Causes and Phenotypes of Work-Related Asthma
Abstract
:1. Introduction
2. Work-Exacerbated Asthma (WEA)
3. Irritant-Induced Asthma (IIA)
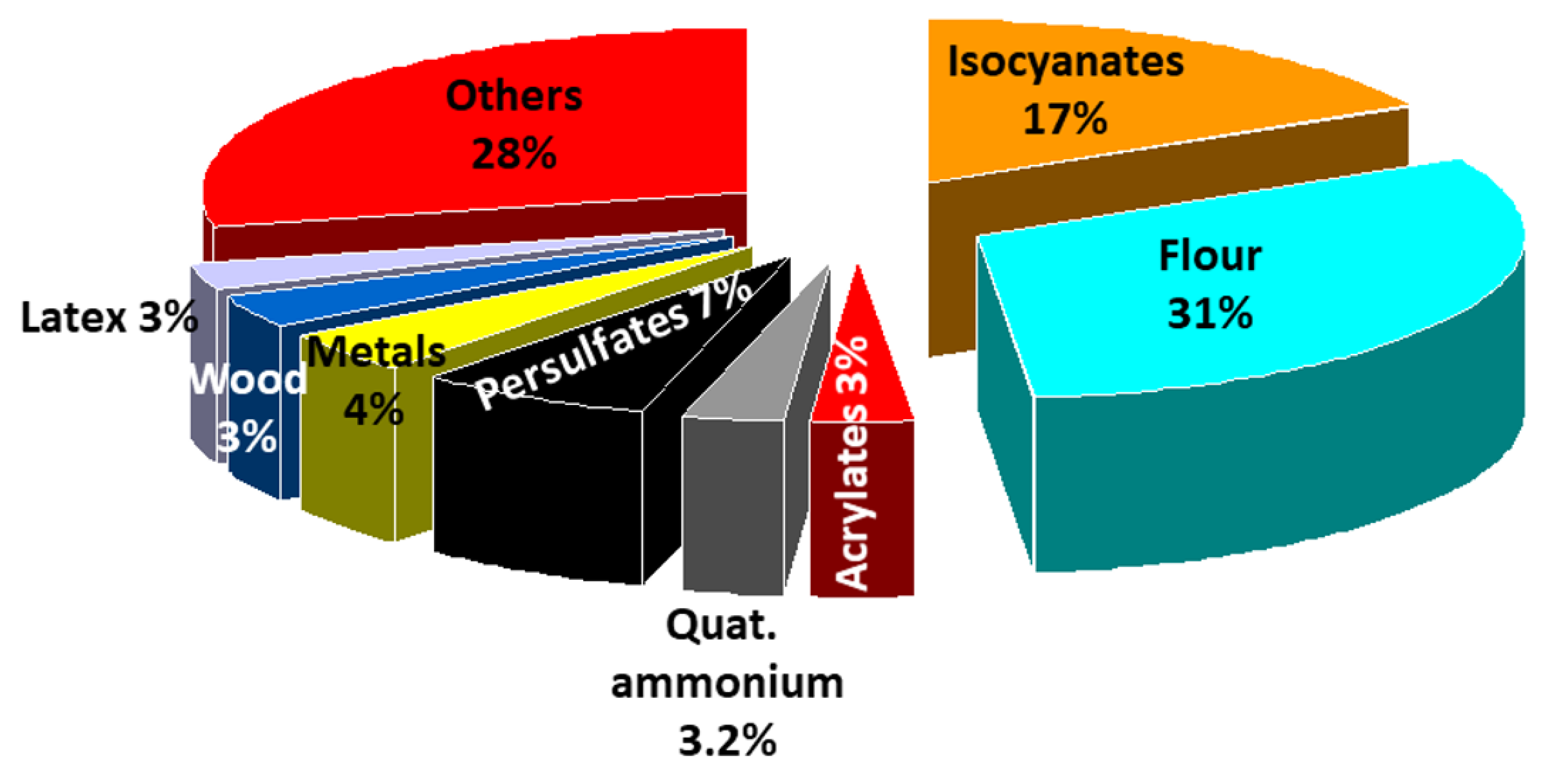
4. Immunologic Occupational Asthma (OA)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Henneberger, P.K.; Redlich, C.A.; Callahan, D.B.; Harber, P.; Lemière, C.; Martin, J.; Tarlo, S.M.; Vandenplas, O.; Torén, K. An official American thoracic society statement: Work-exacerbated asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 368–378. [Google Scholar] [CrossRef]
- Bradshaw, L.; Sumner, J.; Delic, J.; Henneberger, P.; Fishwick, D. Work aggravated asthma in Great Britain: A cross-sectional postal survey. Prim. Health Care Res. Dev. 2018, 19, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talini, D.; Ciberti, A.; Bartoli, D.; Del Guerra, P.; Iaia, T.E.; Lemmi, M.; Innocenti, A.; Di Pede, F.; Latorre, M.; Carrozzi, L.; et al. Work-related asthma in a sample of subjects with established asthma. Respir. Med. 2017, 130, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vila-Rigat, R.; Panadès Valls, R.; Hernandez Huet, E.; Sivecas Maristany, J.; Blanché Prat, X.; Muñoz-Ortiz, L.; Torán Monserrat, P.; Rabell Santacana, V. Prevalence of work-related asthma and its impact in primary health care. Arch. Bronconeumol. Engl. Ed. 2015, 51, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Henneberger, P.K.; Mirabelli, M.C.; Kogevinas, M.; Anto, J.M.; Plana, E.; Dahlman-Hoglund, A.; Jarvis, D.L.; Kromhout, H.; Lillienberg, L.; Norback, D.; et al. The occupational contribution to severe exacerbation of asthma. Eur. Respir. J. 2010, 36, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Lutzker, L.A.; Rafferty, A.P.; Brunner, W.M.; Walters, J.K.; Wasilevich, E.A.; Green, M.K.; Rosenman, K.D. Prevalence of work-related asthma in Michigan, Minnesota, and Oregon. J. Asthma 2010, 47, 156–161. [Google Scholar] [CrossRef]
- Tice, C.J.; Cummings, K.R.; Gelberg, K.H. Surveillance of work-related asthma in New York State. J. Asthma 2010, 47, 310–316. [Google Scholar] [CrossRef]
- Work-Related Asthma: Most Frequently Reported Agents Associated with Work-Related Asthma Cases by Asthma Classification, 2009–2012. Available online: https://wwwn.cdc.gov/eworld/Data/Work-related_asthma_Most_frequently_reported_agents_associated_with_work-related_asthma_cases_by_asthma_classification_20092011/927 (accessed on 18 May 2020).
- Kim, J.-L.; Henneberger, P.K.; Lohman, S.; Olin, A.-C.; Dahlman-Höglund, A.; Andersson, E.; Torén, K.; Holm, M. Impact of occupational exposures on exacerbation of asthma: A population-based asthma cohort study. BMC Pulm. Med. 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- Lipszyc, J.C.; Silverman, F.; Holness, D.L.; Liss, G.M.; Lavoie, K.L.; Tarlo, S.M. Comparison of psychological, quality of life, work-limitation, and socioeconomic status between patients with occupational asthma and work-exacerbated asthma. J. Occup. Environ. Med. 2017, 59, 697–702. [Google Scholar] [CrossRef]
