Ocean Acidification and Human Health
Abstract
1. Introduction
2. Ocean Acidification
3. The Anticipated Influences of Ocean Acidification on Human Health
3.1. Pathway 1—Malnutrition and Poisoning Via Altered Food Quantity and Quality
3.1.1. Quantity and Nutritional Composition of Seafood
3.1.2. Chemical Contamination (Pollutants)
3.1.3. Redistribution and Accumulation of Natural Toxins
3.2. Pathway 2—Respiratory Issues Via Impaired Air Quality
3.3. Pathway 3—Mental Health Impacts Via the Modification of Natural Spaces
3.4. Pathway 4—Decreased Opportunity to Develop and Obtain Medical Resources Via the Loss of Biodiversity
3.5. Interconnection of Pathways to Impacts
4. Strategies to Enhance Benefits to, and Limit Negative Effects on, Human Health under Ocean Acidification
4.1. Biodiversity Conservation and Restoration
4.2. Monitoring and Managing Water Quality at a Local Scale
4.3. Adapting Human Activities
4.4. The Socioeconomic Context of Affected Populations and Individuals—Environmental, Health, Economic, and Social Inequalities
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gascon, M.; Zijlema, W.; Vert, C.; White, M.P.; Nieuwenhuijsen, M.J. Outdoor blue spaces, human health and well-being: A systematic review of quantitative studies. Int. J. Hyg. Environ. Health 2017, 220, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Fleming, L.E.; Maycock, B.; White, M.P.; Depledge, M.H. Fostering human health through ocean sustainability in the 21st century. People Nat. 2019, 1, 276–283. [Google Scholar] [CrossRef]
- Fleming, L.; White, M.; Grellier, J.; Garrett, J.; Elliott, L.; Pahl, S.; Hattam, C.; Wuijts, S.; Friedericks, L.; Braubach, M. Case study: The seas, ocean and coasts as a public health resource. In WHO Policy Brief SDG 14: Health, The Global Ocean and Marine Resources; Matthies, F., Fleming, L., Eds.; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Depledge, M.H.; White, M.P.; Maycock, B.; Fleming, L.E. Time and tide. BMJ 2019, 366. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; De Cicco, P.; Ianaro, A. New drugs from the sea: Pro-apoptotic activity of sponges and algae derived compounds. Mar. Drugs 2019, 17, 31. [Google Scholar] [CrossRef]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. UK 2016, 96, 61–91. [Google Scholar] [CrossRef]
- Cicin-Sain, B.; Bernal, P.; Vandeweerd, V.; Belfiore, S.; Goldstein, K. A guide to oceans, coasts, and islands at the world summit on sustainable development; CSMP; UNESCO; GPA: Newark, DE, USA, 2002. [Google Scholar]
- Leonard, A.F.C.; Zhang, L.; Balfour, A.J.; Garside, R.; Hawkey, P.M.; Murray, A.K.; Ukoumunne, O.C.; Gaze, W.H. Exposure to and colonisation by antibiotic-resistant E. coli in UK coastal water users: Environmental surveillance, exposure assessment, and epidemiological study (Beach Bum Survey). Environ. Int. 2018, 114, 326–333. [Google Scholar] [CrossRef]
- European Marine Board. Linking oceans and human health: A strategic research priority for Europe. Position paper 19; European Marine Board: Ostend, Belgium, 2013. [Google Scholar]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis. Intergovernmental Panel on Climate Change, Working Group I Contribution to the IPCC Fifth Assessment Report (AR5); Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- IPCC. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., et al., Eds.; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef]
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V.J.; Millero, F.J. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 2004, 305, 362–366. [Google Scholar] [CrossRef]
- Le Quéré, C.; Moriarty, R.; Andrew, R.M.; Canadell, J.G.; Sitch, S.; Korsbakken, J.I.; Friedlingstein, P.; Peters, G.P.; Andres, R.J.; Boden, T.A.; et al. Global carbon budget 2015. Earth Syst. Sci. Data 2015, 7, 349–396. [Google Scholar] [CrossRef]
- Falkenberg, L.J.; Dupont, S.; Bellerby, R.G.J. Approaches to reconsider literature on physiological effects of environmental change: Examples from ocean acidification research. Front. Mar. Sci. 2018, 5, 453. [Google Scholar] [CrossRef]
- Wittmann, A.C.; Pörtner, H.O. Sensitivities of extant animal taxa to ocean acidification. Nat. Clim. Chang. 2013, 3, 995–1001. [Google Scholar] [CrossRef]
