Hyperosmolar Treatment for Patients at Risk for Increased Intracranial Pressure: A Single-Center Cohort Study
Abstract
1. Introduction
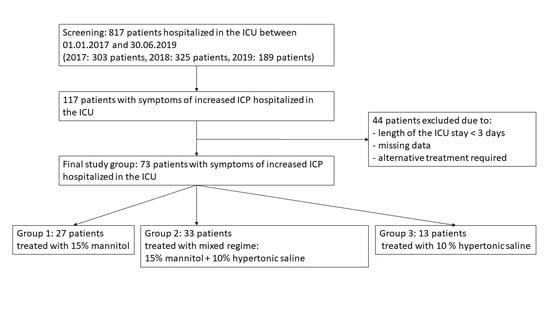
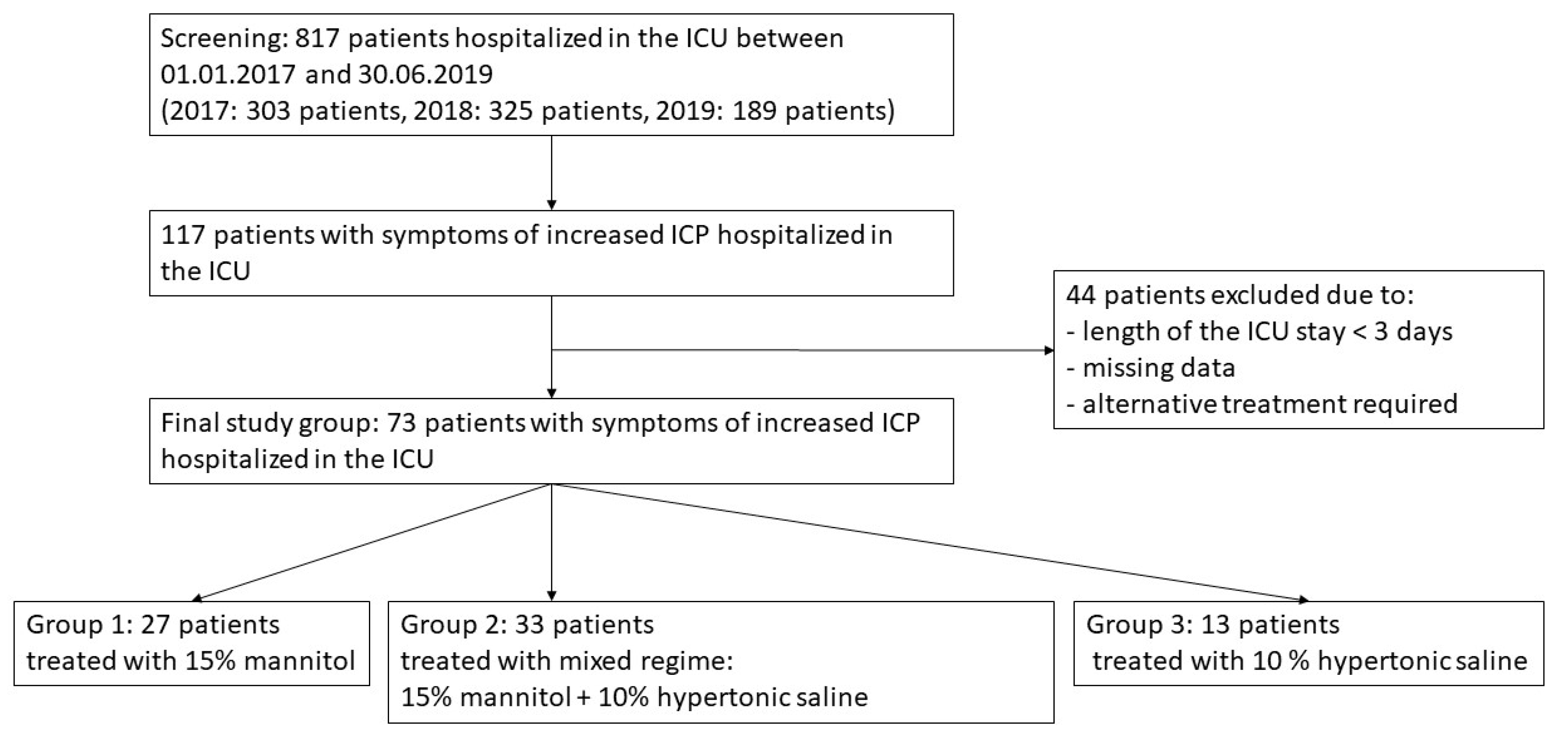
2. Materials and Methods
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peters, N.A.; Farrell, L.B.; Smith, J.P. Hyperosmolar Therapy for the Treatment of Cerebral Edema. US Pharm. 2018, 43, HS8–HS11. [Google Scholar]
- Fink, M.E. Osmotherapy for Intracranial Hypertension: Mannitol Versus Hypertonic Saline. Continuum 2012, 18, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Nau, R. Osmotherapy for Elevated Intracranial Pressure: A critical reappraisal. Clin. Pharmacokinet. 2000, 38, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Xu, M.; Liu, H.; Wang, W.; Wang, Z. A Retrospective Study of Intracranial Pressure in Head-Injured Patients Undergoing Decompressive Craniectomy: A Comparison of Hypertonic Saline and Mannitol. Front. Neurol. 2018, 9, 631. [Google Scholar] [CrossRef]
- Caplan, H.W.; Cox, C.S. Resuscitation Strategies for Traumatic Brain Injury. Curr. Surg. Rep. 2019, 7, 14. [Google Scholar] [CrossRef]
- Bhardwaj, A. Osmotherapy in neurocritical care. Curr. Neurol. Neurosci. Rep. 2007, 7, 513–521. [Google Scholar] [CrossRef]
