Associations between Particulate Matter and Otitis Media in Children: A Meta-Analysis
Abstract
1. Introduction
- (a)
- Cause conductive hearing loss, otalgia, sleep disturbance, loss of appetite, and behavioral problems;
- (b)
- Delay the development of speech, language, balance, and learning abilities [4,5], thus significantly impacting the quality of life of children and their families. OM, with an estimated annual cost of 3.2 billion dollars, is one of the most costly conditions affecting children in the United States [6]. Therefore, the identification of risk factors of OM and potential ways to control for these risk factors may significantly impact global healthcare (e.g., quality of life, medical costs).
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Assessment of Quality and the Risk of Bias
2.4. Data Extraction
2.5. Framework of Analysis
2.6. Statistical Analysis
2.7. Ethical Approval
3. Results
3.1. Search Results
3.2. Characteristics of the Included Studies
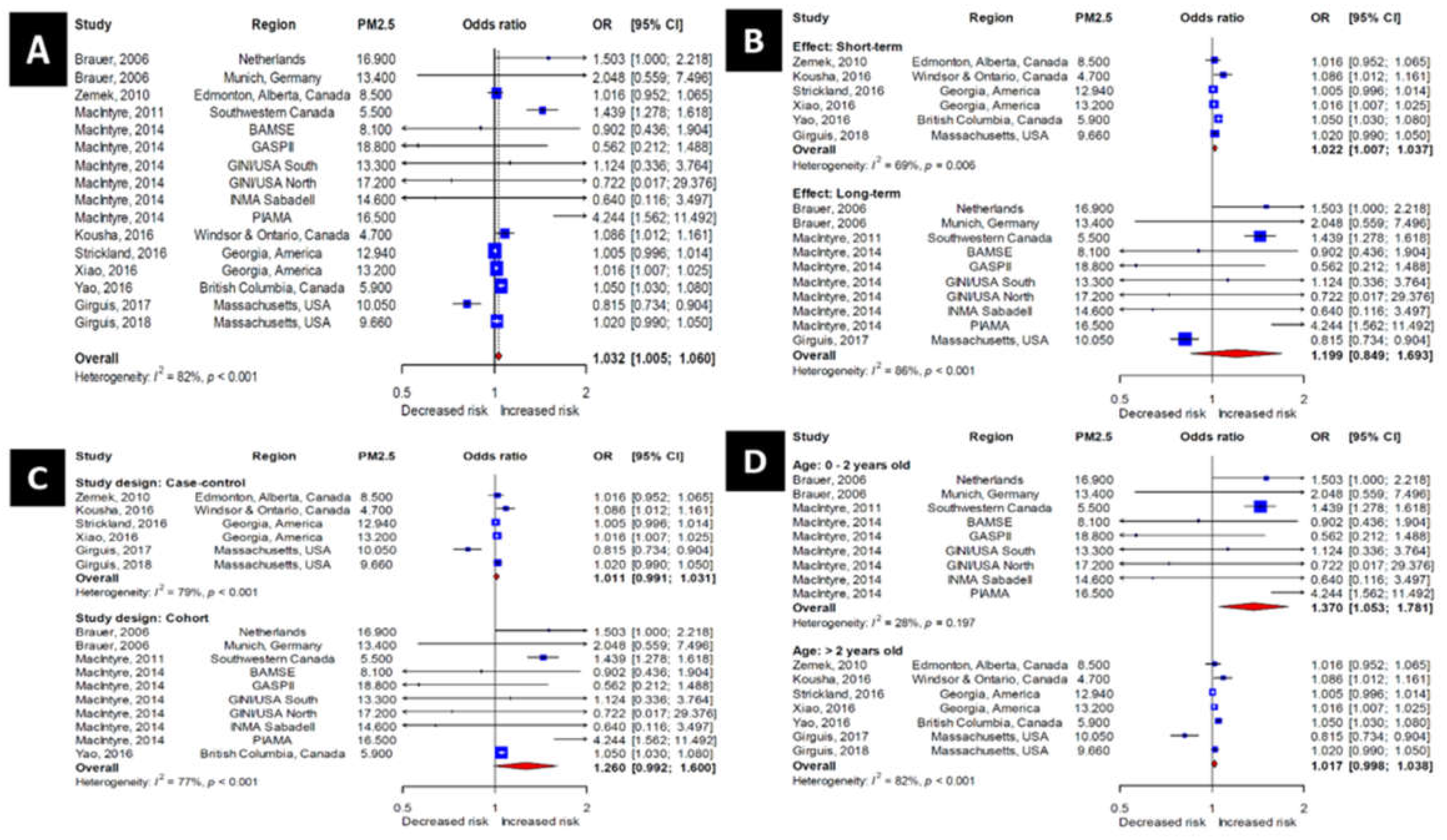
3.3. Association of PM2.5 Exposure with the Incidence of OM
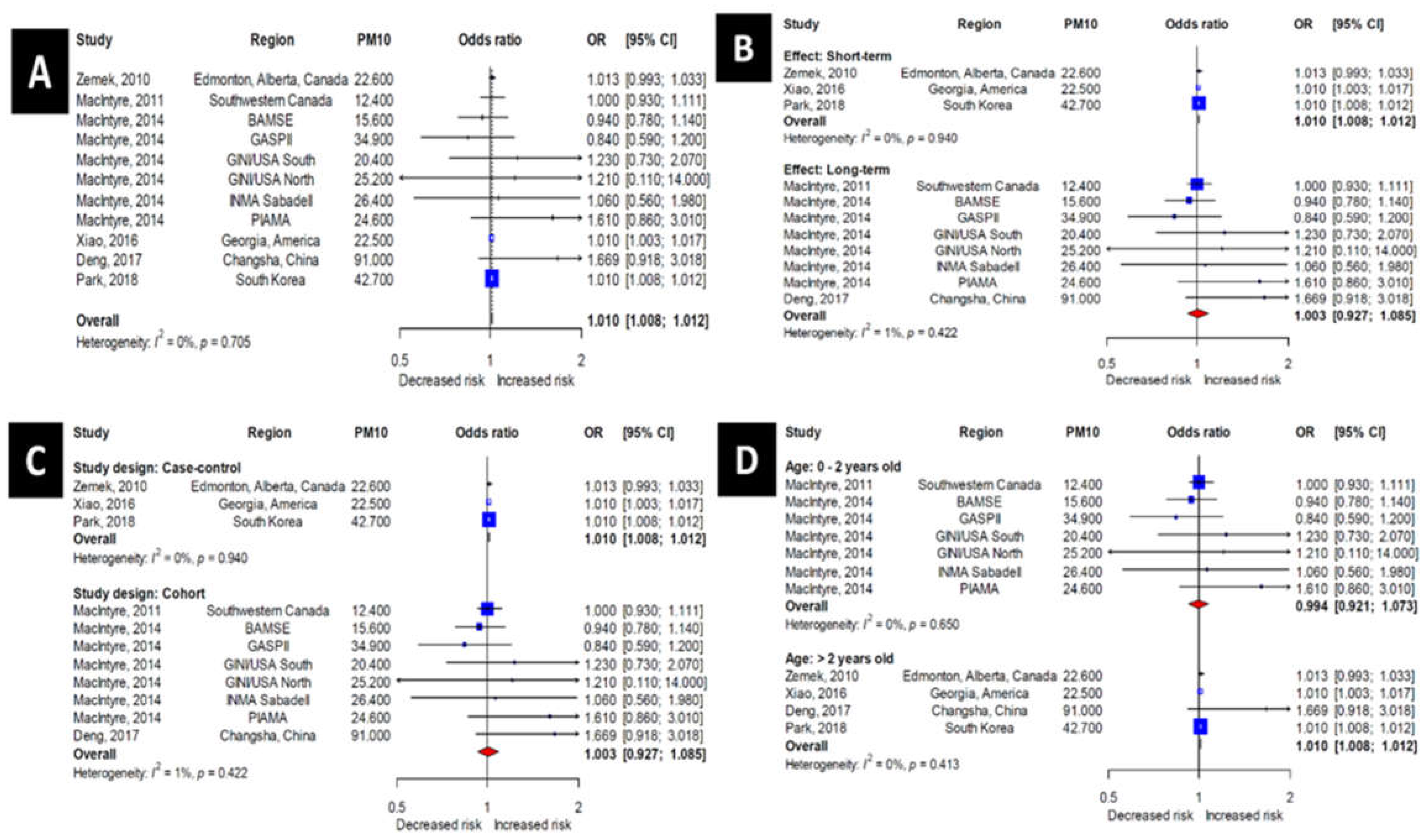
3.4. Association of PM10 Exposure with the Incidence of OM
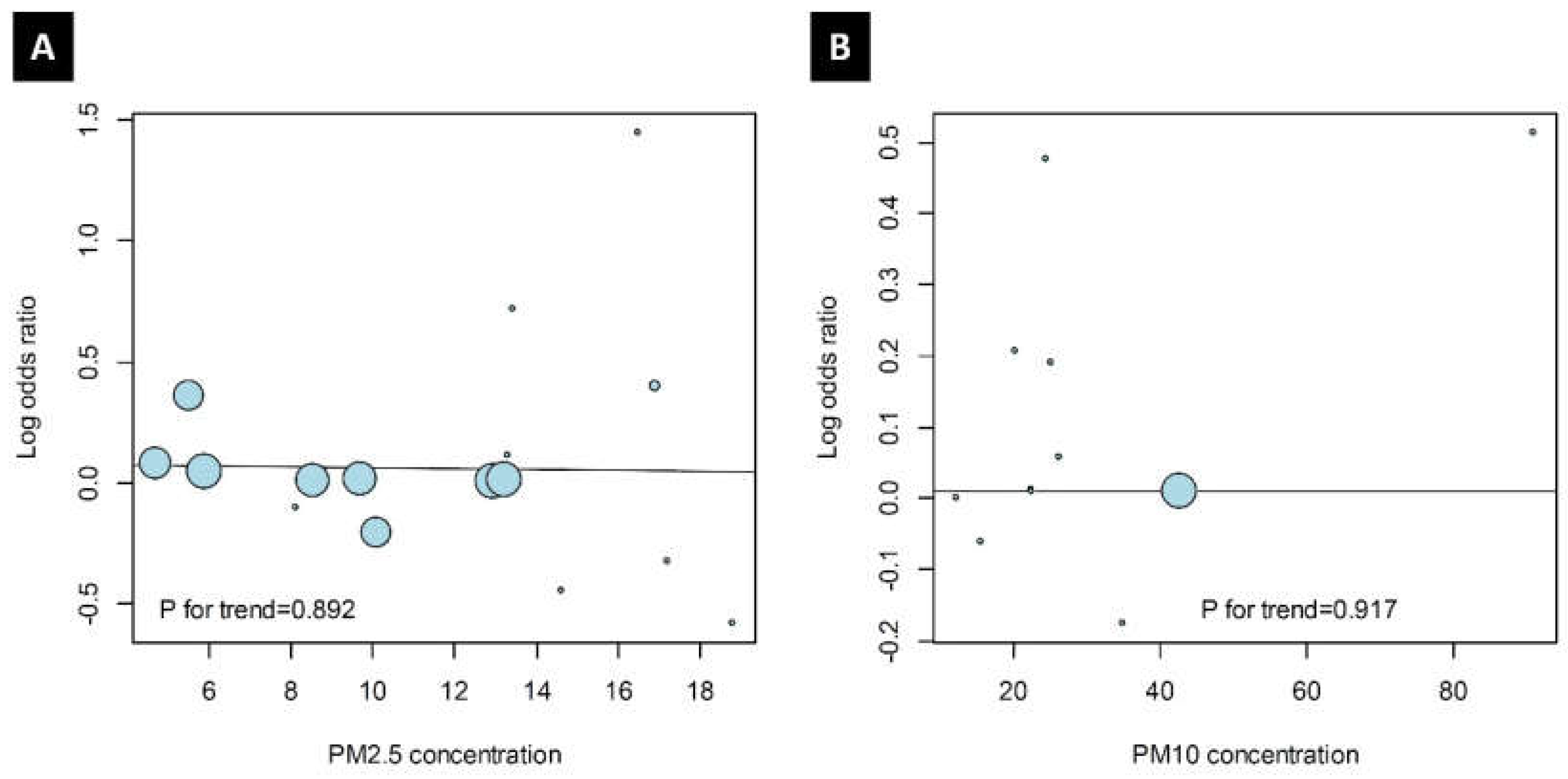
3.5. Trends of Odds Ratio and PM Values
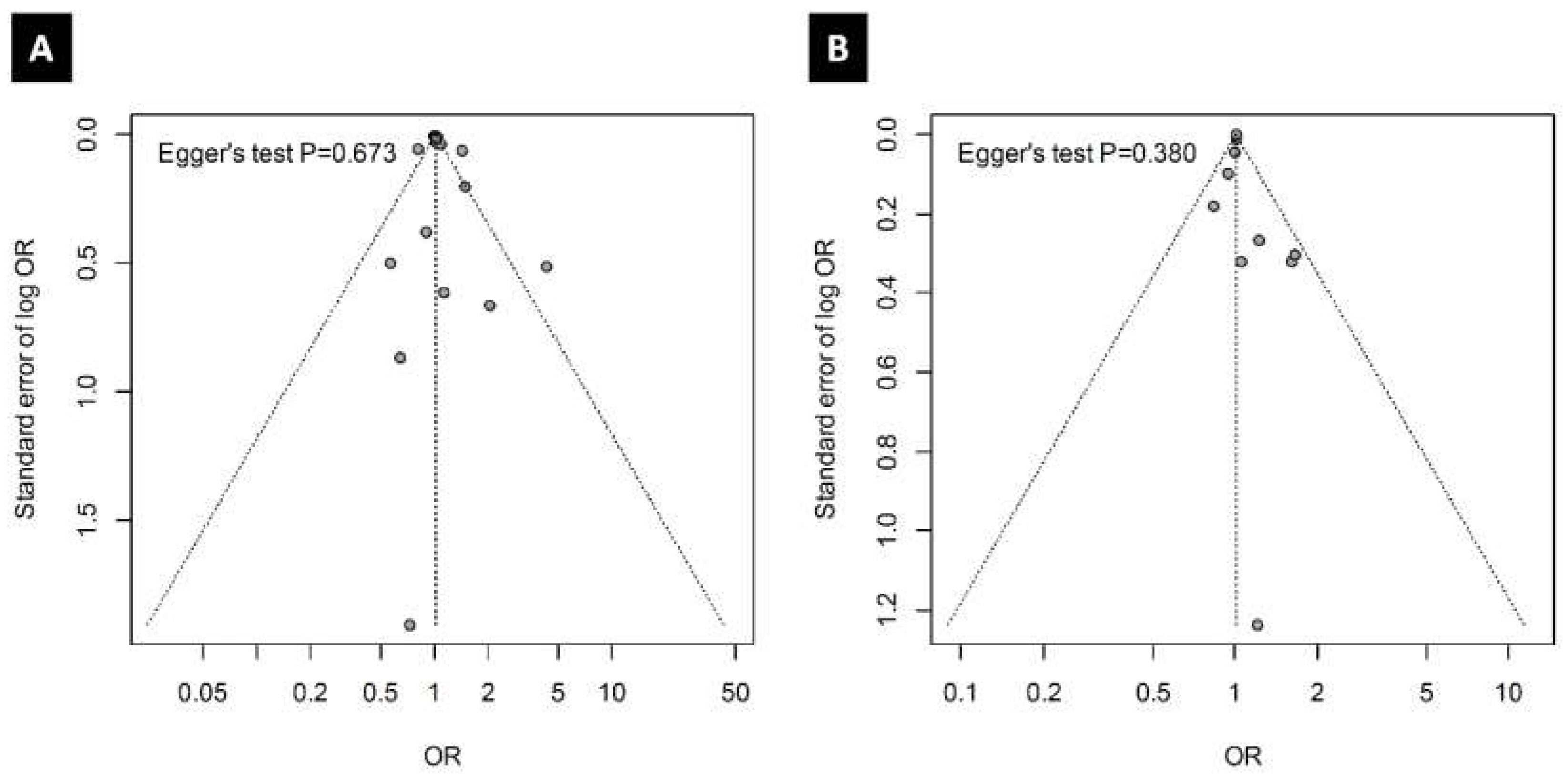
3.6. Publication Bias
3.7. Association between Lag and the Incidence of OM
4. Discussion
- (a)
- the prevalence of OM is high
- (b)
- everyone is exposed to PM. It is also of note that the odds ratio is larger for increased exposure to PM2.5 compared with increased exposure to PM10; this observation is consistent with the widely accepted notion that PM2.5 is more toxic than PM10, likely because PM2.5 can be more easily transported to the lungs and circulatory system, and interact with mucosa and immune cells in the middle ear.