- Connell, S.D.; Kroeker, K.J.; Fabricius, K.E.; Kline, D.I.; Russell, B.D. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Philos. Trans. R. Soc. B: Biol. Sci. 2013, 368. [Google Scholar] [CrossRef]
- Falkenberg, L.J.; Russell, B.D.; Connell, S.D. Contrasting resource limitations of marine primary producers: Implications for competitive interactions under enriched CO2 and nutrient regimes. Oecologia 2013, 172, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.; Waldbusser, G.G.; Feely, R.A.; Weisberg, S.B.; Newton, J.A.; Hales, B.; Cudd, S.; Eudeline, B.; Langdon, C.J.; Jefferds, I.; et al. Impacts of coastal acidification on the Pacific Northwest shellfish industry and adaptation strategies implemented in response. Oceanography 2015, 28, 146–159. [Google Scholar] [CrossRef]
- Wernberg, T.; de Bettignies, T.; Joy, B.A.; Finnegan, P.M. Physiological responses of habitat-forming seaweeds to increasing temperatures. Limnol. Oceanogr. 2016, 61, 2180–2190. [Google Scholar] [CrossRef]
- Falkenberg, L.J.; Russell, B.D.; Connell, S.D. Future herbivory: The indirect effects of enriched CO2 may rival its direct effects. Mar. Ecol. Prog. Ser. 2013, 492, 85–95. [Google Scholar] [CrossRef][Green Version]
- Poore, A.G.B.; Graba-Landry, A.; Favret, M.; Sheppard Brennand, H.; Byrne, M.; Dworjanyn, S.A. Direct and indirect effects of ocean acidification and warming on a marine plant–herbivore interaction. Oecologia 2013, 173, 1113–1124. [Google Scholar] [CrossRef]
- Bibby, R.; Cleall-Harding, P.; Rundle, S.; Widdicombe, S.; Spicer, J. Ocean acidification disrupts induced defences in the intertidal gastropod Littorina littorea. Biol. Lett. 2007, 3, 699–701. [Google Scholar] [CrossRef]
- Doubleday, Z.A.; Nagelkerken, I.; Coutts, M.D.; Goldenberg, S.U.; Connell, S.D. A triple trophic boost: How carbon emissions indirectly change a marine food chain. Glob. Chang. Biol. 2019, 25, 978–984. [Google Scholar] [CrossRef]
- Leung, J.Y.S.; Doubleday, Z.A.; Nagelkerken, I.; Chen, Y.; Xie, Z.; Connell, S.D. How calorie-rich food could help marine calcifiers in a CO2-rich future. Proc. R. Soc. B: Biol. Sci. 2019, 286. [Google Scholar] [CrossRef] [PubMed]
- Tate, R.D.; Benkendorff, K.; Ab Lah, R.; Kelaher, B.P. Ocean acidification and warming impacts the nutritional properties of the predatory whelk, Dicathais orbita. J. Exp. Mar. Biol. Ecol. 2017, 493, 7–13. [Google Scholar] [CrossRef]
- Jin, P.; Wang, T.; Liu, N.; Dupont, S.; Beardall, J.; Boyd, P.W.; Riebesell, U.; Gao, K. Ocean acidification increases the accumulation of toxic phenolic compounds across trophic levels. Nat. Commun. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cinner, J.E.; Barnes, M.L. Social dimensions of resilience in social-ecological systems. One Earth 2019, 1, 51–56. [Google Scholar] [CrossRef]
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.I.; Williams, M. Feeding 9 billion by 2050 – putting fish back on the menu. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef]
- Haigh, R.; Ianson, D.; Holt, C.A.; Neate, H.E.; Edwards, A.M. Effects of ocean acidification on temperate coastal marine ecosystems and fisheries in the northeast pacific. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Billé, R.; Kelly, R.; Biastoch, A.; Harrould-Kolieb, E.; Herr, D.; Joos, F.; Kroeker, K.; Laffoley, D.; Oschlies, A.; Gattuso, J.P. Taking action against ocean acidification: A review of management and policy options. Environ. Manag. 2013, 52, 761–779. [Google Scholar] [CrossRef]
- Richards, R.G.; Davidson, A.T.; Meynecke, J.O.; Beattie, K.; Hernaman, V.; Lynam, T.; van Putten, I.E. Effects and mitigations of ocean acidification on wild and aquaculture scallop and prawn fisheries in Queensland, Australia. Fish. Res. 2015, 161, 42–56. [Google Scholar] [CrossRef]
- Rossoll, D.; Bermúdez, R.; Hauss, H.; Schulz, K.G.; Riebesell, U.; Sommer, U.; Winder, M. Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS ONE 2012, 7, e34737. [Google Scholar] [CrossRef]
- Paz, S.; Rubio, C.; Frías, I.; Gutiérrez, A.J.; González-Weller, D.; Revert, C.; Hardisson, A. Metal concentrations in wild-harvested phaeophyta seaweed from the Atlantic Ocean (Canary Islands, Spain). J. Food Prot. 2018, 81, 1165–1170. [Google Scholar] [CrossRef]
