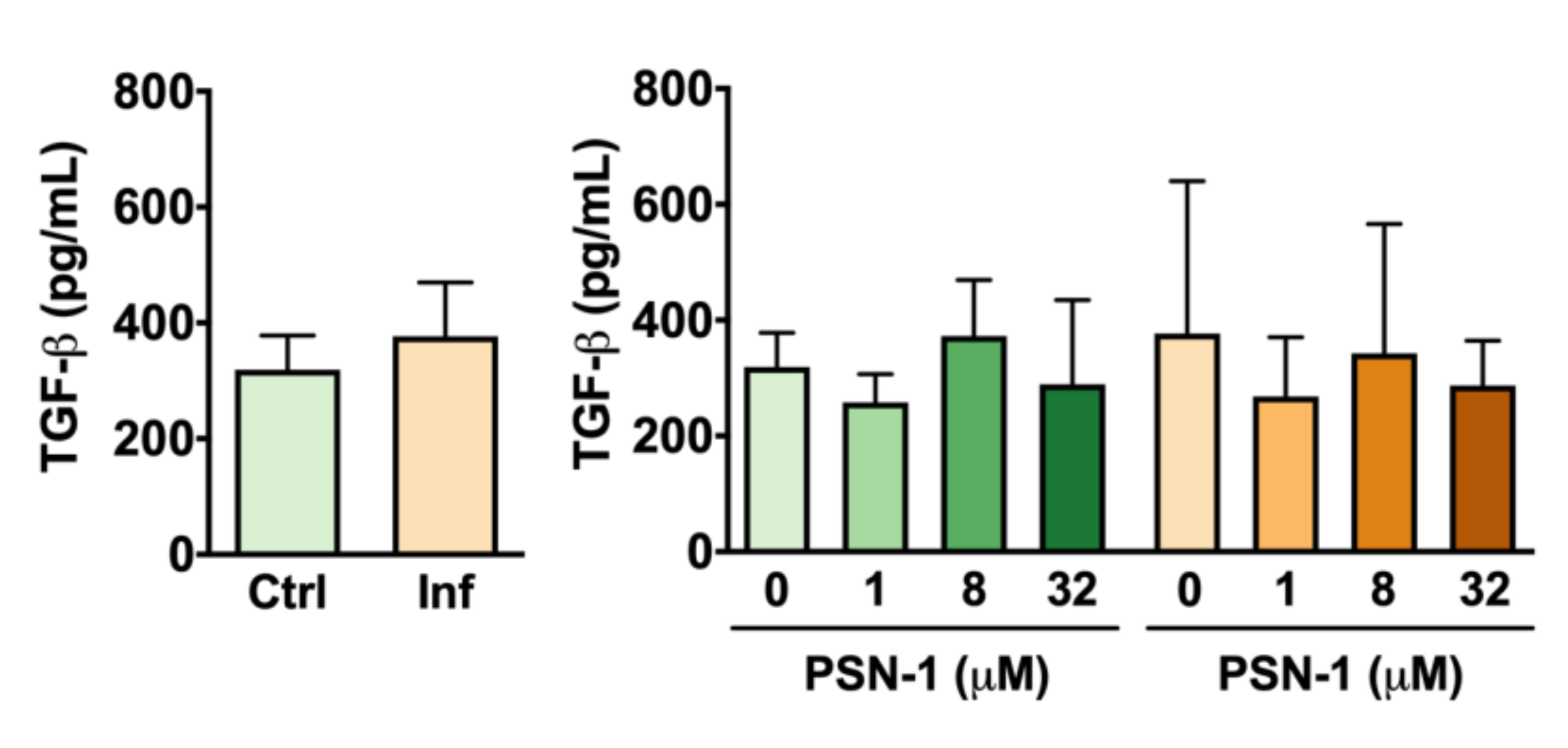
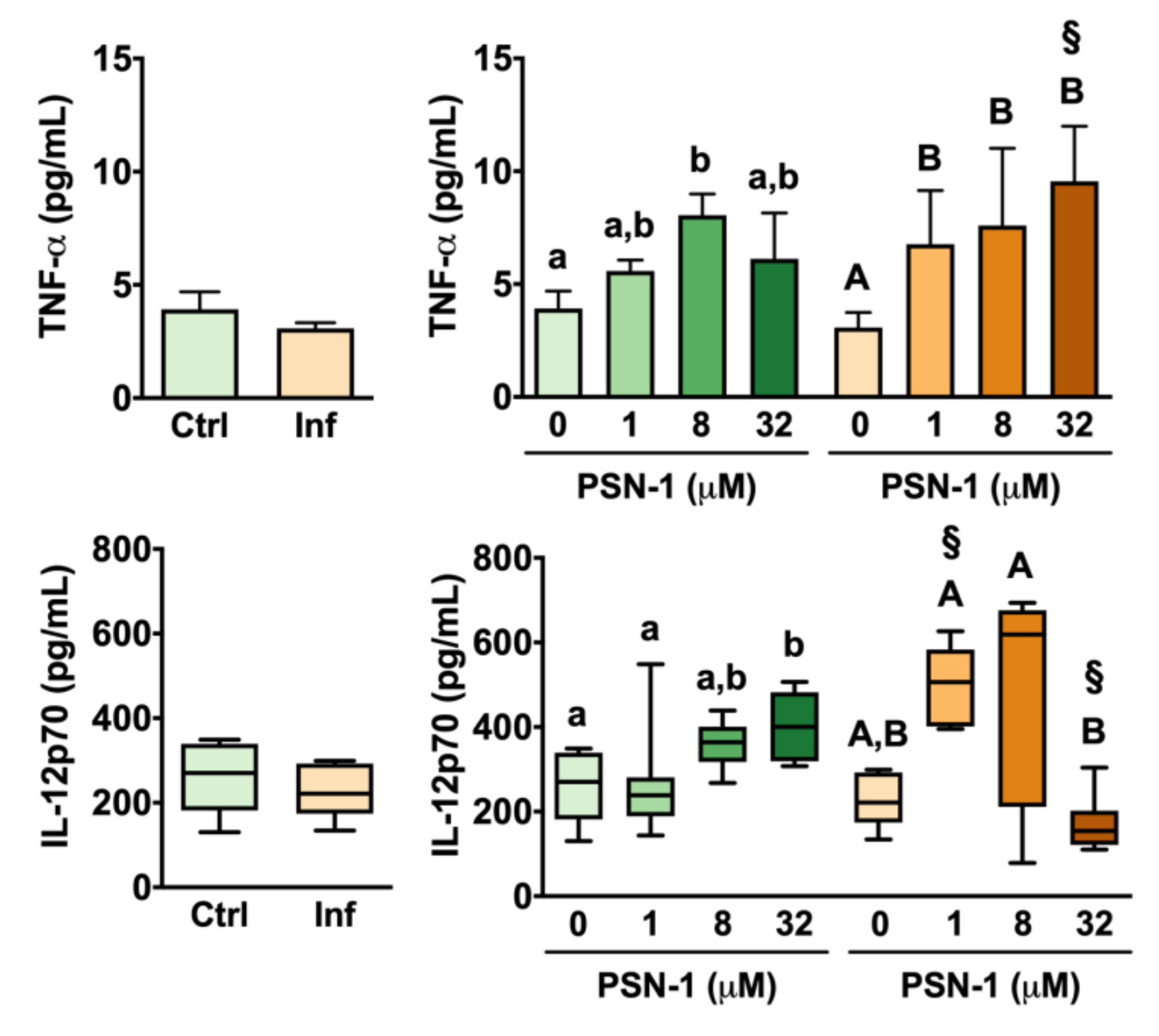
2.7. Cytokines
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santos, D.O.; Coutinho, C.E.R.; Madeira, M.F.; Bottino, C.G.; Vieira, R.T.; Nascimento, S.B.; Bernardino, A.; Bourguignon, S.C.; Corte-Real, S.; Pinho, R.T.; et al. Leishmaniasis treatment—A challenge that remains: A review. Parasitol. Res. 2008, 103, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.M.; Fidock, D.A.; Kyle, D.E.; Kappe, S.H.I.; Alonso, P.L.; Collins, F.H.; Duffy, P.E. Malaria: Progress, perils, and prospects for eradication. J. Clin. Invest. 2008, 118, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Leishmaniasis Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 11 June 2020).