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeAntonio, R.; Yarzabal, J.P.; Cruz, J.P.; Schmidt, J.E.; Kleijnen, J. Epidemiology of otitis media in children from developing countries: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2016, 85, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Auinger, P.; Lanphear, B.P.; Kalkwarf, H.J.; Mansour, M.E. Trends in otitis media among children in the United States. Pediatrics 2003, 112, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Teele, D.W.; Klein, J.O.; Rosner, B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: A prospective, cohort study. J. Infect. Dis. 1989, 160, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Rovers, M.M. The burden of otitis media. Vaccine 2008, 26 (Suppl. 7), G2–G4. [Google Scholar] [CrossRef] [PubMed]
- Grindler, D.J.; Blank, S.J.; Schulz, K.A.; Witsell, D.L.; Lieu, J.E. Impact of Otitis Media Severity on Children’s Quality of Life. Otolaryngol. Head Neck Surg. 2014, 151, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Soni, A. The Five Most Costly Children’s Conditions, 2011: Estimates for U.S. Civilian Noninstitutionalized Children, Ages 0-17. In Statistical Brief (Medical Expenditure Panel Survey (US)); AHRQ: Rockville, MD, USA, 2001. [Google Scholar]
- Bouazza, N.; Foissac, F.; Urien, S.; Guedj, R.; Carbajal, R.; Treluyer, J.M.; Chappuy, H. Fine particulate pollution and asthma exacerbations. Arch. Dis. Child 2018, 103, 828–831. [Google Scholar] [CrossRef]
- Girguis, M.S.; Strickland, M.J.; Hu, X.; Liu, Y.; Chang, H.H.; Kloog, I.; Belanoff, C.; Bartell, S.M.; Vieira, V.M. Exposure to acute air pollution and risk of bronchiolitis and otitis media for preterm and term infants. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 348–357. [Google Scholar] [CrossRef]
