Ceramsite Facilitated Microbial Degradation of Pollutants in Domestic Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ceramsite from Sludge
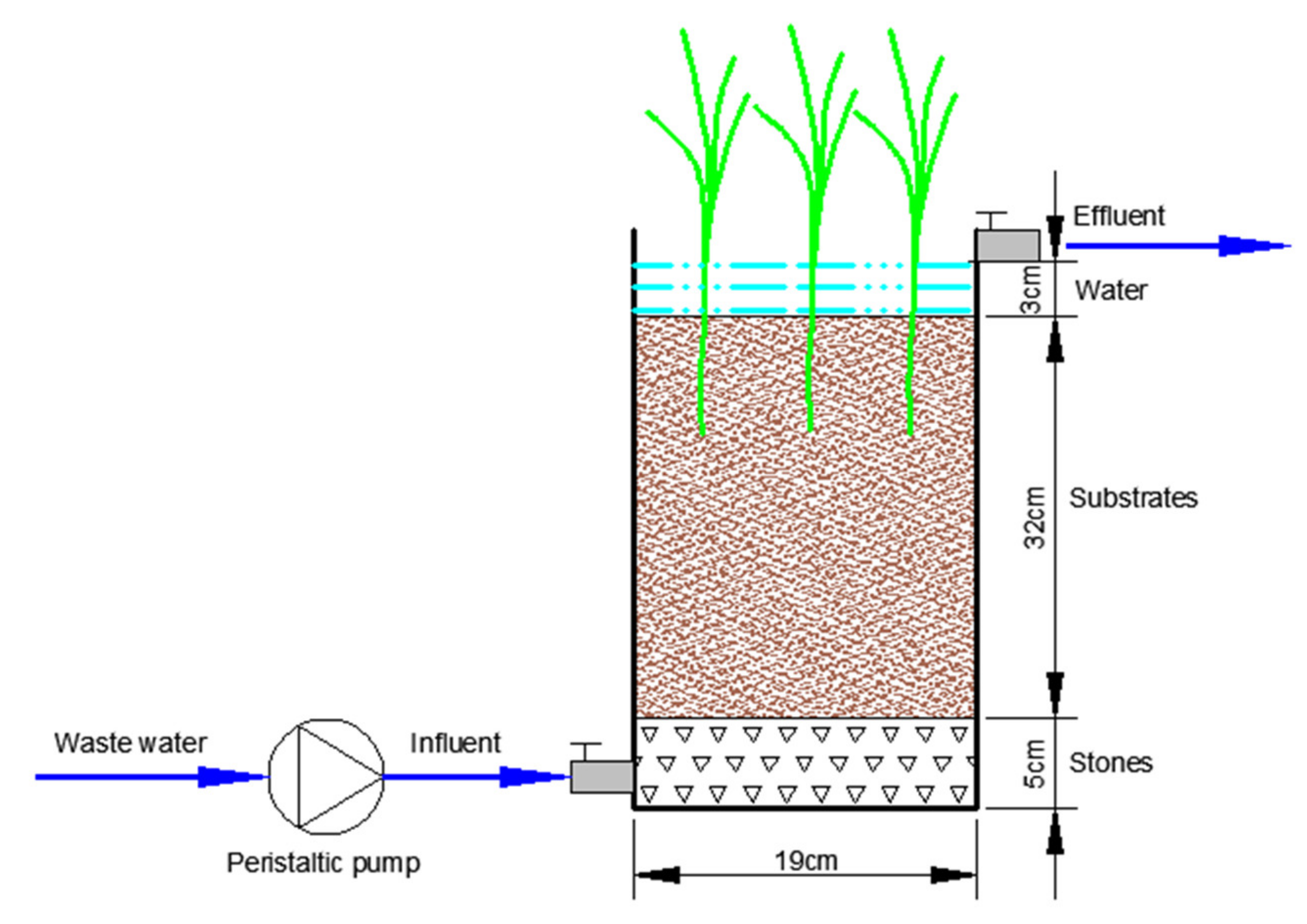
2.2. Set up of Wetland Microcosms
2.3. DNA Extraction and Hiseq Sequencing
2.4. Statistical Analyses
3. Results
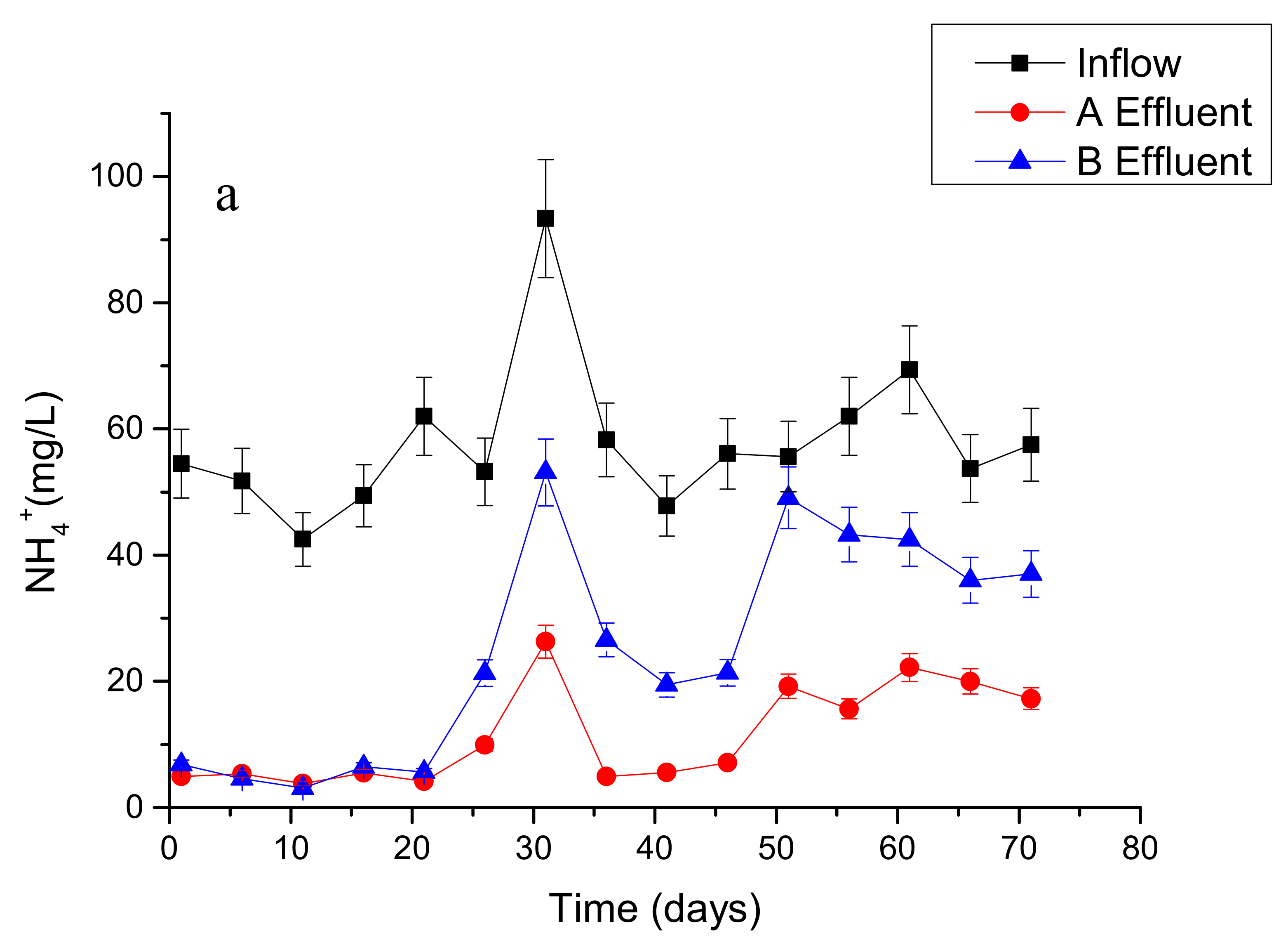
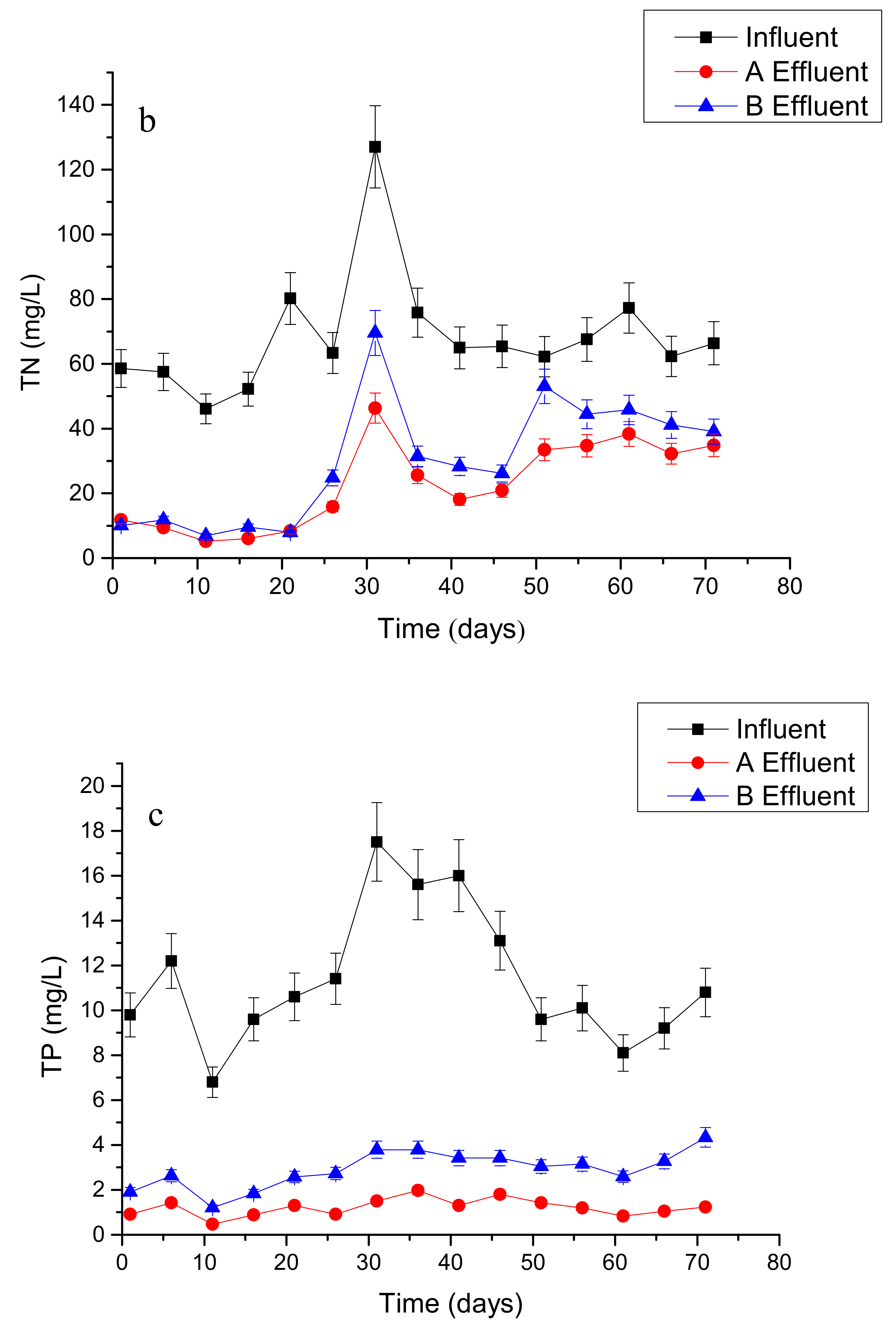
3.1. Overall Performance of Wetland Microcosms with Different Treatments
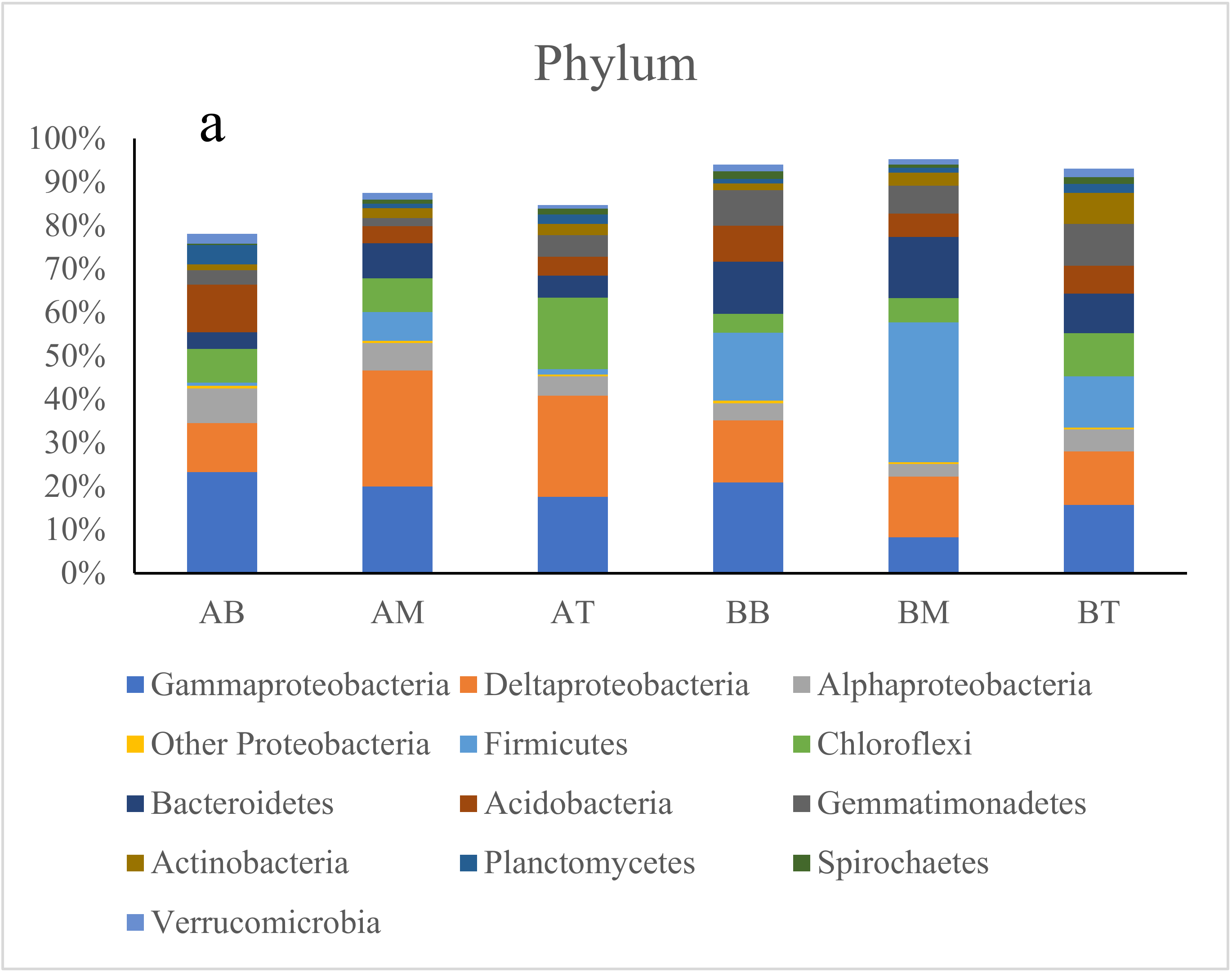
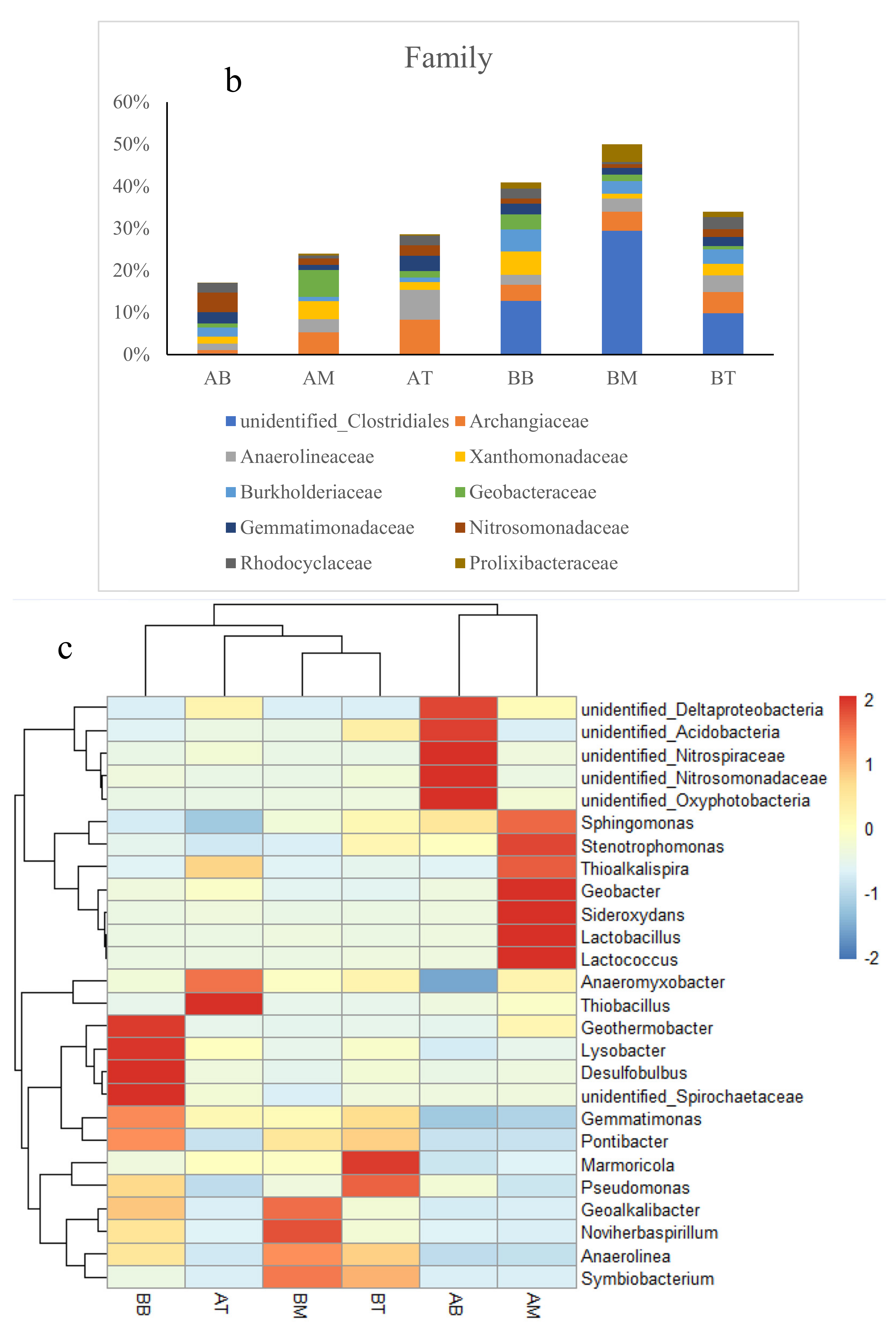
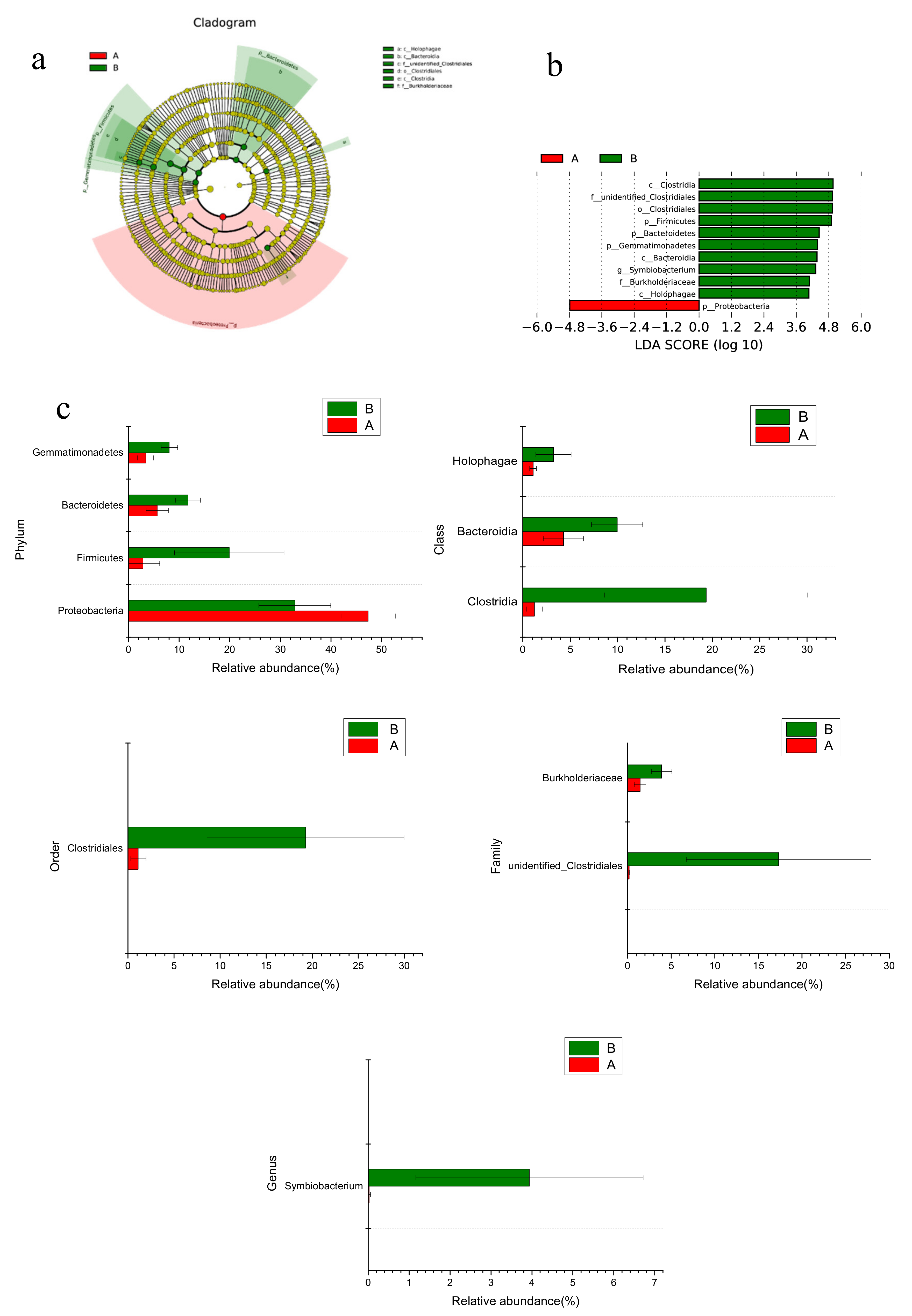
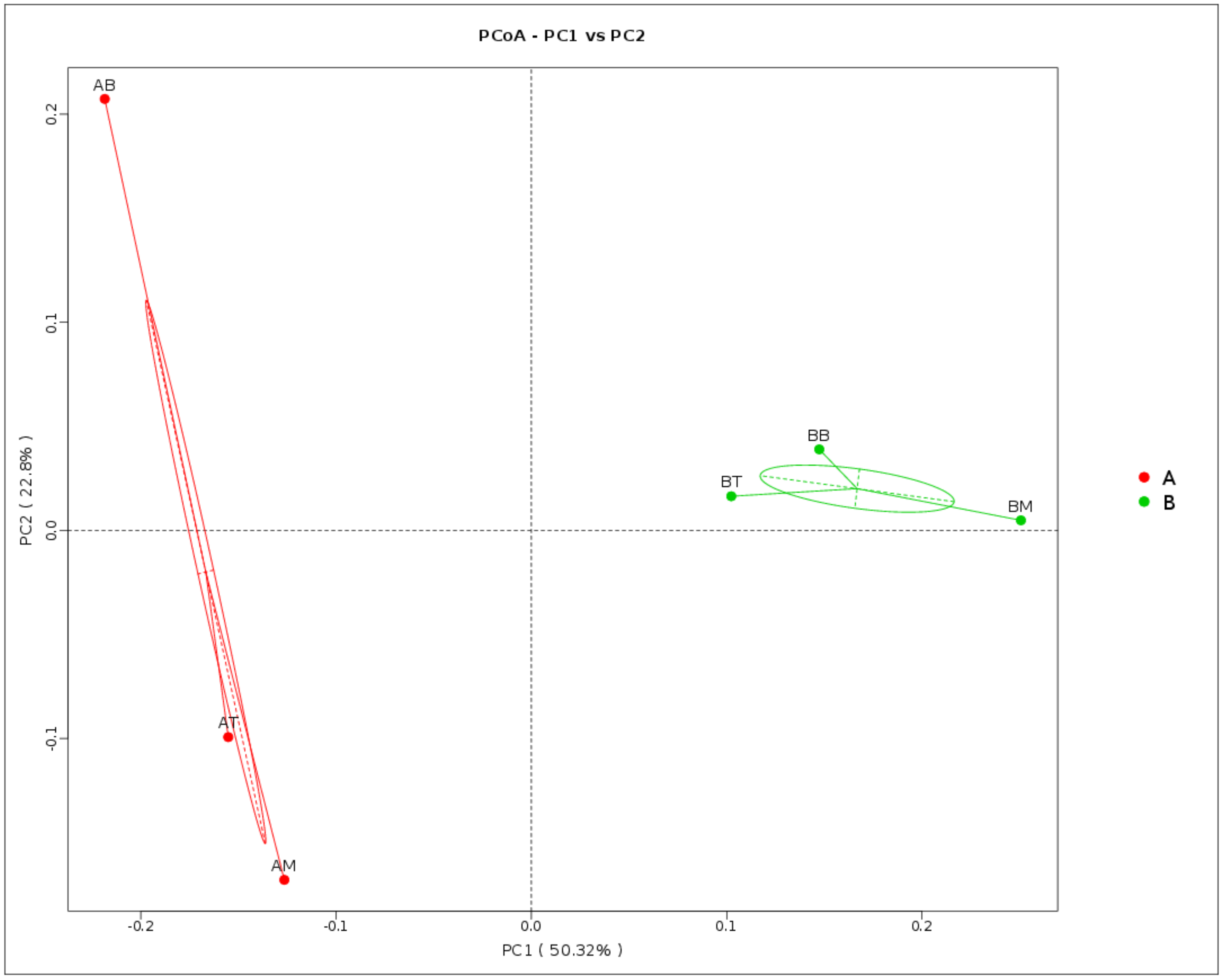
3.2. Microbial Diversity and Composition in Wetland Microcosms
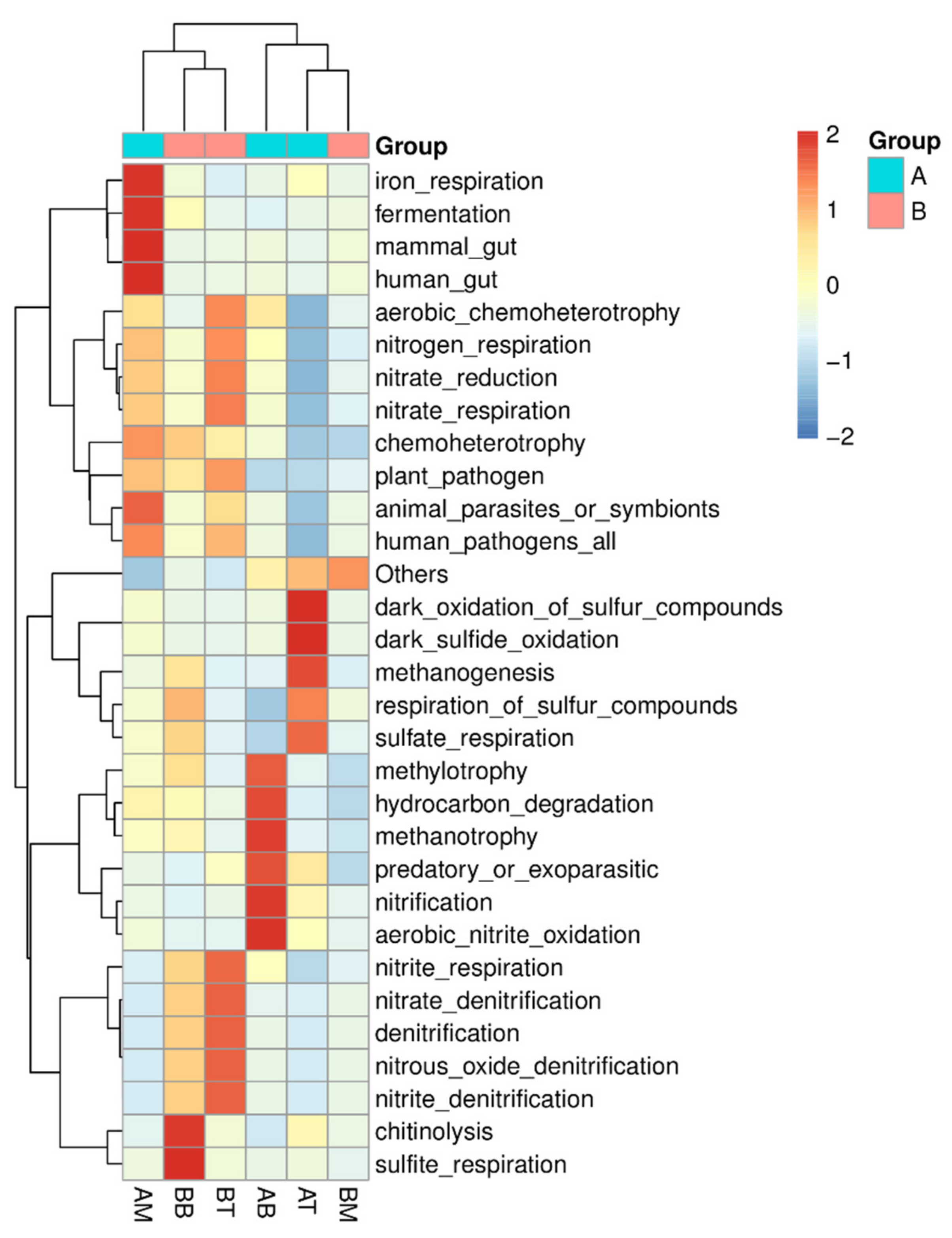
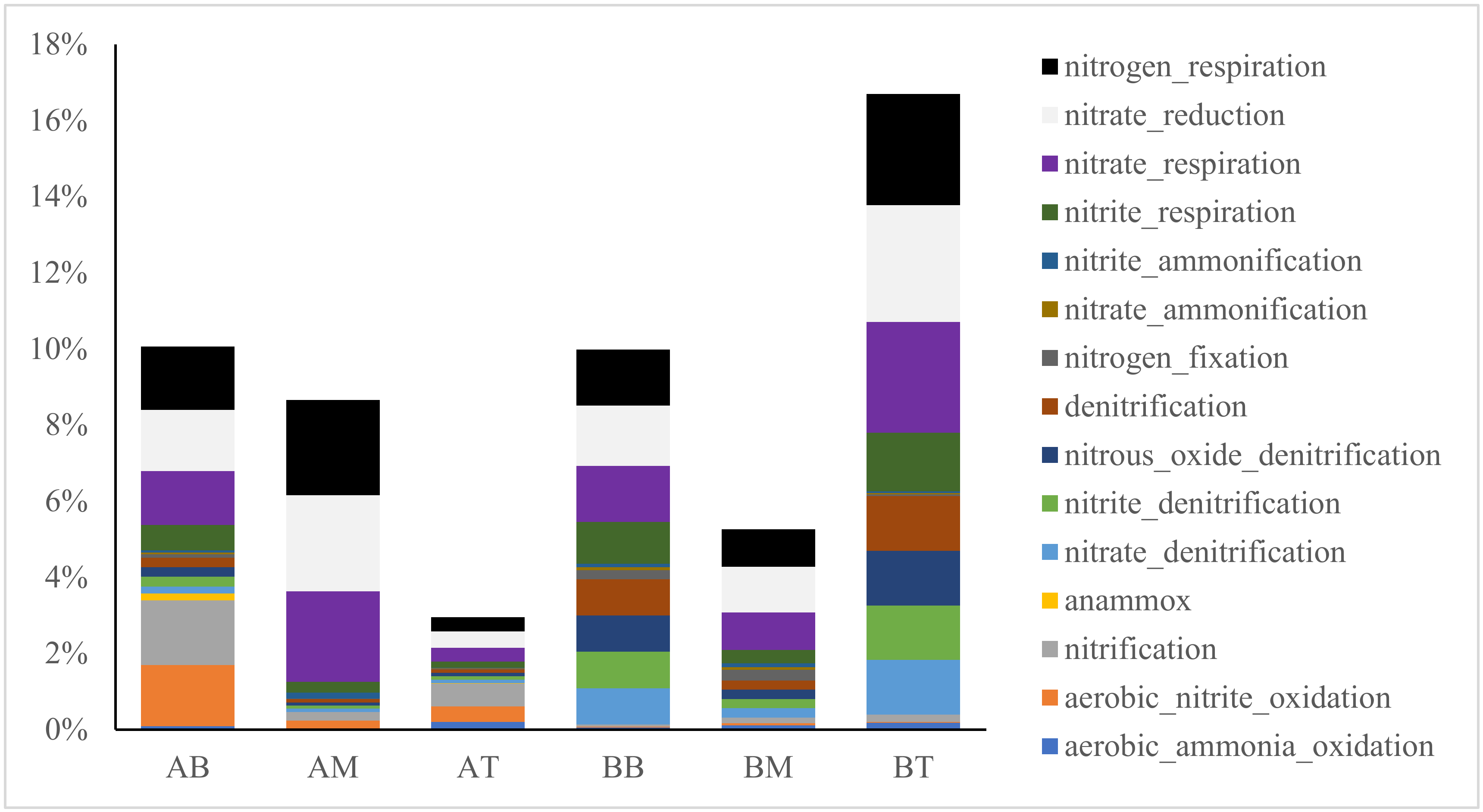
3.3. Functional Prediction
3.4. Correlation of Pollutant Concentrations and Microbial Components
4. Discussion
4.1. Ceramsite Substrate Enhanced Wastewater Treatment Efficiency in CWs
4.2. Changes of Microbial Community in CWs During Operation
4.3. Ceramsite Substrate Enhanced Nitrification in CWs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Burgin, A.J.; Hamilton, S.K. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front. Ecol. Environ. 2007, 5, 89–96. [Google Scholar] [CrossRef]