- Alava, J.J.; Cheung, W.W.L.; Ross, P.S.; Sumaila, U.R. Climate change-contaminant interactions in marine food webs: Toward a conceptual framework. Glob. Chang. Biol. 2017, 23, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.A.; Birchenough, S.N.R.; Lewis, C.; Sanders, M.B.; Bolam, T.; Sheahan, D. Ocean acidification increases the toxicity of contaminated sediments. Glob. Chang. Biol. 2013, 19, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Errera, R.M.; Yvon-Lewis, S.; Kessler, J.D.; Campbell, L. Reponses of the dinoflagellate Karenia brevis to climate change: pCO2 and sea surface temperatures. Harmful Algae 2014, 37, 110–116. [Google Scholar] [CrossRef]
- Pang, M.; Xu, J.; Qu, P.; Mao, X.; Wu, Z.; Xin, M.; Sun, P.; Wang, Z.; Zhang, X.; Chen, H. Effect of CO2 on growth and toxicity of Alexandrium tamarense from the East China Sea, a major producer of paralytic shellfish toxins. Harmful Algae 2017, 68, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Walsh, C.J.; Nierenberg, K.; Clark, J.; Reich, A.; Hollenbeck, J.; Benson, J.; Cheng, Y.S.; et al. Review of Florida red tide and human health effects. Harmful Algae 2011, 10, 224–233. [Google Scholar] [CrossRef]
- United Nations Division for Sustainable Development Goals Department of Economic and Social Affairs. Meeting of the Communities of Ocean Action From Commitments to Action: Implementing SDG14. Incheon, Korea, 2019. [Google Scholar]
- Mayer, F.S.; Frantz, C.M.; Bruehlman-Senecal, E.; Dolliver, K. Why is nature beneficial? The role of connectedness to nature. Environ. Behav. 2009, 41, 607–643. [Google Scholar] [CrossRef]
- White, M.P.; Pahl, S.; Wheeler, B.W.; Fleming, L.E.F.; Depledge, M.H. The “Blue Gym”: What can blue space do for you and what can you do for blue space? J. Mar. Biol. Assoc. UK 2016, 96, 5–12. [Google Scholar] [CrossRef]
- Wyles, K.; White, M.P.; Hattam, C.; Pahl, S.; Austin, M. Nature connectedness and well-being from recent nature visits: The role of environment type and quality. Environ. Behav. 2019, 5, 111–143. [Google Scholar] [CrossRef]
- Connell, S.D.; Doubleday, Z.A.; Foster, N.R.; Hamlyn, S.B.; Harley, C.D.G.; Helmuth, B.; Kelaher, B.P.; Nagelkerken, I.; Rodgers, K.L.; Sarà, G.; et al. The duality of ocean acidification as a resource and a stressor. Ecology 2018, 99, 1005–1010. [Google Scholar] [CrossRef]
- Encarnação, J.; Morais, P.; Baptista, V.; Cruz, J.; Teodósio, M. New evidence of marine fauna tropicalization off the southwestern Iberian Peninsula (southwest Europe). Diversity 2019, 11, 48. [Google Scholar] [CrossRef]
- Kim, J.; Kaplan, R. Physical and psychological factors in sense of community. Environ. Behav. 2004, 36, 313–340. [Google Scholar] [CrossRef]
- De Vries, S.; van Dillen, S.M.E.; Groenewegen, P.P.; Spreeuwenberg, P. Streetscape greenery and health: Stress, social cohesion and physical activity as mediators. Soc. Sci. Med. 2013, 94, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kleypas, J.A.; Yates, K.K. Coral reefs and ocean acidification. Oceanography 2009, 22, 108–117. [Google Scholar] [CrossRef]
- H2020 SOPHIE Consortium. A Strategic Research Agenda for Oceans and Human Health in Europe. H2020 SOPHIE Project; H2020 SOPHIE Consortium: Ostend, Belgium, 2020. [Google Scholar]
- Farmer, T.; Grainger, R.; Plummer, J. The State of World Fisheries and Aquaculture. Opportunities and Challenges; FAO: Rome, Italy, 2014. [Google Scholar]
- Huelsenbeck, M. Ocean-Based Food Security Threatened in a High Ocean, CO2 World: A Ranking of Nations’ Vulnerability to Climate Change and Ocean Acidification; Oceana: Washington, DC, USA, 2012. [Google Scholar]
- United Nations Children’s Fund (UNICEF); World Health Organization; International Bank for Reconstruction and Development/The World Bank. Levels and Trends in Child Malnutrition: Key Findings of the 2019 Edition of the Joint Child Malnutrition Estimates; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization Children: Reducing mortality. Available online: https://www.who.int/news-room/fact-sheets/detail/children-reducing-mortality (accessed on 2 March 2020).