- Moullec, G.; Lavoie, K.; Malo, J.-L.; Gautrin, D.; L’Archevêque, J.; Labrecque, M. Long-term socioprofessional and psychological status in workers investigated for occupational asthma in Quebec. J. Occup. Environ. Med. 2013, 55, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, O.; Wiszniewska, M.; Raulf, M.; De Blay, F.; Van Wijk, R.G.; Moscato, G.; Nemery, B.; Pala, G.; Quirce, S.; Sastre, J.; et al. EAACI position paper: Irritant-induced asthma. Allergy 2014, 69, 1141–1153. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.M.; Weiss, M.A.; Bernstein, I.L. Reactive airways dysfunction syndrome (RADS): Persistent asthma syndrome after high level irritant exposures. Chest 1985, 88, 376–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banauch, G.I.; Dhala, A.; Alleyne, D.; Alva, R.; Santhyadka, G.; Krasko, A.; Weiden, M.; Kelly, K.J.; Prezant, D.J. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit. Care Med. 2005, 33, S102–S106. [Google Scholar] [CrossRef] [PubMed]
- Dumas, O.; Laurent, E.; Bousquet, J.; Metspalu, A.; Milani, L.; Kauffmann, F.; Le Moual, N. Occupational irritants and asthma: An Estonian cross-sectional study of 34,000 adults. Eur. Respir. J. 2014, 44, 647–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.W.H.; Lipszyc, J.C.; Prasad, S.; Tarlo, S.M. Work-related asthma from cleaning agents versus other agents. Occup. Med. 2018, 68, 587–592. [Google Scholar] [CrossRef]
- Carder, M.; Seed, M.J.; Money, A.; Agius, R.M.; Van Tongeren, M. Occupational and work-related respiratory disease attributed to cleaning products. Occup. Environ. Med. 2019, 76, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Folletti, I.; Siracusa, A.; Paolocci, G. Update on asthma and cleaning agents. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Su, F.-C.; Friesen, M.C.; Humann, M.; Stefaniak, A.B.; Stanton, M.L.; Liang, X.; LeBouf, R.F.; Henneberger, P.K.; Virji, M.A. Clustering asthma symptoms and cleaning and disinfecting activities and evaluating their associations among healthcare workers. Int. J. Hyg. Environ. Health 2019, 222, 873–883. [Google Scholar] [CrossRef]
- Dumas, O.; Boggs, K.M.; Quinot, C.; Varraso, R.; Zock, J.-P.; Henneberger, P.K.; Speizer, F.E.; Moual, N.L.; Camargo, C.A. Occupational exposure to disinfectants and asthma incidence in U.S. nurses: A prospective cohort study. Am. J. Ind. Med. 2020, 63, 44–50. [Google Scholar] [CrossRef]
- Dumas, O.; Varraso, R.; Boggs, K.M.; Quinot, C.; Zock, J.-P.; Henneberger, P.K.; Speizer, F.E.; Moual, N.L.; Camargo, C.A. Association of Occupational exposure to disinfectants with incidence of chronic obstructive pulmonary disease among US female nurses. JAMA Netw. Open 2019, 2, e1913563. [Google Scholar] [CrossRef]
