Bird Species Involved in West Nile Virus Epidemiological Cycle in Southern Québec
Abstract
1. Introduction
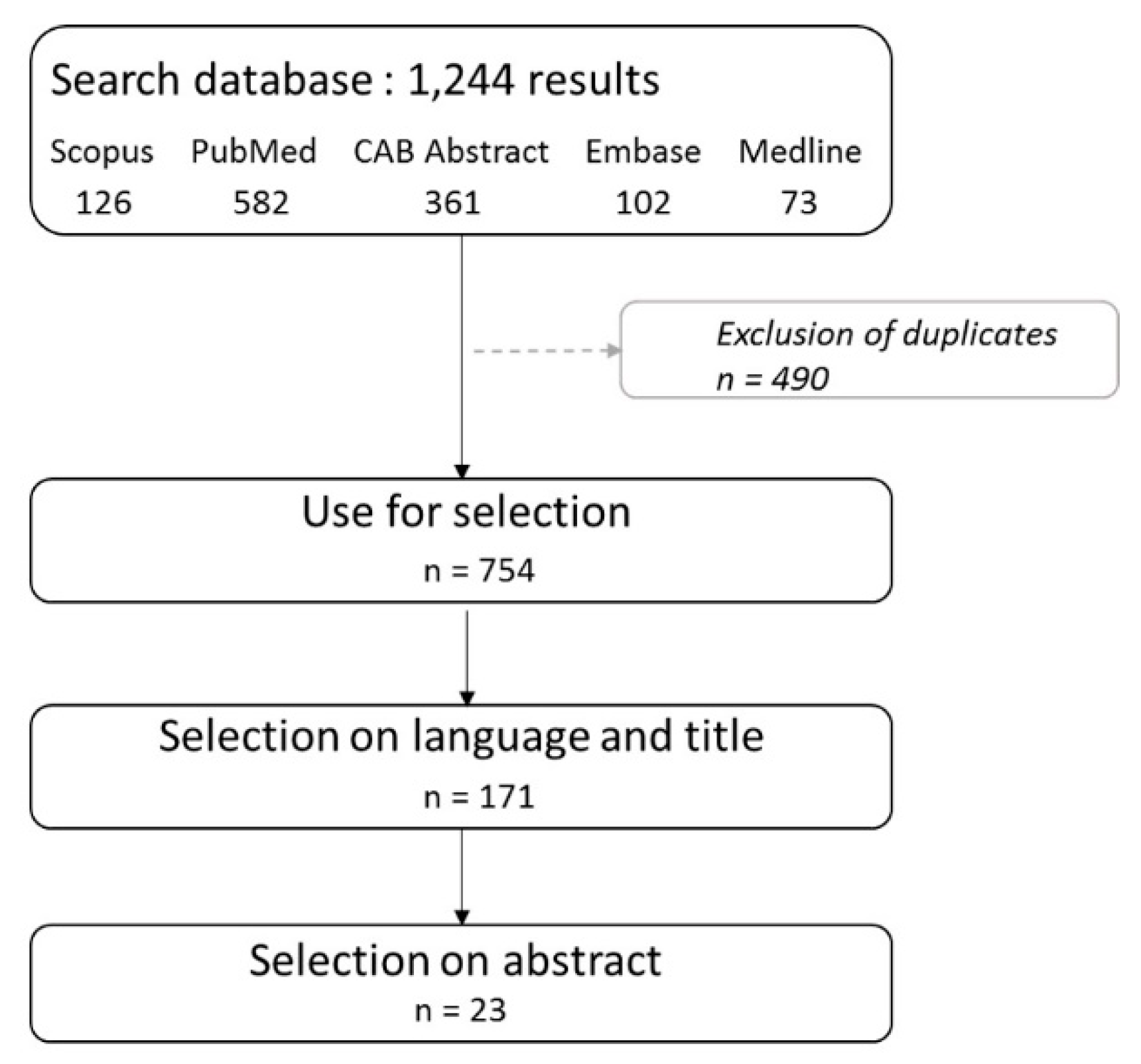