- De Orte, M.R.; Sarmiento, A.M.; Basallote, M.D.; Rodríguez-Romero, A.; Riba, I.; delValls, A. Effects on the mobility of metals from acidification caused by possible CO2 leakage from sub-seabed geological formations. Sci. Total Environ. 2014, 470–471, 356–363. [Google Scholar] [CrossRef]
- Stiasny, M.H.; Mittermayer, F.H.; Sswat, M.; Voss, R.; Jutfelt, F.; Chierici, M.; Puvanendran, V.; Mortensen, A.; Reusch, T.B.H.; Clemmesen, C. Ocean acidification effects on Atlantic cod larval survival and recruitment to the fished population. PLoS ONE 2016, 11, e0155448. [Google Scholar] [CrossRef]
- Munday, P.L.; Donelson, J.M.; Dixson, D.L.; Endo, G.G.K. Effects of ocean acidification on the early life history of a tropical marine fish. Proc. R. Soc. B: Biol. Sci. 2009, 276, 3275–3283. [Google Scholar] [CrossRef]
- Munday, P.L.; Watson, S.-A.; Parsons, D.M.; King, A.; Barr, N.G.; Mcleod, I.M.; Allan, B.J.M.; Pether, S.M.J. Effects of elevated CO2 on early life history development of the yellowtail kingfish, Seriola lalandi, a large pelagic fish. Ices J. Mar. Sci. 2016, 73, 641–649. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J.-P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.N.; Singh, G.G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 2010, 13, 1419–1434. [Google Scholar] [CrossRef]
- Welch, C. Willapa Bay oyster grower sounds alarm, starts hatchery in Hawaii. Seattle Times 2012. [Google Scholar]
- Ellis, R.P.; Urbina, M.A.; Wilson, R.W. Lessons from two high CO2 worlds—Future oceans and intensive aquaculture. Glob. Chang. Biol. 2017, 23, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Hamilton, T.J.; Eom, J.; Lyall, E.M.; Gallup, J.; Jiang, A.; Lee, J.; Close, D.A.; Yun, S.S.; Brauner, C.J. Responses of pink salmon to CO2-induced aquatic acidification. Nat. Clim. Chang. 2015, 5, 950–957. [Google Scholar] [CrossRef]
- Mota, V.C.; Nilsen, T.O.; Gerwins, J.; Gallo, M.; Ytteborg, E.; Baeverfjord, G.; Kolarevic, J.; Summerfelt, S.T.; Terjesen, B.F. The effects of carbon dioxide on growth performance, welfare, and health of Atlantic salmon post-smolt (Salmo salar) in recirculating aquaculture systems. Aquaculture 2019, 498, 578–586. [Google Scholar] [CrossRef]
- Sunday, J.M.; Fabricius, K.E.; Kroeker, K.J.; Anderson, K.M.; Brown, N.E.; Barry, J.P.; Connell, S.D.; Dupont, S.; Gaylord, B.; Hall-Spencer, J.M.; et al. Ocean acidification can mediate biodiversity shifts by changing biogenic habitat. Nat. Clim. Chang. 2017, 7, 81–85. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Russell, B.D.; Gillanders, B.M.; Connell, S.D. Ocean acidification alters fish populations indirectly through habitat modification. Nat. Clim. Chang. 2016, 6, 89–93. [Google Scholar] [CrossRef]
- Dutkiewicz, S.; Morris, J.J.; Follows, M.J.; Scott, J.; Levitan, O.; Dyhrman, S.T.; Berman-Frank, I. Impact of ocean acidification on the structure of future phytoplankton communities. Nat. Clim. Chang. 2015, 5, 1002–1006. [Google Scholar] [CrossRef]
- Bermúdez, J.R.; Riebesell, U.; Larsen, A.; Winder, M. Ocean acidification reduces transfer of essential biomolecules in a natural plankton community. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Bermúdez, R.; Winder, M.; Stuhr, A.; Almén, A.-K.; Engström-Öst, J.; Riebesell, U. Effect of ocean acidification on the structure and fatty acid composition of a natural plankton community in the Baltic Sea. Biogeosciences 2016, 13, 6625–6635. [Google Scholar] [CrossRef]
- Wang, T.; Tong, S.; Liu, N.; Li, F.; Wells, M.L.; Gao, K. The fatty acid content of plankton is changing in subtropical coastal waters as a result of OA: Results from a mesocosm study. Mar. Environ. Res. 2017, 132, 51–62. [Google Scholar] [CrossRef]
- Taucher, J.; Haunost, M.; Boxhammer, T.; Bach, L.T.; Algueró-Muñiz, M.; Riebesell, U. Influence of ocean acidification on plankton community structure during a winter-to-summer succession: An imaging approach indicates that copepods can benefit from elevated CO2 via indirect food web effects. PLoS ONE 2017, 12. [Google Scholar] [CrossRef][Green Version]