- Shuai, W.T.; Jaffe, P.R. Anaerobic ammonium oxidation coupled to iron reduction in constructed wetland mesocosms. Sci. Total Environ. 2019, 648, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zhao, Y.; Pan, F.; Yang, B.; Wang, H.; Wang, S.; He, F. Enhanced optimal removal of nitrogen and organics from intermittently aerated vertical flow constructed wetlands: Relative COD/N ratios and microbial responses. Chemosphere 2020, 244, 125556. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. The use constructed wetlands with horizontal sub-surface flow for various types of wastewater. Ecol. Eng. 2009, 35, 1–17. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, Z.; Sheng, S.; Pan, F.; Chen, F.; Fu, J. Comprehensive evaluation of substrate materials for contaminants removal in constructed wetlands. Sci. Total Environ. 2020, 701, 134736. [Google Scholar] [CrossRef]
- Meng, P.; Pei, H.; Hu, W.; Shao, Y.; Li, Z. How to increase microbial degradation in constructed wetlands: Influencing factors and improvement measures. Bioresour. Technol. 2014, 157, 316–326. [Google Scholar] [CrossRef]
- Truu, M.; Juhanson, J.; Truu, J. Microbial biomass, activity and community composition in constructed wetlands. Sci. Total Environ. 2009, 407, 3958–3971. [Google Scholar] [CrossRef]
- Wu, Y.; He, T.; Chen, C.; Fang, X.; Wei, D.; Yang, J.; Zhang, R.; Han, R. Impacting microbial communities and absorbing pollutants by Canna Indica and Cyperus Alternifolius in a full-scale constructed wetland system. Int. J. Environ. Res. Public Health 2019, 16, 802. [Google Scholar] [CrossRef]