- Marko, N.F. Hypertonic saline, not mannitol, should be considered gold-standard medical therapy for intracranial hypertension. Crit. Care 2012, 16, 113. [Google Scholar] [CrossRef]
- Mangat, H.S.; Wu, X.; Gerber, L.M.; Schwarz, J.T.; Fakhar, M.; Murthy, S.B.; Stieg, P.E.; Ghajar, J.; Härtl, R. Hypertonic Saline is Superior to Mannitol for the Combined Effect on Intracranial Pressure and Cerebral Perfusion Pressure Burdens in Patients With Severe Traumatic Brain Injury. Neurosurgery 2019, 2, 221–230. [Google Scholar] [CrossRef]
- Witherspoon, B.; Ashby, N. The Use of Mannitol and Hypertonic Saline Therapies in Patients with Elevated Intracranial Pressure: A Review of the Evidence. Nurs. Clin. N. Am. 2017, 52, 249–260. [Google Scholar] [CrossRef]
- Maguigan, K.L.; Dennis, B.M.; Hamblin, S.E.; Guillamondegui, O.D. Method of Hypertonic Saline Administration: Effects on Osmolality in Traumatic Brain Injury Patients. J. Clin. Neurosci. 2017, 39, 147–150. [Google Scholar] [CrossRef]
- Wagner, I.; Hauer, E.-M.; Staykov, D.; Volbers, B.; Dörfler, A.; Schwab, S.; Bardutzky, J. Effects of Continuous Hypertonic Saline Infusion on Perihemorrhagic Edema Evolution. Stroke 2011, 42, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Act of 5 December 1996 on the Medical Profession. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU19970280152 (accessed on 28 October 2019).