- Gattuso, J.P.; Magnan, A.K.; Bopp, L.; Cheung, W.W.L.; Duarte, C.M.; Hinkel, J.; Mcleod, E.; Micheli, F.; Oschlies, A.; Williamson, P.; et al. Ocean solutions to address climate change and its effects on marine ecosystems. Front. Mar. Sci. 2018, 5, 337. [Google Scholar] [CrossRef]
- Cooley, S.R.; Lucey, N.; Kite-Powell, H.; Doney, S.C. Nutrition and income from molluscs today imply vulnerability to ocean acidification tomorrow. Fish Fish. 2012, 13, 182–215. [Google Scholar] [CrossRef]
- Speers, A.E.; Besedin, E.Y.; Palardy, J.E.; Moore, C. Impacts of climate change and ocean acidification on coral reef fisheries: An integrated ecological-economic model. Ecol. Econ. 2016, 128, 33–43. [Google Scholar] [CrossRef]
- Ab Lah, R.; Kelaher, B.P.; Bucher, D.; Benkendorff, K. Ocean warming and acidification affect the nutritional quality of the commercially-harvested turbinid snail Turbo militaris. Mar. Environ. Res. 2018, 141, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Maki, K.C.; Palacios, O.M.; Bell, M.; Toth, P.P. Use of supplemental long-chain omega-3 fatty acids and risk for cardiac death: An updated meta-analysis and review of research gaps. J. Clin. Lipidol. 2017, 11, 1152–1160.e2. [Google Scholar] [CrossRef]
- Bowen, K.J.; Harris, W.S.; Kris-Etherton, P.M. Omega-3 fatty acids and cardiovascular disease: Are there benefits? Curr. Treat. Options Cardiovasc. Med. 2016, 18, 1–16. [Google Scholar] [CrossRef]
- Kainz, M.; Arts, M.T.; Mazumder, A. Essential fatty acids in the planktonic food web and their ecological role for higher trophic levels. Limnol. Oceanogr. 2004, 49, 1784–1793. [Google Scholar] [CrossRef]
- Weir, T.; Dovey, L.; Orcherton, D. Social and cultural issues raised by climate change in Pacific Island countries: An overview. Reg. Environ. Chang. 2017, 17, 1017–1028. [Google Scholar] [CrossRef]
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate change and global food systems: Potential impacts on food security and undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, L.; Comte, A.; Langdon, C.; Ekstrom, J.A.; Cooley, S.R.; Suatoni, L.; Beck, M.W.; Brander, L.M.; Burke, L.; Cinner, J.E.; et al. Coral reefs and people in a high-CO2 world: Where can science make a difference to people? PLoS ONE 2016, 11, e0164699. [Google Scholar] [CrossRef] [PubMed]
- Mathis, J.T.; Cooley, S.R.; Lucey, N.; Colt, S.; Ekstrom, J.; Hurst, T.; Hauri, C.; Evans, W.; Cross, J.N.; Feely, R.A. Ocean acidification risk assessment for Alaska’s fishery sector. Prog. Oceanogr. 2015, 136, 71–91. [Google Scholar] [CrossRef]
- Chan, A.; Hon, K.; Leung, T.; Ho, M.; Rosa Duque, J.; Lee, T. The effects of global warming on allergic diseases. Hong Kong Med J. 2018, 24, 277–284. [Google Scholar] [CrossRef]
- McDonald, M.D.; Riemer, D.D. The fate of pharmaceuticals and personal care products in the environment. In Oceans and human health: Risks and remedies from the seas; Walsh, P.J., Smith, S., Fleming, L., Solo-Gabriele, H., Gerwick, W.H., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 161–179. [Google Scholar]
- Gaw, S.; Thomas, K.V.; Hutchinson, T.H. Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Philos. Trans. R. Soc. B: Biol. Sci. 2014, 369. [Google Scholar] [CrossRef]
- Jewett, S.C.; Duffy, L.K. Mercury in fishes of Alaska, with emphasis on subsistence species. Sci. Total Environ. 2007, 387, 3–27. [Google Scholar] [CrossRef]
- Castro-González, M.I.; Méndez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharm. 2008, 26, 263–271. [Google Scholar] [CrossRef]
- Hosomi, R.; Yoshida, M.; Fukunaga, K. Seafood consumption and components for health. Global J. Health Sci. 2012, 4, 72–86. [Google Scholar] [CrossRef]
- Tsygankov, V.Y.; Lukyanova, O.N.; Boyarova, M.D. Organochlorine pesticide accumulation in seabirds and marine mammals from the Northwest Pacific. Mar. Pollut. Bull. 2018, 128, 208–213. [Google Scholar] [CrossRef]
- Vetter, W.; Luckas, B.; Heidemann, G.; Skírnisson, K. Organochlorine residues in marine mammals from the northern hemisphere—A consideration of the composition of organochlorine residues in the blubber of marine mammals. Proc. Sci. Total Environ. 1996, 186, 29–39. [Google Scholar] [CrossRef]