- Faulwetter, J.L.; Gagnon, V.; Sundberg, C.; Chazarenc, F.; Burr, M.D.; Brisson, J.; Camper, A.K.; Stein, O.R. Microbial processes influencing performance of treatment wetlands: A review. Ecol. Eng. 2009, 35, 987–1004. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, Y.; Guo, Q.; Li, Z.; Qi, M.; Ma, X.; Wang, H.; Kong, D.; Wang, A.; Liang, B. Bioremediation of contaminated urban river sediment with methanol stimulation: Metabolic processes accompanied with microbial community changes. Sci. Total Environ. 2019, 653, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, C.; Jaffé, P.R. Seasonal distribution of nitrifiers and denitrifiers in urban river sediments affected by agricultural activities. Sci. Total Environ. 2018, 642, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C.M. Full-scale partial nitritation/anammox experiences—An application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, S.; Wu, Q.; Zhang, R. Nitrate reduction coupled with microbial oxidation of sulfide in river sediment. J. Soils Sediments 2012, 12, 1435–1444. [Google Scholar] [CrossRef]
- Lovley, D.R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Mol. Biol. Rev. 1991, 55, 259–287. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, H.J.; Lee, D.S.; Jeon, C.O. Characterization of the denitrification-associated phosphorus uptake properties of “Candidatus Accumulibacter phosphatis” clades in sludge subjected to enhanced biological phosphorus removal. Appl. Environ. Microbiol. 2013, 79, 1969–1979. [Google Scholar] [CrossRef]