- Matulonga, B.; Rava, M.; Siroux, V.; Bernard, A.; Dumas, O.; Pin, I.; Zock, J.-P.; Nadif, R.; Leynaert, B.; Le Moual, N. Women using bleach for home cleaning are at increased risk of non-allergic asthma. Respir. Med. 2016, 117, 264–271. [Google Scholar] [CrossRef]
- Moore, V.C.; Burge, P.S.; Robertson, A.S.; Walters, G.I. What causes occupational asthma in cleaners? Thorax 2017, 72, 581–583. [Google Scholar] [CrossRef]
- Seys, S.F.; Feyen, L.; Keirsbilck, S.; Adams, E.; Dupont, L.J.; Nemery, B. An outbreak of swimming-pool related respiratory symptoms: An elusive source of trichloramine in a municipal indoor swimming pool. Int. J. Hyg. Environ. Health 2015, 218, 386–391. [Google Scholar] [CrossRef]
- Baur, X. A compendium of causative agents of occupational asthma. J. Occup. Med. Toxicol. 2013, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Zholos, A.V. TRP channels in respiratory pathophysiology: The role of oxidative, chemical irritant and temperature stimuli. Curr. Neuropharmacol. 2015, 13, 279–291. Available online: http://www.eurekaselect.com/129926/article (accessed on 18 May 2020). [CrossRef] [PubMed]
- Molfino, N.A.; Wright, S.C.; Katz, I.; Tarlo, S.; Silverman, F.; McClean, P.A.; Slutsky, A.S.; Zamel, N.; Szalai, J.P.; Raizenne, M. Effect of low concentrations of ozone on inhaled allergen responses in asthmatic subjects. Lancet 1991, 338, 199–203. [Google Scholar] [CrossRef]
- Witten, A.; Solomon, C.; Abbritti, E.; Arjomandi, M.; Zhai, W.; Kleinman, M.; Balmes, J. Effects of nitrogen dioxide on allergic airway responses in subjects with asthma. J. Occup. Environ. Med. 2005, 47, 1250–1259. [Google Scholar] [CrossRef]
- Rava, M.; Ahmed, I.; Kogevinas, M.; Le Moual, N.; Bouzigon, E.; Curjuric, I.; Dizier, M.-H.; Dumas, O.; Gonzalez, J.R.; Imboden, M.; et al. Genes interacting with occupational exposures to low molecular weight agents and irritants on adult-onset asthma in three European studies. Environ. Health Perspect. 2017, 125, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.S.; Lee, T.-H.; Jun, J.A.; Baek, A.R.; Park, J.-S.; Koo, S.-M.; Kim, Y.-K.; Lee, H.S.; Park, C.-S. Neutrophilic inflammation in asthma: Mechanisms and therapeutic considerations. Expert Rev. Respir. Med. 2017, 11, 29–40. [Google Scholar] [CrossRef]
- Tarlo, S.M.; Lemiere, C. Occupational asthma. N. Engl. J. Med. 2014, 370, 640–649. [Google Scholar] [CrossRef]
- Vandenplas, O.; Godet, J.; Hurdubaea, L.; Rifflart, C.; Suojalehto, H.; Wiszniewska, M.; Munoz, X.; Sastre, J.; Klusackova, P.; Moore, V.; et al. Are high- and low-molecular-weight sensitizing agents associated with different clinical phenotypes of occupational asthma? Allergy 2019, 74, 261–272. [Google Scholar] [CrossRef]