- Patto, N.V.; Nascimento, L.F.; Mantovani, K.C.; Vieira, L.C.; Moreira, D.S. Exposure to fine particulate matter and hospital admissions due to pneumonia: Effects on the number of hospital admissions and its costs. Rev. Assoc. Med. Bras. 2016, 62, 342–346. [Google Scholar] [CrossRef]
- Choi, J.; Oh, J.Y.; Lee, Y.S.; Min, K.H.; Hur, G.Y.; Lee, S.Y.; Kang, K.H.; Shim, J.J. Harmful impact of air pollution on severe acute exacerbation of chronic obstructive pulmonary disease: Particulate matter is hazardous. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 1053–1059. [Google Scholar] [CrossRef]
- Park, M.; Han, J.; Jang, M.J.; Suh, M.W.; Lee, J.H.; Oh, S.H.; Park, M.K. Air pollution influences the incidence of otitis media in children: A national population-based study. PLoS ONE 2018, 13, e0199296. [Google Scholar] [CrossRef]
- Song, J.J.; Lee, J.D.; Lee, B.D.; Chae, S.W.; Park, M.K. Effect of diesel exhaust particles on human middle ear epithelial cells. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J. Nonallergic respiratory morbidity improved along with a decline of traditional air pollution levels: A review. Eur. Respir. J. Suppl. 2003, 40, 64s–69s. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J.; Raghuyamshi, V.S. Air pollution and otitis media: A review of evidence from epidemiologic studies. Curr. Allergy Asthma. Rep. 2004, 4, 302–309. [Google Scholar] [CrossRef]
- Jang, A.S.; Jun, Y.J.; Park, M.K. Effects of air pollutants on upper airway disease. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 13–17. [Google Scholar] [CrossRef]