- Albuquerque, A.; Oliveira, J.; Semitela, S.; Amaral, L. Influence of bed media characteristics on ammonia and nitrate removal in shallow horizontal subsurface flow constructed wetlands. Bioresour. Technol. 2009, 100, 6269–6277. [Google Scholar] [CrossRef]
- Du, L.; Chen, Q.; Liu, P.; Zhang, X.; Wang, H.; Zhou, Q.; Xu, D.; Wu, Z. Phosphorus removal performance and biological dephosphorization process in treating reclaimed water by Integrated Vertical-flow Constructed Wetlands (IVCWs). Bioresour. Technol. 2017, 243, 204–211. [Google Scholar] [CrossRef]
- Zhi, W.; Yuan, L.; Ji, G.; He, C. Enhanced long-term nitrogen removal and its quantitative molecular mechanism in tidal flow constructed wetlands. Environ. Sci. Technol. 2015, 49, 4575–4583. [Google Scholar] [CrossRef]
- Sandoval-Herazo, L.C.; Alvarado-Lassman, A.; Marin-Muniz, J.L.; Mendez-Contreras, J.M.; Zamora-Castro, S.A. Effects of the use of ornamental plants and different substrates in the removal of wastewater pollutants through microcosms of constructed wetlands. Sustainability 2018, 10, 1594. [Google Scholar] [CrossRef]