- Beard, J. DDT and human health. Sci. Total Environ. 2006, 355, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, M.P.; Rogan, W.J.; Lucier, G. The human health effects of DDT (dichlorodiphenyltrichloroethane and PCBs (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu. Rev. Public Health 1997, 18, 211–244. [Google Scholar] [CrossRef]
- Celo, V.; Lean, D.R.S.; Scott, S.L. Abiotic methylation of mercury in the aquatic environment. Sci. Total Environ. 2006, 368, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhao, X.; Han, Y.; Che, Z.; Chai, X.; Liu, G. Ocean acidification increases cadmium accumulation in marine bivalves: A potential threat to seafood safety. Sci. Rep. 2016, 6, 20197. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, M.; Lucotte, M.; Garceau, S.; Laliberté, D. Fish growth rates modulate mercury concentrations in walleye (Sander vitreus) from eastern Canadian lakes. Environ. Res. 2005, 98, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Jones, D.; de Rosa, C. Effects of polychlorinated biphenyls on the nervous system. Toxicol. Ind. Health 2000, 16, 305–333. [Google Scholar] [CrossRef]
- Hoagland, P.; Kirkpatrick, B.; Jin, D.; Kirkpatrick, G.; Fleming, L.E.; Ullmann, S.G.; Beet, A.; Hitchcock, G.; Harrison, K.K.; Li, Z.; et al. Lessening the hazards of Florida red tides: A common sense approach. Front. Mar. Sci. 2020, 7, 538. [Google Scholar]
- Tatters, A.O.; Fu, F.-X.; Hutchins, D.A. High CO2 and silicate limitation synergistically increase the toxicity of Pseudo-nitzschia fraudulenta. PLoS ONE 2012, 7, e32116. [Google Scholar] [CrossRef]
- Braga, A.C.; Camacho, C.; Marques, A.; Gago-Martínez, A.; Pacheco, M.; Costa, P.R. Combined effects of warming and acidification on accumulation and elimination dynamics of paralytic shellfish toxins in mussels Mytilus galloprovincialis. Environ. Res. 2018, 164, 647–654. [Google Scholar] [CrossRef]
- Solo-Gabriele, H. Infectious microbes in coastal waters. In Oceans and Human Health: Risks and Remedies from the Seas; Walsh, P.J., Smith, S., Fleming, L., Solo-Gabriele, H., Gerwick, W.H., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 331–357. [Google Scholar]
- Segner, W.P.; Schmidt, C.F.; Boltz, J.K. Minimal growth temperature, sodium chloride tolerance, pH sensitivity, and toxin production of marine and terrestrial strains of Clostridium botulinum Type C. Appl. Environ. Microbiol. 1971, 22. [Google Scholar] [CrossRef]
- Woodcock, A.H. Note concerning human respiratory irritation associated with high concentrations of plankton and mass mortality of marine organisms. J. Mar. Res. 1948, 7, 56–62. [Google Scholar]
- Backer, L.C.; Fleming, L.E.; Rowan, A.; Cheng, Y.S.; Benson, J.; Pierce, R.H.; Zaias, J.; Bean, J.; Bossart, G.D.; Johnson, D.; et al. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae 2003, 2, 19–28. [Google Scholar] [CrossRef]
- Howell, A.J.; Dopko, R.L.; Passmore, H.A.; Buro, K. Nature connectedness: Associations with well-being and mindfulness. Personal. Individ. Differ. 2011, 51, 166–171. [Google Scholar] [CrossRef]
- Wilson, E.O. Biophilia: The Human Bond with Other Species; Harvard Un.: Cambridge, MA, USA, 1984. [Google Scholar]
- Needleman, R.K.; Neylan, I.P.; Erickson, T. Potential environmental and ecological effects of global climate change on venomous terrestrial species in the wilderness. Wilderness Environ. Med. 2018, 29, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Dallimer, M.; Irvine, K.N.; Skinner, A.M.J.; Davies, Z.G.; Rouquette, J.R.; Maltby, L.L.; Warren, P.H.; Armsworth, P.R.; Gaston, K.J. Biodiversity and the feel-good factor: Understanding associations between self-reported human well-being and species richness. BioScience 2012, 62, 47–55. [Google Scholar] [CrossRef]
- White, M.P.; Weeks, A.; Hooper, T.; Bleakley, L.; Cracknell, D.; Lovell, R.; Jefferson, R.L. Marine wildlife as an important component of coastal visits: The role of perceived biodiversity and species behaviour. Mar. Policy 2017, 78, 80–89. [Google Scholar] [CrossRef]