- Lochner, P.; Czosnyka, M.; Naldi, A.; Lyros, E.; Pelosi, P.; Mathur, S.; Fassbender, K.; Robba, C. Optic nerve sheath diameter: Present and future perspectives for neurologists and critical care physicians. Neurol. Sci. 2019, 40, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Yao, Y.; Feng, L.-S.; Wang, Y.-Z.; Zheng, N.-N.; Feng, J.-C.; Xing, Y.-Q. Noninvasive and quantitative intracranial pressure estimation using ultrasonographic measurement of optic nerve sheath diameter. Sci. Rep. 2017, 7, 42063. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Bragazzi, N.L.; Bertuccio, A.; Cardim, D.; Donnelly, J.; Sekhon, M.; Lavinio, A.; Duane, D.; Burnstein, R.; Matta, B.; et al. Effects of Prone Position and Positive End-Expiratory Pressure on Noninvasive Estimators of ICP: A Pilot Study. J. Neurosurg. Anesthesiol. 2017, 29, 243–250. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Vitiello, L.; Rosa, N. Optic nerve ultrasound measurement in multiple sclerosis. Acta Neurol. Scand. 2019, 139, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Lochner, P.; Fassbender, K.; Knodel, S.; Andrejewski, A.; Lesmeister, M.; Wagenpfeil, G.; Nardone, R.; Brigo, F. B-Mode Transorbital Ultrasonography for the Diagnosis of Idiopathic Intracranial Hypertension: A Systematic Review and Meta-Analysis. Ultraschall Med. 2018, 40, 247–252. [Google Scholar] [CrossRef]
- Robba, C.; Santori, G.; Czosnyka, M.; Corradi, F.; Bragazzi, N.; Padayachy, L.; Taccone, F.S.; Citerio, G. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2018, 44, 1284–1294. [Google Scholar] [CrossRef]
- Toms, D.A. The mechanical index, ultrasound practices, and the ALARA principle. J. Ultrasound Med. 2006, 25, 560–561. [Google Scholar] [CrossRef]
- The Safety Group of the British Medical Ultrasound Society. Guidelines for the safe use of diagnostic ultrasound equipment. Ultrasound 2010, 18, 52–59. [Google Scholar] [CrossRef]
- Weidner, N.; Bomberg, H.; Antes, S.; Meiser, A.; Volk, T.; Groesdonk, H. Real-time evaluation of optic nerve sheath diameter (ONSD) in awake neurosurgical patients. Intensive Care Med. Exp. 2015, 3, A608. [Google Scholar] [CrossRef]
- Czempik, P.F.; Gąsiorek, J.; Bąk, A.; Krzych, Ł.J. Ultrasonic Assessment of Optic Nerve Sheath Diameter in Patients at Risk of Sepsis-Associated Brain Dysfunction: A Preliminary Report. Int. J. Environ. Res. Public Health 2020, 17, 3656. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A. Acids and Bases. In Handbook of Blood Gas/Acid-Base Interpretation; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2009; pp. 117–141. [Google Scholar]
- Xi, G.; Keep, R.F.; Hoff, J.T. Pathophysiology of brain edema formation. Neurosurg. Clin. N. Am. 2002, 13, 371–383. [Google Scholar] [CrossRef]
- Vik, A.; Nag, T.; Fredriksli, O.A.; Skandsen, T.; Moen, K.G.; Schirmer-Mikalsen, K.; Manley, G.T. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J. Neurosurg. 2008, 109, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, E.B.; Emond, M.; Lauzier, F.; Savard, M.; Turgeon, A.F. Hyperosmolar Therapy in Severe Traumatic Brain Injury: A Survey of Emergency Physicians from a Large Canadian Province. PLoS ONE 2014, 9, e95778. [Google Scholar] [CrossRef]
- Burgess, S.; Abu-Laban, R.; Slavik, R.S.; Vu, E.N.; Zed, P.J. A Systematic Review of Randomized Controlled Trials Comparing Hypertonic Sodium Solutions and Mannitol for Traumatic Brain Injury: Implications for Emergency Department Management. Ann. Pharmacother. 2016, 50, 291–300. [Google Scholar] [CrossRef]
- De Lima-Oliveira, M.; Salinet, A.S.; Nogueira, R.; De Azevedo, D.; Paiva, W.S.; Teixeira, M.J.; Bor-Seng-Shu, E. Intracranial Hypertension and Cerebral Autoregulation: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 113, 110–124. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.J.; Bell, M.J. Guidelines for the Management of Severe Traumatic Brain Injury, 4th ed.; Brain Trauma Foundation: New York, NY, USA, September 2016; Available online: Braintrauma.org (accessed on 22 March 2020).