2. Materials and Methods
2.1. Study Area
2.2. Identification of Priority List of Wild Bird Reservoir Species
2.2.1. Wild Bird Mortality Data: List
2.2.2. Blood Meal Data: List
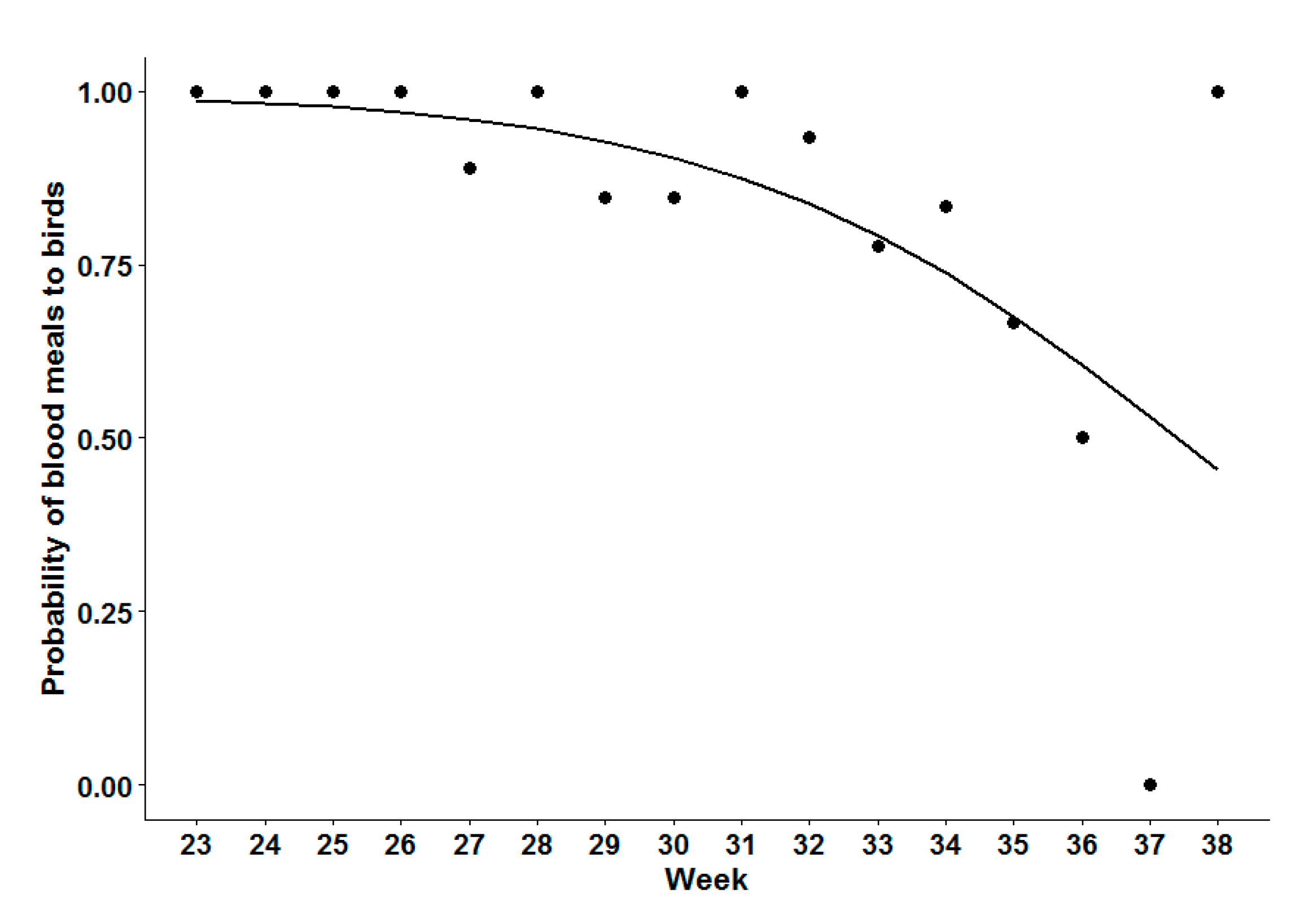
Analysis of Seasonal Bird-to-Mammal Feeding Shift of CPR Mosquitoes