- Cracknell, D.L.; Pahl, S.; White, M.P.; Depledge, M.H. Reviewing the role of aquaria as restorative settings: How subaquatic diversity in public aquaria can influence preferences, and human health and well-being. Hum. Dimens. Wildl. 2018, 23, 446–460. [Google Scholar] [CrossRef]
- Besley, J.C.; Nisbet, M. How scientists view the public, the media and the political process. Public Underst. Sci. 2011, 22, 644–659. [Google Scholar] [CrossRef]
- Nisbet, E.K.; Zelenski, J.M.; Murphy, S.A. The nature relatedness scale: Linking individuals’ connection with nature to environmental concern and behavior. Environ. Behav. 2009, 41, 715–740. [Google Scholar] [CrossRef]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.M.; Calado, R. Trends in the discovery of new marine natural products from invertebrates over the last two decades—Where and what are we bioprospecting? PLoS ONE 2012, 7, e030580. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Poloczanska, E.S.; Skirving, W.; Dove, S. Coral reef ecosystems under climate change and ocean acidification. Front. Mar. Sci. 2017, 4, 158. [Google Scholar] [CrossRef]
- Ussiri, D.A.N.; Lal, R. Mitigation of climate change: Introduction. In Carbon Sequestration for Climate Change Mitigation and Adaptation; Springer International Publishing: Berlin, Germany, 2017; pp. 287–325. [Google Scholar]
- Gattuso, J.-P.; Magnan, A.; Billé, R.; Cheung, W.W.L.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M.; et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 2015, 349, aac4722. [Google Scholar] [CrossRef] [PubMed]
- Heller, N.E.; Zavaleta, E.S. Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biol. Conserv. 2009, 142, 14–32. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Adams, W.M.; Aronson, R.B.; Aveling, R.; Blackburn, T.M.; Broad, S.; Ceballos, G.; Cote, I.M.; Cowling, R.M.; Da Fonseca, G.A.B.; et al. One hundred questions of importance to the conservation of global biological diversity. Conserv. Biol. 2009, 23, 557–567. [Google Scholar] [CrossRef] [PubMed]
- West, J.M.; Salm, R.V. Resistance and resilience to coral bleaching: Implications for coral reef conservation and management. Conserv. Biol. 2003, 17, 956–967. [Google Scholar] [CrossRef]
- McLeod, E.; Salm, R.; Green, A.; Almany, J. Designing marine protected area networks to address the impacts of climate change. Front. Ecol. Environ. 2009, 7, 362–370. [Google Scholar] [CrossRef]
- Hendriks, I.E.; Olsen, Y.S.; Ramajo, L.; Basso, L.; Steckbauer, A.; Moore, T.S.; Howard, J.; Duarte, C.M. Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences 2014, 11, 333–346. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Smith, J.E.; Johnson, K.S.; Send, U.; Levin, L.A.; Micheli, F.; Paytan, A.; Price, N.N.; Peterson, B.; Takeshita, Y.; et al. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLoS ONE 2011, 6, e28983. [Google Scholar] [CrossRef]
- Kelly, R.P.; Foley, M.M.; Fisher, W.S.; Feely, R.A.; Halpern, B.S.; Waldbusser, G.G.; Caldwell, M.R. Mitigating local causes of ocean acidification with existing laws. Science 2011, 332, 1036–1037. [Google Scholar] [CrossRef]
- Vatanpour, N.; Malvandi, A.M.; Hedayati Talouki, H.; Gattinoni, P.; Scesi, L. Impact of rapid urbanization on the surface water’s quality: A long-term environmental and physicochemical investigation of Tajan river, Iran (2007–2017). Environ. Sci. Pollut. Res. 2020, 27, 8439–8450. [Google Scholar] [CrossRef]
- Russell, B.D.; Thompson, J.-A.I.; Falkenberg, L.J.; Connell, S.D. Synergistic effects of climate change and local stressors: CO2 and nutrient-driven change in subtidal rocky habitats. Glob. Chang. Biol. 2009, 15, 2153–2162. [Google Scholar] [CrossRef]
- Falkenberg, L.J.; Connell, S.D.; Russell, B.D. Disrupting the effects of synergies between stressors: Improved water quality dampens the effects of future CO2 on a marine habitat. J. Appl. Ecol. 2013, 50, 51–58. [Google Scholar] [CrossRef]
- Falkenberg, L.J.; Russell, B.D.; Connell, S.D. Stability of strong species interactions resist the synergistic effects of local and global pollution in kelp forests. PLoS ONE 2012, 7, e33841. [Google Scholar] [CrossRef] [PubMed]