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Ribeiro-de-Jesus, A.; Almeida, R.P.; Lessa, H.; Bacellar, O.; Carvalho, E.M. Cytokine profile and pathology in human leishmaniasis. Braz. J. Med. Biol. Res. 1998, 31, 143–148. [Google Scholar] [CrossRef]
- Croft, S.; Yardley, V. Chemotherapy of leishmaniasis. Curr. Pharm. Des. 2002, 8, 319–342. [Google Scholar] [CrossRef]
- Haldar, A.K.; Sen, P.; Roy, S. Use of antimony in the treatment of leishmaniasis: Current status and future directions. Mol. Biol. Int. 2011, 2011, 1–23. [Google Scholar] [CrossRef]
- Uliana, S.R.B.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef]
- Leite, J.R.S.A.; Silva, L.P.; Rodrigues, M.I.S.; Prates, M.V.; Brand, G.D.; Lacava, B.M.; Azevedo, R.B.; Bocca, A.L.; Albuquerque, S.; Bloch, C. Phylloseptins: A novel class of anti-bacterial and anti-protozoan peptides from the Phyllomedusa genus. Peptides 2005, 26, 565–573. [Google Scholar] [CrossRef]
- Kückelhaus, S.; Leite, J.R.S.A.; Neves, M.P.; Frota, K.S.; Abdala, L.F.; Muniz-Junqueira, M.I.; Bloch, C.; Tosta, C.E. Toxicity evaluation to mice of phylloseptin-1, an antimicrobial peptide from the skin secretion of Phyllomedusa hypochondrialis (Amphibia). Int. J. Pept. Res. Ther. 2007, 13, 423–429. [Google Scholar] [CrossRef]
- Chernysh, S.; Kim, S.I.; Bekker, G.; Pleskach, V.A.; Filatova, N.A.; Anikin, V.B.; Platonov, V.G.; Bulet, P. Antiviral and antitumor peptides from insects. Proc. Natl. Acad. Sci. USA 2002, 99, 12628–12632. [Google Scholar] [CrossRef]
- Peschel, A. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 2002, 10, 179–186. [Google Scholar] [CrossRef]
- Lee, D.G.; Shin, S.Y.; Kim, D.-H.; Seo, M.Y.; Kang, J.H.; Lee, Y.; Kim, K.L.; Hahm, K.-S. Antifungal mechanism of a cysteine-rich antimicrobial peptide, Ib-AMP1, from Impatiens balsamina against Candida albicans. Biotechnol. Lett. 1999, 21, 1047–1050. [Google Scholar] [CrossRef]
- Ghosh, J.K.; Shaool, D.; Guillaud, P.; Cicéron, L.; Mazier, D.; Kustanovich, I.; Shai, Y.; Mor, A. Selective cytotoxicity of dermaseptin S3 toward Intraerythrocytic Plasmodium falciparum and the underlying molecular basis. J. Biol. Chem. 1997, 272, 31609–31616. [Google Scholar] [CrossRef] [PubMed]
- Brand, G.D.; Leite, J.R.S.A.; Silva, L.P.; Albuquerque, S.; Prates, M.V.; Azevedo, R.B.; Carregaro, V.; Silva, J.S.; Sá, V.C.L.; Brandão, R.A.; et al. Dermaseptins from Phyllomedusa oreades and Phyllomedusa distincta: Anti-Trypanosoma cruzi activity without cytotoxicity to mammalian cells. J. Biol. Chem. 2002, 277, 49332–49340. [Google Scholar] [CrossRef] [PubMed]
- Amiche, M.; Ladram, A.; Nicolas, P. A consistent nomenclature of antimicrobial peptides isolated from frogs of the subfamily Phyllomedusinae. Peptides 2008, 29, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Kückelhaus, S.A.S.; Leite, J.R.S.A.; Muniz-Junqueira, M.I.; Sampaio, R.N.; Bloch, C.; Tosta, C.E. Antiplasmodial and antileishmanial activities of phylloseptin-1, an antimicrobial peptide from the skin secretion of Phyllomedusa azurea (Amphibia). Exp. Parasitol. 2009, 123, 11–16. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, M.; Wang, L.; McGrath, S.; Chen, T.; Chen, X.; Shaw, C. Phylloseptin-1 (PSN-1) from Phyllomedusa sauvagei skin secretion: A novel broad-spectrum antimicrobial peptide with antibiofilm activity. Mol. Immunol. 2010, 47, 2030–2037. [Google Scholar] [CrossRef]