- Wu, H.; Fan, J.; Zhang, J.; Ngo, H.H.; Guo, W.; Liang, S.; Lv, J.; Lu, S.; Wu, W.; Wu, S. Intensified organics and nitrogen removal in the intermittent-aerated constructed wetland using a novel sludge-ceramsite as substrate. Bioresour. Technol. 2016, 210, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yue, Q.; Han, S.; Yue, M.; Gao, B.; Yu, H.; Shao, T. Preparation and mechanism of ultra-lightweight ceramics produced from sewage sludge. J. Hazard. Mater. 2010, 176, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jaffe, P.R. Isolation and characterization of an ammonium-oxidizing iron reducer: Acidimicrobiaceae sp A6. PLoS ONE 2018, 13, e0194007. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Wu, S.; Qi, Y.; Yue, Q.; Gao, B.; Gao, Y.; Fan, C.; He, S. Preparation of ceramic filler from reusing sewage sludge and application in biological aerated filter for soy protein secondary wastewater treatment. J. Hazard. Mater. 2015, 283, 608–616. [Google Scholar] [CrossRef]
- Zou, J.L.; Xu, G.R.; Pan, K.; Zhou, W.; Dai, Y.; Wang, X.; Zhang, D.; Hu, Y.-C.; Ma, M. Nitrogen removal and biofilm structure affected by COD/NH4+–N in a biofilter with porous sludge-ceramsite. Sep. Purif. Technol. 2012, 94, 9–15. [Google Scholar] [CrossRef]
- Öövel, M.; Tooming, A.; Mauring, T.; Mander, Ü. Schoolhouse wastewater purification in a LWA-filled hybrid constructed wetland in Estonia. Ecol. Eng. 2007, 29, 17–26. [Google Scholar] [CrossRef]