- Shyue, S.-W.; Lee, C.-L.; Chen, H.-C. An Approach to a Coastal Water Quality Index for Taiwan; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2020; pp. 904–907. [Google Scholar]
- Gupta, A.K.; Gupta, S.K.; Patil, R.S. A comparison of water quality indices for coastal water. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2003, 38, 2711–2725. [Google Scholar] [CrossRef] [PubMed]
- Cinner, J.E.; Lau, J.D.; Bauman, A.G.; Feary, D.A.; Januchowski-Hartley, F.A.; Rojas, C.A.; Barnes, M.L.; Bergseth, B.J.; Shum, E.; Lahari, R.; et al. Sixteen years of social and ecological dynamics reveal challenges and opportunities for adaptive management in sustaining the commons. Proc. Natl. Acad. Sci. USA 2019, 116, 26474–26483. [Google Scholar] [CrossRef] [PubMed]
- Doubleday, Z.A.; Connell, S.D. Weedy futures: Can we benefit from the species that thrive in the marine Anthropocene? Front. Ecol. Environ. 2018, 16, 599–604. [Google Scholar] [CrossRef]
- Gibbons, M.J.; Boero, F.; Brotz, L. We should not assume that fishing jellyfish will solve our jellyfish problem. Ices J. Mar. Sci. 2016, 73, 1012–1018. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Rodhouse, P.G.K.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L.; Arguelles, J.; Bower, J.R.; Castillo, G.; Ceriola, L.; et al. World squid fisheries. In Reviews in Fisheries Science and Aquaculture; Taylor and Francis Inc.: Abingdon, UK, 2015. [Google Scholar]
- Berry, H.L.; Bowen, K.; Kjellstrom, T. Climate change and mental health: A causal pathways framework. Int. J. Public Health 2010, 55, 123–132. [Google Scholar] [CrossRef]
- Friel, S.; Marmot, M.; McMichael, A.J.; Kjellstrom, T.; Vågerö, D. Global health equity and climate stabilisation: A common agenda. Lancet 2008, 372, 1677–1683. [Google Scholar] [CrossRef]
- Bayles, B.R.; Brauman, K.A.; Adkins, J.N.; Allan, B.F.; Ellis, A.M.; Goldberg, T.L.; Golden, C.D.; Grigsby-Toussaint, D.S.; Myers, S.S.; Osofsky, S.A.; et al. Ecosystem services connect environmental change to human health outcomes. EcoHealth 2016, 13, 443–449. [Google Scholar] [CrossRef]
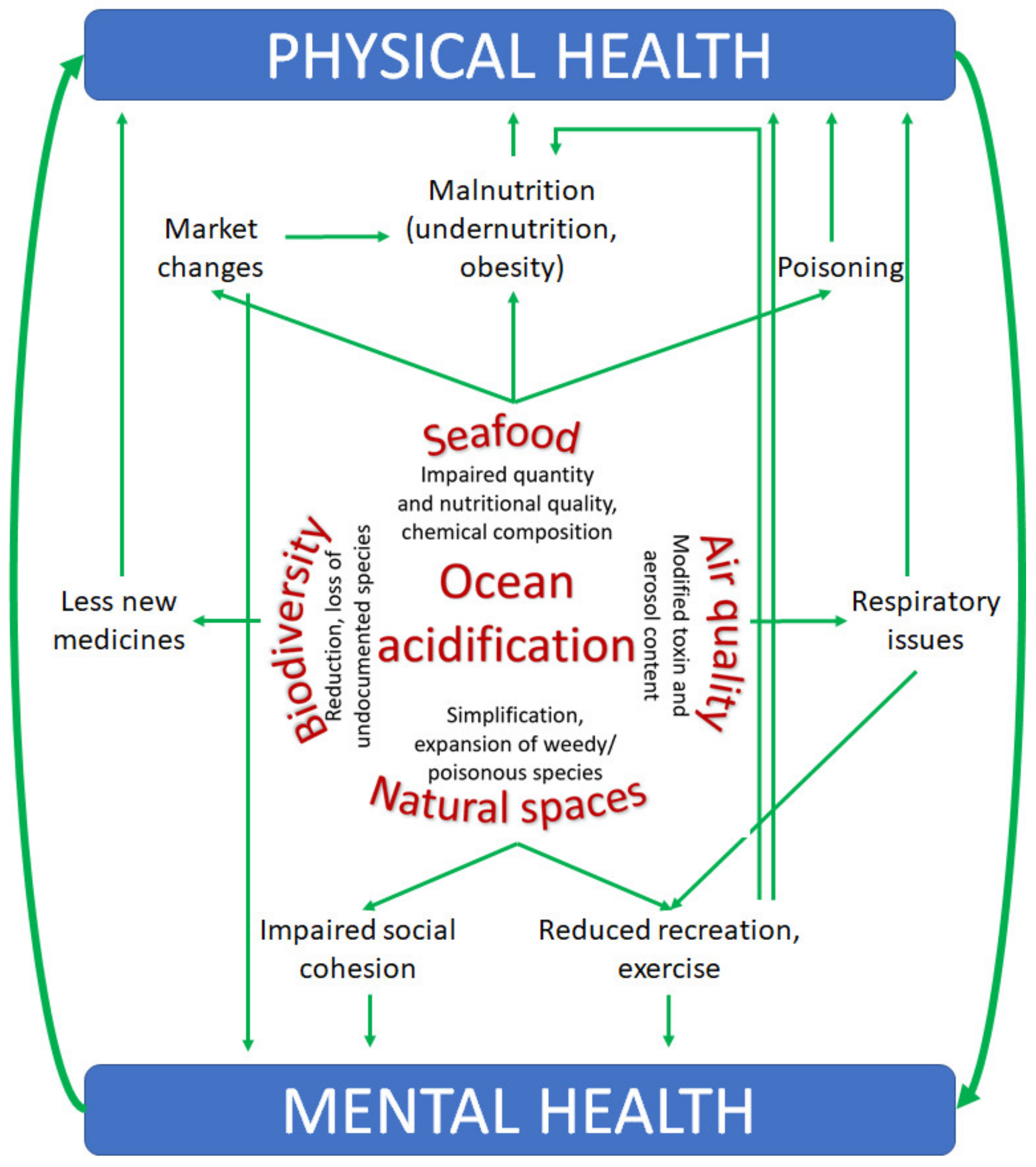
| Pathway of Ocean Acidification Impact | References |
|---|---|
| Pathway 1—malnutrition and poisoning via altered food quantity and quality | |
| • Reduced quantity | [30,31,32,33] |
| • Impaired nutritional composition | [27,34] |
| • Chemical contamination (pollutants) | [20,35,36,37] |
| • Redistribution and accumulation of natural toxins | [38,39] |
| Pathway 2—respiratory issues via impaired air quality | |
| • Enhanced aerosolization of natural toxins | [38,40] |
| Pathway 3—mental health impacts via modification of natural spaces | |
| • Loss of livelihoods | [41] |
| • Disruption of nature-based recreation, exercise, and connection | [42,43,44,45,46] |
| • Reduced social connections | [47,48] |
| Pathway 4—decreased opportunity to develop and obtain medical resources via loss of biodiversity | |
| • Loss of source of potential medical resources | [49,50] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falkenberg, L.J.; Bellerby, R.G.J.; Connell, S.D.; Fleming, L.E.; Maycock, B.; Russell, B.D.; Sullivan, F.J.; Dupont, S. Ocean Acidification and Human Health. Int. J. Environ. Res. Public Health 2020, 17, 4563. https://doi.org/10.3390/ijerph17124563
Falkenberg LJ, Bellerby RGJ, Connell SD, Fleming LE, Maycock B, Russell BD, Sullivan FJ, Dupont S. Ocean Acidification and Human Health. International Journal of Environmental Research and Public Health. 2020; 17(12):4563. https://doi.org/10.3390/ijerph17124563
Chicago/Turabian StyleFalkenberg, Laura J., Richard G.J. Bellerby, Sean D. Connell, Lora E. Fleming, Bruce Maycock, Bayden D. Russell, Francis J. Sullivan, and Sam Dupont. 2020. "Ocean Acidification and Human Health" International Journal of Environmental Research and Public Health 17, no. 12: 4563. https://doi.org/10.3390/ijerph17124563
APA StyleFalkenberg, L. J., Bellerby, R. G. J., Connell, S. D., Fleming, L. E., Maycock, B., Russell, B. D., Sullivan, F. J., & Dupont, S. (2020). Ocean Acidification and Human Health. International Journal of Environmental Research and Public Health, 17(12), 4563. https://doi.org/10.3390/ijerph17124563