- Bowatte, G.; Tham, R.; Perret, J.L.; Bloom, M.S.; Dong, G.; Waidyatillake, N.; Bui, D.; Morgan, G.G.; Jalaludin, B.; Lodge, C.J.; et al. Air Pollution and Otitis Media in Children: A Systematic Review of Literature. Int. J. Environ. Res. Public Health 2018, 15, 257. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Gehring, U.; Brunekreef, B.; de Jongste, J.; Gerritsen, J.; Rovers, M.; Wichmann, H.E.; Wijga, A.; Heinrich, J. Traffic-related air pollution and otitis media. Environ. Health Perspect 2006, 114, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Zemek, R.; Szyszkowicz, M.; Rowe, B.H. Air pollution and emergency department visits for otitis media: A case-crossover study in Edmonton, Canada. Environ. Health Perspect 2010, 118, 1631–1636. [Google Scholar] [CrossRef]
- MacIntyre, E.A.; Karr, C.J.; Koehoorn, M.; Demers, P.A.; Tamburic, L.; Lencar, C.; Brauer, M. Residential air pollution and otitis media during the first two years of life. Epidemiology 2011, 22, 81–89. [Google Scholar] [CrossRef]
- MacIntyre, E.A.; Gehring, U.; Molter, A.; Fuertes, E.; Klumper, C.; Kramer, U.; Quass, U.; Hoffmann, B.; Gascon, M.; Brunekreef, B.; et al. Air pollution and respiratory infections during early childhood: An analysis of 10 European birth cohorts within the ESCAPE Project. Environ. Health Perspect. 2014, 122, 107–113. [Google Scholar] [CrossRef]
- Kousha, T.; Castner, J. The Air Quality Health Index and Emergency Department Visits for Otitis Media. J. Nurs. Sch. 2016, 48, 163–171. [Google Scholar] [CrossRef]
- Strickland, M.J.; Hao, H.; Hu, X.; Chang, H.H.; Darrow, L.A.; Liu, Y. Pediatric Emergency Visits and Short-Term Changes in PM2.5 Concentrations in the U.S. State of Georgia. Environ. Health Perspect 2016, 124, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Liu, Y.; Mulholland, J.A.; Russell, A.G.; Darrow, L.A.; Tolbert, P.E.; Strickland, M.J. Pediatric emergency department visits and ambient Air pollution in the U.S. State of Georgia: A case-crossover study. Environ. Health 2016, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Eyamie, J.; Henderson, S.B. Evaluation of a spatially resolved forest fire smoke model for population-based epidemiologic exposure assessment. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ye, Z.; Lu, H.; Lu, J.; Huang, L.; Gong, J.; Deng, Q.; Xu, L. Association between body mass index (BMI) and vital capacity of college students of Zhuang nationality in China: A cross-section study. Oncotarget 2017, 8, 80923–80933. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Girguis, M.S.; Strickland, M.J.; Hu, X.; Liu, Y.; Chang, H.H.; Belanoff, C.; Bartell, S.M.; Vieira, V.M. Chronic PM2.5 exposure and risk of infant bronchiolitis and otitis media clinical encounters. Int. J. Hyg. Environ. Health 2017, 220, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A., 3rd; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef]
- Deng, Q.; Lu, C.; Jiang, W.; Zhao, J.; Deng, L.; Xiang, Y. Association of outdoor air pollution and indoor renovation with early childhood ear infection in China. Chemosphere 2017, 169, 288–296. [Google Scholar] [CrossRef]
- Park, M.K.; Chae, S.W.; Kim, H.B.; Cho, J.G.; Song, J.J. Middle ear inflammation of rat induced by urban particles. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.Y.; Kwon, J.Y.; Kim, Y.J.; Hun Kang, S.; Jang, W.H.; Lee, J.H.; Seo, M.W.; Song, J.J.; Seo, Y.R.; et al. Identification of Potential Novel Biomarkers and Signaling Pathways Related to Otitis Media Induced by Diesel Exhaust Particles Using Transcriptomic Analysis in an In Vivo System. PLoS ONE 2016, 11, e0166044. [Google Scholar] [CrossRef] [PubMed]