- Lee, D.G.; Hahm, K.-S.; Shin, S.Y. Structure and fungicidal activity of a synthetic antimicrobial peptide, P18, and its truncated peptides. Biotechnol. Lett. 2004, 26, 337–341. [Google Scholar] [CrossRef]
- Raja, Z.; André, S.; Piesse, C.; Sereno, D.; Nicolas, P.; Foulon, T.; Oury, B.; Ladram, A. Structure, antimicrobial activities and mode of interaction with membranes of novel phylloseptins from the painted-belly leaf frog, Phyllomedusa sauvagii. PLoS ONE 2013, 8, e70782. [Google Scholar] [CrossRef]
- Waddell, W.J. A simple ultraviolet spectrophotometric method for the determination of protein. J. Lab. Clin. Med. 1956, 48, 311–314. [Google Scholar]
- Brand, G.D.; Ramada, M.H.S.; Manickchand, J.R.; Correa, R.; Ribeiro, D.J.S.; Santos, M.A.; Vasconcelos, A.G.; Abrão, F.Y.; Prates, M.V.; Murad, A.M.; et al. Intragenic antimicrobial peptides (IAPs) from human proteins with potent antimicrobial and anti-inflammatory activity. PLoS ONE 2019, 14, e0220656. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.A.; de Jesus Santos, R.; Sampaio, R.; Pontes-de-Carvalho, L.; dos-Santos, W. A simple and reproducible method to obtain large numbers of axenic amastigotes of different Leishmania species. Parasitol. Res. 2002, 88, 963–968. [Google Scholar] [CrossRef]
- Muniz-Junqueira, M.I.; Peçanha, L.M.F.; da Silva-Filho, V.L.; de Almeida Cardoso, M.C.; Tosta, C.E. Novel microtechnique for assessment of postnatal maturation of the phagocytic function of neutrophils and monocytes. Clin. Diagn. Lab. Immunol. 2003, 10, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [CrossRef]
- Archer, S. Measurement of nitric oxide in biological models. FASEB J. 1993, 7, 349–360. [Google Scholar] [CrossRef]
- Kückelhaus, C.S.; Kückelhaus, S.A.S.; Tosta, C.E.; Muniz-Junqueira, M.I. Pravastatin modulates macrophage functions of Leishmania (L.) amazonensis-infected BALB/c mice. Exp. Parasitol. 2013, 134, 18–25. [Google Scholar] [CrossRef]
- Henry, Y.; Ducrocq, C.; Drapier, J.C.; Servent, D.; Pellat, C.; Guissani, A. Nitric oxide, a biological effector. Electron paramagnetic resonance detection of nitrosyl-iron-protein complexes in whole cells. Eur. Biophys. J. 1991, 20, 1–15. [Google Scholar] [CrossRef]
- Williamson, P.; Schlegel, R.A. Back and forth: The regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol. Membr. Biol. 1994, 11, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Sansom, M.S.P. Alamethicin and related peptaibols – model ion channels. Eur. Biophys. J. 1993, 22. [Google Scholar] [CrossRef]
- Dathe, M.; Meyer, J.; Beyermann, M.; Maul, B.; Hoischen, C.; Bienert, M. General aspects of peptide selectivity towards lipid bilayers and cell membranes studied by variation of the structural parameters of amphipathic helical model peptides. Biochim. Biophys. Acta 2002, 1558, 171–186. [Google Scholar] [CrossRef]
- Brand, G.D.; Leite, J.R.S.A.; de Sá Mandel, S.M.; Mesquita, D.A.; Silva, L.P.; Prates, M.V.; Barbosa, E.A.; Vinecky, F.; Martins, G.R.; Galasso, J.H.; et al. Novel dermaseptins from Phyllomedusa hypochondrialis (Amphibia). Biochem. Biophys. Res. Commun. 2006, 347, 739–746. [Google Scholar] [CrossRef]