- Meng, P.; Pei, H.; Hu, W.; Shao, Y.; Li, Z. Performance evaluation of light-weight aggregates-based horizontal flow constructed wetlands for domestic wastewater treatment. CLEAN—Soil Air Water 2015, 43, 217–222. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Kuenen, J.G. Haloalkaliphilic sulfur-oxidizing bacteria in soda lakes. FEMS Microbiol. Rev. 2005, 29, 685–702. [Google Scholar] [CrossRef] [PubMed]
- Limpiyakorn, T.; Sonthiphand, P.; Rongsayamanont, C.; Polprasert, C. Abundance of amoA genes of ammonia-oxidizing archaea and bacteria in activated sludge of full-scale wastewater treatment plants. Bioresour. Technol. 2011, 102, 3694–3701. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.F.; Luo, X.; Wu, G.X.; Li, T.; Peng, Y.Z. Abundance and diversity based on amoA genes of ammonia-oxidizing archaea and bacteria in ten wastewater treatment systems. Appl. Microbiol. Biotechnol. 2014, 98, 3339–3354. [Google Scholar] [CrossRef]
- Kartal, B.; Kuenen, J.G.; van Loosdrecht, M.C.M. Sewage treatment with anammox. Science 2010, 328, 702–703. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- Van Kessel, M.A.H.J.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.M.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef]
- Pester, M.; Maixner, F.; Berry, D.; Rattei, T.; Koch, H.; Lucker, S.; Nowka, B.; Richter, A.; Spieck, E.; Lebedeva, E.; et al. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ. Microbiol. 2014, 16, 3055–3071. [Google Scholar] [CrossRef]