- Malo, J.-L.; Lemière, C.; Desjardins, A.; Cartier, A. Prevalence and intensity of rhinoconjunctivitis in subjects with occupational asthma. Eur. Respir. J. 1997, 10, 1513–1515. [Google Scholar] [CrossRef] [Green Version]
- Talini, D.; Novelli, F.; Bacci, E.; Dente, F.L.; De Santis, M.; Di Franco, A.; Melosini, L.; Vagaggini, B.; Paggiaro, P.L. Comparison between airway responses to high versus low molecular weight compounds in occupational asthma. J. Allergy 2011, 2011, 781470. [Google Scholar] [CrossRef] [Green Version]
- Dufour, M.-H.; Lemiere, C.; Prince, P.; Boulet, L.-P. Comparative airway response to high-versus low-molecular weight agents in occupational asthma. Eur. Respir. J. 2009, 33, 734–739. [Google Scholar] [CrossRef] [Green Version]
- Meca, O.; Cruz, M.-J.; Sánchez-Ortiz, M.; González-Barcala, F.-J.; Ojanguren, I.; Munoz, X. Do low molecular weight agents cause more severe asthma than high molecular weight agents? PLoS ONE 2016, 11, e0156141. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenplas, O.; Godet, J.; Hurdubaea, L.; Rifflart, C.; Suojalehto, H.; Walusiak-Skorupa, J.; Munoz, X.; Sastre, J.; Klusackova, P.; Moore, V.; et al. Severe occupational asthma: Insights from a multicenter European cohort. J. Allergy Clin. Immunol. Pract. 2019, 7, 2309–2318.e4. [Google Scholar] [CrossRef] [Green Version]
- Von Bülow, A.; Backer, V.; Bodtger, U.; Søes-Petersen, N.U.; Assing, K.D.; Skjold, T.; Porsbjerg, C. The level of diagnostic assessment in severe asthma: A nationwide real-life study. Respir. Med. 2017, 124, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Lemière, C.; Boulet, L.-P.; Chaboillez, S.; Forget, A.; Chiry, S.; Villeneuve, H.; Prince, P.; Maghni, K.; Kennedy, W.A.; Blais, L. Work-exacerbated asthma and occupational asthma: Do they really differ? J. Allergy Clin. Immunol. 2013, 131, 704–710.e3. [Google Scholar] [CrossRef]
- Tai, A.; Tran, H.; Roberts, M.; Clarke, N.; Gibson, A.-M.; Vidmar, S.; Wilson, J.; Robertson, C.F. Outcomes of childhood asthma to the age of 50 years. J. Allergy Clin. Immunol. 2014, 133, 1572–1578.e3. [Google Scholar] [CrossRef]
- Trupin, L.; Katz, P.P.; Balmes, J.R.; Chen, H.; Yelin, E.H.; Omachi, T.; Blanc, P.D. Mediators of the socioeconomic gradient in outcomes of adult asthma and rhinitis. Am. J. Public Health 2013, 103, e31–e38. [Google Scholar] [CrossRef]
- Mincheva, R.; Ekerljung, L.; Bossios, A.; Lundbäck, B.; Lötvall, J. High prevalence of severe asthma in a large random population study. J. Allergy Clin. Immunol. 2018, 141, 2256–2264.e2. [Google Scholar] [CrossRef] [Green Version]