| Number | Published Year | Author | Study Period | Study Region | Study Design | OM Diagnosis Source | Age of Subjects (yr) | Number of Subjects | Type of PM | Mean PM | Period of PM Measurement (d) | Classification of PM Measurement Period * | Measure of Association | Per Increase | Study Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2006 | Brauer [17] | 2002–2003 | Netherlands | Cohort study | Parent report | 0–1 | 2984 | PM2.5 | 16.9 | 365, 730 | Long–term | Odds ratio | 3 | 7 |
| 2 | 2010 | Zemek [18] | 1992–2002 | Edmonton, Alberta, Canada | Case-control study | Medical record | 1–3 | 14,527 | PM10 | 22.6 | 0–4 | Short-term | Odds ratio | 15 | 7 |
| 3 | 2011 | MacIntyre [19] | 1999–2000 | Southwestern Canada | Cohort study | Medical record | 0–2 | 44,917 | PM10 | 12.4 | 60 | Long-term | Odds ratio | 2.8 | 8 |
| 4 | 2014 | MacIntyre [20] | 2008–2011 | Six Countries in Western Europe | Cohort study | Parent report | 0 | 8772 | PM2.5 | 8.1–18.8 | 365 | Long-term | Odds ratio | 5 | 8 |
| 5 | 2016 | Kousha [21] | 2004–2010 | Windsor & Ontario, Canada | Case-control study | Medical record | 0–3 | 4815 | PM2.5 | 4.7 | 0–7 | Short-term | Odds ratio | 8.2 | 8 |
| 6 | 2016 | Strickland [22] | 2002–2010 | Georgia, America | Case-control study | Medical record | 0–18 | 237,833 | PM2.5 | 12.94 | 1–2 | Short-term | Odds ratio | 10 | 7 |
| 7 | 2016 | Xiao [23] | 2002–2008 | Georgia, America | Case–control study | Medical record | 0–18 | 422,268 | PM10 | 22.5 | 3 | Short-term | Odds ratio | 11.5 | 7 |
| 8 | 2016 | Yao [24] | 2003–2010 | British Columbia, Canada | Cohort study | Medical record | 0–10 | 175 | PM2.5 | 5.9 | 1 | Short-term | Risk ratio | 10 | 8 |
| 9 | 2017 | Deng [25] | 2011–2012 | Changsha, China | Cohort study | Parent report | 0 | 1617 | PM10 | 106 | 90–1095 | Long-term | Odds ratio | 15 | 7 |
| 10 | 2017 | Girguis [26] | 2001–2006 | Massachusetts, USA | Case-control study | Medical record | 0–3 | 40,042 | PM2.5 | 10.1 | 0–1095 | Long-term | Odds ratio | 2 | 8 |