- Daims, H.; Nielsen, J.L.; Nielsen, P.H.; Schleifer, K.H.; Wagner, M. In situ characterization of Nitrospira-like nitrite oxidizing bacteria active in wastewater treatment plants. Appl. Environ. Microbiol. 2001, 67, 5273–5284. [Google Scholar] [CrossRef]
- Yamada, T.; Hamada, M.; Nakagawa, M.; Sato, N.; Ando, A.; Ogawa, J.; Yasuda, M.; Kawagishi, T. 16S rRNA gene amplicon sequencing of microbiota in polybutylene succinate adipate-packed denitrification reactors used for water treatment of land-based recirculating aquaculture systems. Microbiol. Resour. Announc. 2019, 8, e01295-19. [Google Scholar] [CrossRef]
| NH4+ | TN | TP | |
|---|---|---|---|
| Class Level | |||
| Clostridia | 0.145 | 0.145 | 0.000 |
| Deltaproteobacteria | −0.406 | −0.406 | −0.239 |
| Gammaproteobacteria | 0.580 | 0.580 | 0.717 |
| Anaerolineae | −0.986 ** | −0.986 ** | −0.956 ** |
| Bacteroidia | 0.145 | 0.145 | 0.000 |
| Alphaproteobacteria | 0.029 | 0.029 | 0.120 |
| Holophagae | 0.638 | 0.638 | 0.478 |
| unidentified_Actinobacteria | −0.870 * | −0.870 * | −0.956 ** |
| Bacilli | 0.058 | 0.058 | 0.000 |
| unidentified_Gemmatimonadetes | −0.058 | −0.058 | 0.000 |
| Genus | |||
| Anaeromyxobacter | −0.928 ** | −0.928 ** | −0.837 * |
| Symbiobacterium | 0.232 | 0.232 | 0.000 |
| Geobacter | −0.116 | −0.116 | 0.120 |
| Lysobacter | −0.232 | −0.232 | −0.239 |
| Stenotrophomonas | 0.116 | 0.116 | 0.120 |