- Alnemari, A.M.; Krafcik, B.M.; Mansour, T.R.; Gaudin, D. A Comparison of Pharmacologic Therapeutic Agents Used for the Reduction of Intracranial Pressure After Traumatic Brain Injury. World Neurosurg. 2017, 106, 509–528. [Google Scholar] [CrossRef]
- Alotaibi, N.; Wang, J.Z.; Pasarikovski, C.R.; Guha, D.; Al-Mufti, F.; Mamdani, M.; Saposnik, G.; Schweizer, T.A.; Macdonald, R.L. Management of raised intracranial pressure in aneurysmal subarachnoid hemorrhage: Time for a consensus? Neurosurg. Focus 2017, 43, E13. [Google Scholar] [CrossRef]
- Mortazavi, M.M.; Romeo, A.K.; Deep, A.; Griessenauer, C.J.; Shoja, M.M.; Tubbs, R.S.; Fisher, W. Hypertonic saline for treating raised intracranial pressure: Literature review with meta-analysis. J. Neurosurg. 2012, 116, 210–221. [Google Scholar] [CrossRef]
- Grande, P.-O.; Romner, B. Osmotherapy in Brain Edema: A questionable therapy. J. Neurosurg. Anesthesiol. 2012, 24, 407–412. [Google Scholar] [CrossRef]
- Jarvela, K.; Rantanen, M.; Kööbi, T.; Huhtala, H.; Sisto, T. Hypertonic saline-hydroxyethyl starch solution attenuates fluid accumulation in cardiac surgery patients: A randomized controlled double-blind trial. Anaesthesiol. Intensive Ther. 2018, 50, 122–127. [Google Scholar] [CrossRef] [PubMed]
- White, H.; Cook, D.; Venkatesh, B. The Use of Hypertonic Saline for Treating Intracranial Hypertension After Traumatic Brain Injury. Anesth. Analg. 2006, 102, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Picetti, E.; Zoerle, T.; Carbonara, M.; Zanier, E.R.; Stocchetti, N. Fluid Management in Acute Brain Injury. Curr. Neurol. Neurosci. Rep. 2018, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Diringer, M. The Evolution of the Clinical Use of Osmotic Therapy in the Treatment of Cerebral Edema. Acta Neurochir. Suppl. 2016, 121, 3–6. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, X.; Ying, M.; Chang, H.-T.; Zhang, W. Association between serum osmolarity and mortality in patients who are critically ill: A retrospective cohort study. BMJ Open 2017, 7, 015729. [Google Scholar] [CrossRef]
- White, H.; Cook, D.; Venkatesh, B. The role of hypertonic saline in neurotrauma. Eur. J. Anaesthesiol. 2008, 25, 104–109. [Google Scholar] [CrossRef]
- Gu, J.; Huang, H.; Huang, Y.; Sun, H.; Xu, H. Hypertonic saline or mannitol for treating elevated intracranial pressure in traumatic brain injury: A meta-analysis of randomized controlled trials. Neurosurg. Rev. 2018, 42, 499–509. [Google Scholar] [CrossRef]
- Asehnoune, K.; Lasocki, S.; Seguin, P.; Geeraerts, T.; Perrigault, P.F.; Dahyot-Fizelier, C.; Burtz, C.P.; Cook, F.; latte, D.D.; Cinotti, R.; et al. Association between continuous hyperosmolar therapy and survival in patients with traumatic brain injury—A multicentre prospective cohort study and systematic review. Crit. Care 2017, 21, 328. [Google Scholar] [CrossRef]
- Boone, M.D.; Oren-Grinberg, A.; Robinson, T.M.; Chen, C.C.; Kasper, E.M. Mannitol or hypertonic saline in the setting of traumatic brain injury: What have we learned? Surg. Neurol. Int. 2015, 6, 177. [Google Scholar] [CrossRef]