- Pérez-Payá, E.; Houghten, R.A.; Blondelle, S.E. Determination of the secondary structure of selected melittin analogues with different haemolytic activities. Biochem. J. 1994, 299, 587–591. [Google Scholar] [CrossRef]
- Tenenholz, T.C.; Klenk, K.C.; Matteson, D.R.; Blaustein, M.P.; Weber, D.J. Structural determinants of scorpion toxin affinity: The charybdotoxin (α-KTX) family of K+-channel blocking peptides. Rev. Physiol. Biochem. Pharmacol. 2000, 140, 135–185. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.C.; Mangoni, M.L.; Rufo, A.; Luzi, C.; Barra, D.; Zhao, H.; Kinnunen, P.K.J.; Bozzi, A.; Giulio, A.D.; Simmaco, M. Temporin L: Antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem. J. 2002, 368, 91–100. [Google Scholar] [CrossRef]
- El Amri, C.; Lacombe, C.; Zimmerman, K.; Ladram, A.; Amiche, M.; Nicolas, P.; Bruston, F. The plasticins: Membrane adsorption, lipid disorders, and biological activity. Biochemistry 2006, 45, 14285–14297. [Google Scholar] [CrossRef]
- Dolis, D.; Moreau, C.; Zachowski, A.; Devaux, P.F. Aminophospholipid translocase and proteins involved in transmembrane phospholipid traffic. Biophys. Chem. 1997, 68, 221–231. [Google Scholar] [CrossRef]
- Matsuzaki, K. Why and how are peptide–lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta 1999, 1462, 1–10. [Google Scholar] [CrossRef]
- Ilgoutz, S.C.; McConville, M.J. Function and assembly of the Leishmania surface coat. Int. J. Parasitol. 2001, 31, 899–908. [Google Scholar] [CrossRef]
- Guerrero, E.; Saugar, J.M.; Matsuzaki, K.; Rivas, L. Role of positional hydrophobicity in the leishmanicidal activity of magainin 2. Antimicrob. Agents Chemother. 2004, 48, 2980–2986. [Google Scholar] [CrossRef] [PubMed]
- Resende, J.M.; Moraes, C.M.; Prates, M.V.; Cesar, A.; Almeida, F.C.L.; Mundim, N.C.C.R.; Valente, A.P.; Bemquerer, M.P.; Piló-Veloso, D.; Bechinger, B. Solution NMR structures of the antimicrobial peptides phylloseptin-1, -2, and -3 and biological activity: The role of charges and hydrogen bonding interactions in stabilizing helix conformations. Peptides 2008, 29, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, G.; Lecar, H. Electrically gated ionic channels in lipid bilayers. Quart. Rev. Biophys. 1977, 10, 1–34. [Google Scholar] [CrossRef]
- Probst, C.M.; Silva, R.A.; B Menezes, J.P.; Almeida, T.F.; Gomes, I.N.; Dallabona, A.C.; Ozaki, L.S.; Buck, G.A.; Pavoni, D.P.; Krieger, M.A.; et al. A comparison of two distinct murine macrophage gene expression profiles in response to Leishmania amazonensis infection. BMC Microbiol. 2012, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Junqueira, M.I.; de Paula-Coelho, V.N. Meglumine antimonate directly increases phagocytosis, superoxide anion and TNF-α production, but only via TNF-α it indirectly increases nitric oxide production by phagocytes of healthy individuals, in vitro. Int. Immunopharmacol. 2008, 8, 1633–1638. [Google Scholar] [CrossRef]
- Stamper, B.D.; Davis, M.; Scott-Collins, S.; Tran, J.; Ton, C.; Simidyan, A.; Roberts, S.C. Model-based evaluation of gene expression changes in response to Leishmania infection. Gene Regul. Syst. Biol. 2019, 13, 117762501982835. [Google Scholar] [CrossRef]
- Conceição-Silva, F.; Morgado, F.N. Leishmania Spp-host interaction: There is always an onset, but is there an end? Front. Cell. Infect. Microbiol. 2019, 9, 330. [Google Scholar] [CrossRef]