- Descatha, A.; Leproust, H.; Choudat, D.; Garnier, R.; Pairon, J.-C.; Ameille, J. Factors associated with severity of occupational asthma with a latency period at diagnosis. Allergy 2007, 62, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Maestrelli, P.; Schlunssen, V.; Mason, P.; Sigsgaard, T. Contribution of host factors and workplace exposure to the outcome of occupational asthma. Eur. Respir. Rev. 2012, 21, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Rachiotis, G.; Savani, R.; Brant, A.; MacNeill, S.J.; Newman Taylor, A.; Cullinan, P. Outcome of occupational asthma after cessation of exposure: A systematic review. Thorax 2007, 62, 147–152. [Google Scholar] [CrossRef] [Green Version]
| Agent Category2 | Examples of agents | No. | No. as % of 3253 |
|---|---|---|---|
| Miscellaneous chemicals and materials | Chemicals n.o.s., perfume n.o.s., pesticides n.o.s., glues n.o.s. | 523 | 16.1 |
| Mineral and inorganic dusts | Dust n.o.s., cement dust, copier toner | 477 | 14.7 |
| Cleaning materials | Sodium hypochlorite, disinfectant cleaners, floor strippers, floor wax, carpet cleaners | 440 | 13.5 |
| Pyrolysis products | Cigarette smoke, diesel exhaust, plastic smoke | 347 | 10.7 |
| Indoor air pollutants | Indoor air pollutants, indoor air pollutants from renovations | 241 | 7.4 |
| Molds | Mold n.o.s. | 179 | 5.5 |
| Solvents, n.o.s. | Paint, lacquer, solvents n.o.s. | 165 | 5.1 |
| Plant materials | Capsicum, wood dust, pollen, flour | 119 | 3.7 |
| Ergonomics | Exercise, stress | 98 | 3.0 |
| Aliphatic and alicyclic hydrocarbons | 4–Phenylcyclohexane, gasoline, petroleum fractions, n.o.s. | 78 | 2.4 |
| Acids, bases, and oxidizing agents | Sulfuric acid, hydrochloric acid, anhydrous ammonia, nitric acid, phosphoric acid | 70 | 2.2 |
| Animal materials | Cat, rat antigens, rat feces, insects n.o.s., animal dander n.o.s. | 68 | 2.1 |
| Physical factors | Cold and hot temperatures, high humidity | 59 | 1.8 |
| Metals and metalloids | Welding, metal dust n.o.s. | 53 | 1.6 |
| Other and unknown | n.a. | 336 | 10.3 |
| TOTAL | n.a. | 3253 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maestrelli, P.; Henneberger, P.K.; Tarlo, S.; Mason, P.; Boschetto, P. Causes and Phenotypes of Work-Related Asthma. Int. J. Environ. Res. Public Health 2020, 17, 4713. https://doi.org/10.3390/ijerph17134713
Maestrelli P, Henneberger PK, Tarlo S, Mason P, Boschetto P. Causes and Phenotypes of Work-Related Asthma. International Journal of Environmental Research and Public Health. 2020; 17(13):4713. https://doi.org/10.3390/ijerph17134713
Chicago/Turabian StyleMaestrelli, Piero, Paul K. Henneberger, Susan Tarlo, Paola Mason, and Piera Boschetto. 2020. "Causes and Phenotypes of Work-Related Asthma" International Journal of Environmental Research and Public Health 17, no. 13: 4713. https://doi.org/10.3390/ijerph17134713
APA StyleMaestrelli, P., Henneberger, P. K., Tarlo, S., Mason, P., & Boschetto, P. (2020). Causes and Phenotypes of Work-Related Asthma. International Journal of Environmental Research and Public Health, 17(13), 4713. https://doi.org/10.3390/ijerph17134713