- Ziai, W.C.; Toung, T.J.; Bhardwaj, A. Hypertonic saline: First-line therapy for cerebral edema? J. Neurol. Sci. 2007, 261, 157–166. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Kolias, A.G.; Timofeev, I.S.; Corteen, E.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.O.; Belli, A.; Eynon, C.A.; et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N. Engl. J. Med. 2016, 375, 1119–1130. [Google Scholar] [CrossRef]
- Meiburger, K.M.; Naldi, A.; Michielli, N.; Coppo, L.; Fassbender, K.; Molinari, F.; Lochner, P. Automatic Optic Nerve Measurement: A New Tool to Standardize Optic Nerve Assessment in Ultrasound B-Mode Images. Ultrasound Med. Biol. 2020, 46, 1533–1544. [Google Scholar] [CrossRef]
| Variable | Unit | G1 (n = 27) | G2 (n = 33) | G3 (n = 13) | ‘p’ |
|---|---|---|---|---|---|
| Sex | Males | 14 (52%) | 16 (48%) | 6 (46%) | 0.9 |
| Females | 13 (48%) | 17 (52%) | 7 (54%) | ||
| Age | (years) | 52 (35–66) | 65 (51.5–70) | 50 (42–57) | 0.4 |
| Duration of the therapy | (days) | 5 (3–14) | 6 (2–12) | 3 (2.75–4.25) | 0.06 |
| Total dose of mannitol | (grams) | 30 (22.8–42.4) | 20 (13.8–30.9) | N/A | – |
| APACHE II score | (points) | 17 (15.0–23.0) | 19.5 (15.5–26) | 16 (12–18.5) | 0.03 |
| Mortality | Death | 21 (78%) | 22 (67%) | 6 (46%) | 0.1 |
| Survival | 6 (22%) | 11 (33%) | 7 (54%) |
| Variable | G1 (n = 27) | G2 (n = 33) | G3 (n = 13) | ‘p’ |
|---|---|---|---|---|
| NaCv | 2.86 (2.35–4.61) | 3.31 (2.44–4.59) | 3.88 (2.32–6.32) | 0.7 |
| NaMean | 140.9 (137.3–148.1) | 142.6 (138.6–146.9) | 147.8 (144.1–151.6) | 0.03 |
| ClCv | 4.35 (3.15–6.21) | 4.37 (3.72–6.45) | 4.45 (3.56–7.61) | 0.7 |
| ClMean | 109 (105–118) | 110 (105–116) | 115 (112–120) | 0.09 |
| mOsmCv | 3.29 (2.32–5.22) | 3.39 (2.32–4.47) | 3.89 (2.27–6.28) | 0.8 |
| mOsmMean | 293.6 (282.0–306.7) | 292.8 (286.0–301.4) | 302.8 (295.4–311.2) | 0.07 |
| SIDCv | 10.93 (8.33–13.99) | 10.25 (7.68–12.58) | 10.19 (8.83–14.39) | 0.7 |
| SIDMean | 32.72 (30.82–34.24) | 34.08 (33.55–35.68) | 33.95 (32.14–35.30) | 0.06 |
| Variable | G1 (n = 27) | G2 (n = 33) | G3 (n = 13) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Survival n = 6 | Death n = 21 | ‘p’ | Survival n = 11 | Death n = 22 | ‘p’ | Survival n = 7 | Death n = 6 | ‘p’ | |
| NaCv | 2.07 (1.51–2.35) | 3.74 (2.76–5.32) | 0.002 | 3.90 (2.43–5.04) | 3.30 (2.71–3.84) | 0.6 | 2.74 (2.07–3.72) | 6.40 (5.91–6.67) | 0.03 |
| NaMean | 137.8 (136.5–139.1) | 142.8 (138.3–149.4) | 0.1 | 139.4 (136.7–142.2) | 143.0 (140.2–150.1) | 0.06 | 144.4 (142.3–147.2) | 151.9 (150.0–152.9) | 0.004 |
| ClCv | 3.09 (1.63–3.57) | 5.65 (3.30–6.88) | 0.02 | 4.11 (3.74–6.91) | 4.45 (3.70–5.99) | 0.9 | 3.80 (3.49–4.43) | 8.40 (5.38–10.25) | 0.04 |
| ClMean | 108 (103–109) | 113 (106–122) | 0.1 | 108 (104–111) | 112 (106–117) | 0.1 | 113 (112–115) | 120 (118–121) | 0.04 |
| mOsmCv | 2.05 (2.03–2.22) | 4.23 (3.03–5.55) | 0.001 | 4.04 (2.38–4.89) | 3.39 (2.28–4.03) | 0.6 | 2.40 (2.02–3.72) | 6.29 (5.40–6.94) | 0.02 |
| mOsmMean | 282.3 (279.6–286.4) | 295.0 (284.4–309.0) | 0.06 | 286.4 (279.4–291.7) | 295.7 (288.6–308.7) | 0.03 | 295.7 (290.3–301.4) | 311.3 (309.8–314.0) | 0.003 |