| Thiobacillus | −0.294 | −0.294 | −0.061 |
| Marmoricola | −0.725 | −0.725 | −0.837 * |
| Pontibacter | 0.000 | 0.000 | −0.120 |
| Thioalkalispira | −0.691 | −0.691 | −0.546 |
| Pseudomonas | 0.406 | 0.406 | 0.239 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Q.; Han, Q.; Luo, H.; He, T.; Xue, F.; Ye, Z.; Chen, C.; Huang, S. Ceramsite Facilitated Microbial Degradation of Pollutants in Domestic Wastewater. Int. J. Environ. Res. Public Health 2020, 17, 4692. https://doi.org/10.3390/ijerph17134692
Wan Q, Han Q, Luo H, He T, Xue F, Ye Z, Chen C, Huang S. Ceramsite Facilitated Microbial Degradation of Pollutants in Domestic Wastewater. International Journal of Environmental Research and Public Health. 2020; 17(13):4692. https://doi.org/10.3390/ijerph17134692
Chicago/Turabian StyleWan, Qiong, Qingji Han, Hailin Luo, Tao He, Feng Xue, Zihuizhong Ye, Chen Chen, and Shan Huang. 2020. "Ceramsite Facilitated Microbial Degradation of Pollutants in Domestic Wastewater" International Journal of Environmental Research and Public Health 17, no. 13: 4692. https://doi.org/10.3390/ijerph17134692
APA StyleWan, Q., Han, Q., Luo, H., He, T., Xue, F., Ye, Z., Chen, C., & Huang, S. (2020). Ceramsite Facilitated Microbial Degradation of Pollutants in Domestic Wastewater. International Journal of Environmental Research and Public Health, 17(13), 4692. https://doi.org/10.3390/ijerph17134692