- Loeuillet, C.; Bañuls, A.-L.; Hide, M. Study of Leishmania pathogenesis in mice: Experimental considerations. Parasit. Vectors 2016, 9, 144. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Dillon, L.A.L.; Belew, A.T.; Bravo, H.C.; Mosser, D.M.; El-Sayed, N.M. Dual transcriptome profiling of Leishmania-infected human macrophages reveals distinct reprogramming signatures. mBio 2016, 7, e00027-16. [Google Scholar] [CrossRef]
- de Saldanha, R.R.; Martins-Papa, M.C.; Sampaio, R.N.R.; Muniz-Junqueira, M.I. Meglumine antimonate treatment enhances phagocytosis and TNF-α production by monocytes in human cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 596–603. [Google Scholar] [CrossRef]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host–pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef]
- Watford, W.T.; Hissong, B.D.; Bream, J.H.; Kanno, Y.; Muul, L.; O’Shea, J.J. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev. 2004, 202, 139–156. [Google Scholar] [CrossRef] [PubMed]
- McDyer, J.F.; Wu, C.Y.; Seder, R.A. The regulation of IL-12: Its role in infectious, autoimmune, and allergic diseases. J. Allergy Clin. Immunol. 1998, 102, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Chehimi, J.; Starr, S.E.; Frank, I.; D’Andrea, A.; Ma, X.; MacGregor, R.R.; Sennelier, J.; Trinchieri, G. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J. Exp. Med. 1994, 179, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Tait Wojno, E.D.; Hunter, C.A.; Stumhofer, J.S. The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kückelhaus, S.A.S.; de Aquino, D.S.; Borges, T.K.; Moreira, D.C.; Leite, L.d.M.; Muniz-Junqueira, M.I.; Kückelhaus, C.S.; Romero, G.A.S.; Prates, M.V.; Bloch, C., Jr.; et al. Phylloseptin-1 is Leishmanicidal for Amastigotes of Leishmania amazonensis Inside Infected Macrophages. Int. J. Environ. Res. Public Health 2020, 17, 4856. https://doi.org/10.3390/ijerph17134856
Kückelhaus SAS, de Aquino DS, Borges TK, Moreira DC, Leite LdM, Muniz-Junqueira MI, Kückelhaus CS, Romero GAS, Prates MV, Bloch C Jr., et al. Phylloseptin-1 is Leishmanicidal for Amastigotes of Leishmania amazonensis Inside Infected Macrophages. International Journal of Environmental Research and Public Health. 2020; 17(13):4856. https://doi.org/10.3390/ijerph17134856
Chicago/Turabian StyleKückelhaus, Selma A. S., Daniela Sant’Ana de Aquino, Tatiana K. Borges, Daniel C. Moreira, Luciana de Magalhães Leite, Maria Imaculada Muniz-Junqueira, Carlos S. Kückelhaus, Gustavo A. Sierra Romero, Maura V. Prates, Carlos Bloch, Jr., and et al. 2020. "Phylloseptin-1 is Leishmanicidal for Amastigotes of Leishmania amazonensis Inside Infected Macrophages" International Journal of Environmental Research and Public Health 17, no. 13: 4856. https://doi.org/10.3390/ijerph17134856
APA StyleKückelhaus, S. A. S., de Aquino, D. S., Borges, T. K., Moreira, D. C., Leite, L. d. M., Muniz-Junqueira, M. I., Kückelhaus, C. S., Romero, G. A. S., Prates, M. V., Bloch, C., Jr., & Leite, J. R. S. A. (2020). Phylloseptin-1 is Leishmanicidal for Amastigotes of Leishmania amazonensis Inside Infected Macrophages. International Journal of Environmental Research and Public Health, 17(13), 4856. https://doi.org/10.3390/ijerph17134856