| SIDCv | 7.69 (7.47–9.90) | 11.20 (9.72–15.4) | 0.01 | 10.87 (9.69–12.60) | 9.34 (7.13–12.45) | 0.2 | 10.19 (8.38–12.75) | 12.47 (9.28–15.59) | 0.3 |
| SIDMean | 34.88 (32.36–37.79) | 32.18 (30.60–34.03) | 0.1 | 34.08 (33.68–34.29) | 34.16 (33.27–35.93) | 0.7 | 33.95 (31.94–35.71) | 34.36 (32.34–35.03) | 1.0 |
| Variable | G1 (n = 27) | G2 (n = 33) | G3 (n = 13) | |||
|---|---|---|---|---|---|---|
| AUC (95% CI) | ‘p’ | AUC (95% CI) | ‘p’ | AUC (95% CI) | ‘p’ | |
| NaCv | 0.929 (0.761–0.992) | <0.0001 | 0.554 (0.371–0.726) | 0.6 | 0.857 (0.558–0.984) | 0.01 |
| NaMean | 0.722 (0.518–0.876) | 0.03 | 0.705 (0.521–0.850) | 0.03 | 0.976 (0.715–1.000) | <0.001 |
| ClCv | 0.817 (0.622–0.939) | 0.0005 | 0.514 (0.335–0.691) | 0.9 | 0.833 (0.531–0.976) | 0.04 |
| ClMean | 0.718 (0.513–0.873) | 0.04 | 0.661 (0.476–0.816) | 0.1 | 0.845 (0.544–0.980) | 0.03 |
| mOsmCv | 0.937 (0.772–0.994) | p < 0.0001 | 0.562 (0.379–0.733) | 0.6 | 0.881 (0.587–0.991) | 0.002 |
| mOsmMean | 0.754 (0.551–0.898) | 0.01 | 0.738 (0.556–0.875) | 0.008 | 1.000 (0.753–1.000) | <0.001 |
| SIDCv | 0.849 (0.660–0.957) | <0.0001 | 0.630 (0.445–0.791) | 0.2 | 0.667 (0.362–0.894) | 0.3 |
| SIDMean | 0.722 (0.518–0.876) | 0.09 | 0.539 (0.358–0.713) | 0.7 | 0.500 (0.221–0.779) | 1.0 |
| Variable | Mortality Prediction |
|---|---|
| Sex (Female = 0/Male = 1) | OR = 1.74; 95% CI; 0.47 to 6.43 p = 0.4 |
| Age (per 1 year) | OR = 1.01; 95% CI 0.97–1.06; p = 0.6 |
| ICU length of stay (per 1 day) | OR = 0.98; 95% CI 0.82–1.17; p = 0.8 |
| Duration of osmotherapy (per 1 day) | OR = 0.98; 95% CI 0.83–1.16; p = 0.8 |
| NaCv (per 1%) | OR = 0.09; 95% CI 0.01–0.61; p = 0.01 |
| NaMean (per 1 mmol/L) | OR = 0.22; 95% CI 0.07–0.66; p = 0.007 |
| mOsmCv (per 1%) | OR = 8.22; 95% CI 1.51–44.81; p = 0.01 |
| mOsmMean (per 1 mmol/L) | OR = 2.40; 95% CI 1.34–4.29; p = 0.003 |
| ClCv (per 1%) | OR = 1.42; 95% CI 0.83–2.42 p = 0.2 |
| ClMean (per 1 mmol/L) | OR = 0.85; 95% CI 0.63–1.14 p = 0.3 |
| AUC for the final logistic model | 0.886; 95% CI 0.790–0.949; p = 0.0002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiórek, A.; Jaworski, T.; Krzych, Ł.J. Hyperosmolar Treatment for Patients at Risk for Increased Intracranial Pressure: A Single-Center Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 4573. https://doi.org/10.3390/ijerph17124573
Wiórek A, Jaworski T, Krzych ŁJ. Hyperosmolar Treatment for Patients at Risk for Increased Intracranial Pressure: A Single-Center Cohort Study. International Journal of Environmental Research and Public Health. 2020; 17(12):4573. https://doi.org/10.3390/ijerph17124573
Chicago/Turabian StyleWiórek, Agnieszka, Tomasz Jaworski, and Łukasz J. Krzych. 2020. "Hyperosmolar Treatment for Patients at Risk for Increased Intracranial Pressure: A Single-Center Cohort Study" International Journal of Environmental Research and Public Health 17, no. 12: 4573. https://doi.org/10.3390/ijerph17124573
APA StyleWiórek, A., Jaworski, T., & Krzych, Ł. J. (2020). Hyperosmolar Treatment for Patients at Risk for Increased Intracranial Pressure: A Single-Center Cohort Study. International Journal of Environmental Research and Public Health, 17(12), 4573. https://doi.org/10.3390/ijerph17124573