| 11 | 2018 | Girguis [8] | 2001–2008 | Massachusetts, USA | Case-control study | Medical record | 0–3 | 37,040 | PM2.5 | 9.56–9.76 | 0–7 | Short-term | Odds ratio | 10 | 8 |
| 12 | 2018 | Park [11] | 2011–2012 | South Korea | Case-control study | Medical record | 0–14 | 160,875 | PM10 | 42.7 | 7 | Short-term | Odds ratio | 30 | 8 |
| Study | Group | Subgroup | |
|---|---|---|---|
| Overall 975,865 | PM2.5 813,181 | Short-term 716,708 | Long-term 96,473 |
| Case-control 756,575 | Cohort 56,606 | ||
| <3 years old 56,431 | ≥3 years old 756,750 | ||
| PM10 653,942 | Short-term 597,670 | Long-term 56,272 | |
| Case-control 597,670 | Cohort 56,272 | ||
| <3 years old 54,655 | ≥3 years old 599,287 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-Y.; Jang, M.-j.; Oh, S.H.; Lee, J.H.; Suh, M.-W.; Park, M.K. Associations between Particulate Matter and Otitis Media in Children: A Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 4604. https://doi.org/10.3390/ijerph17124604
Lee S-Y, Jang M-j, Oh SH, Lee JH, Suh M-W, Park MK. Associations between Particulate Matter and Otitis Media in Children: A Meta-Analysis. International Journal of Environmental Research and Public Health. 2020; 17(12):4604. https://doi.org/10.3390/ijerph17124604
Chicago/Turabian StyleLee, Sang-Youp, Myoung-jin Jang, Seung Ha Oh, Jun Ho Lee, Myung-Whan Suh, and Moo Kyun Park. 2020. "Associations between Particulate Matter and Otitis Media in Children: A Meta-Analysis" International Journal of Environmental Research and Public Health 17, no. 12: 4604. https://doi.org/10.3390/ijerph17124604
APA StyleLee, S.-Y., Jang, M.-j., Oh, S. H., Lee, J. H., Suh, M.-W., & Park, M. K. (2020). Associations between Particulate Matter and Otitis Media in Children: A Meta-Analysis. International Journal of Environmental Research and Public Health, 17(12), 4604. https://doi.org/10.3390/ijerph17124604