2.2.3. Literature Review: List
3. Results
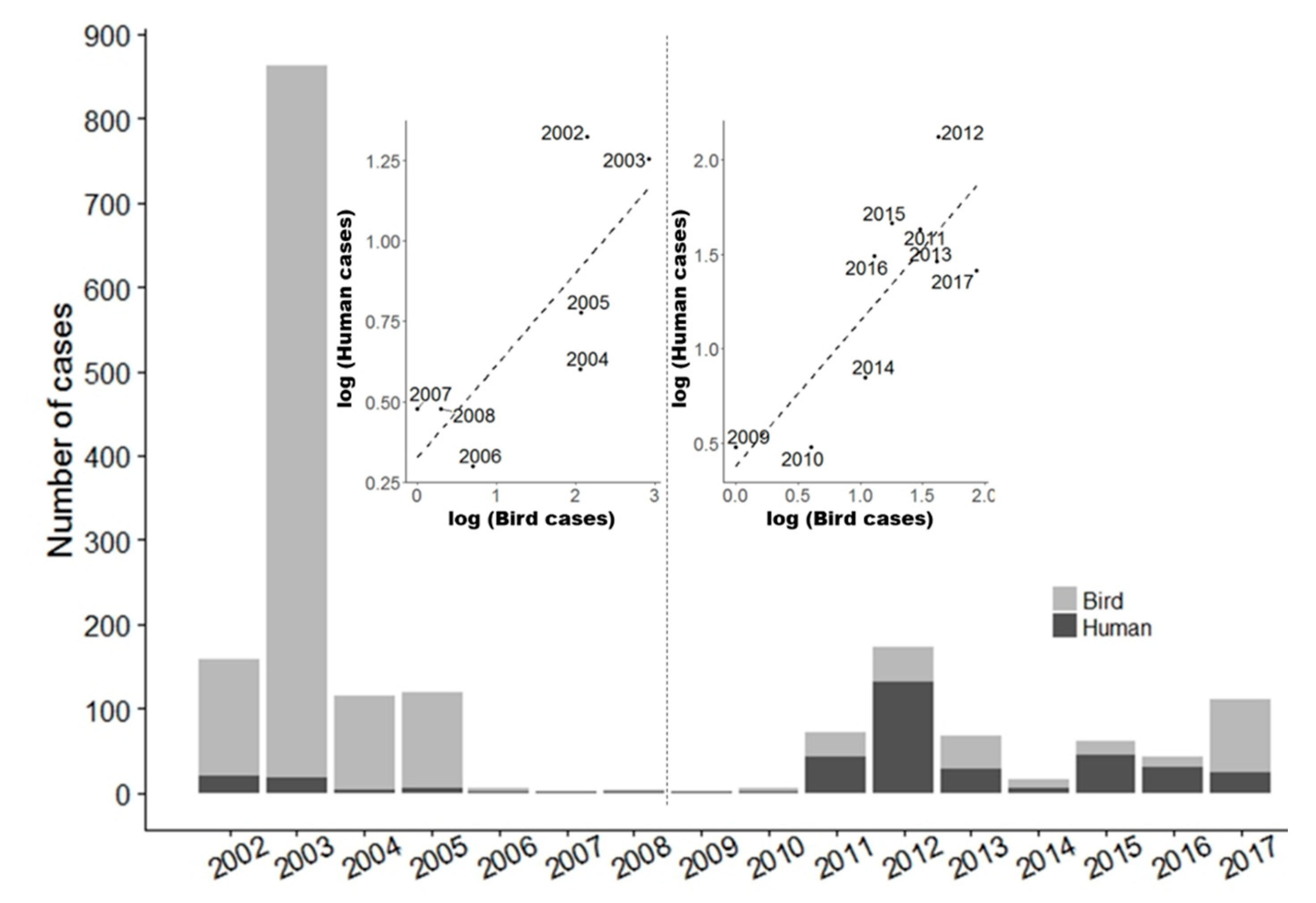
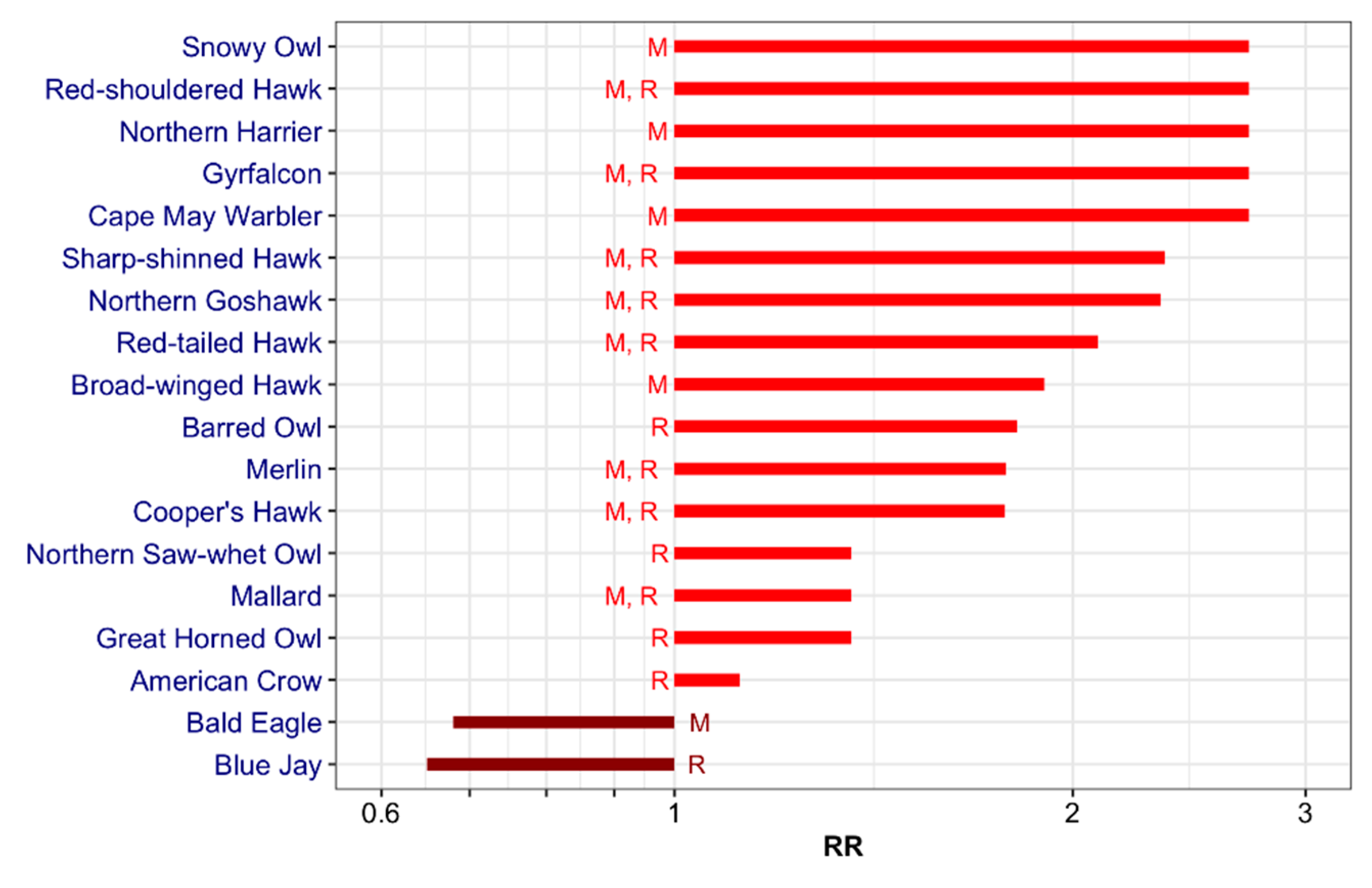
3.1. Wild Bird Mortality Data: List
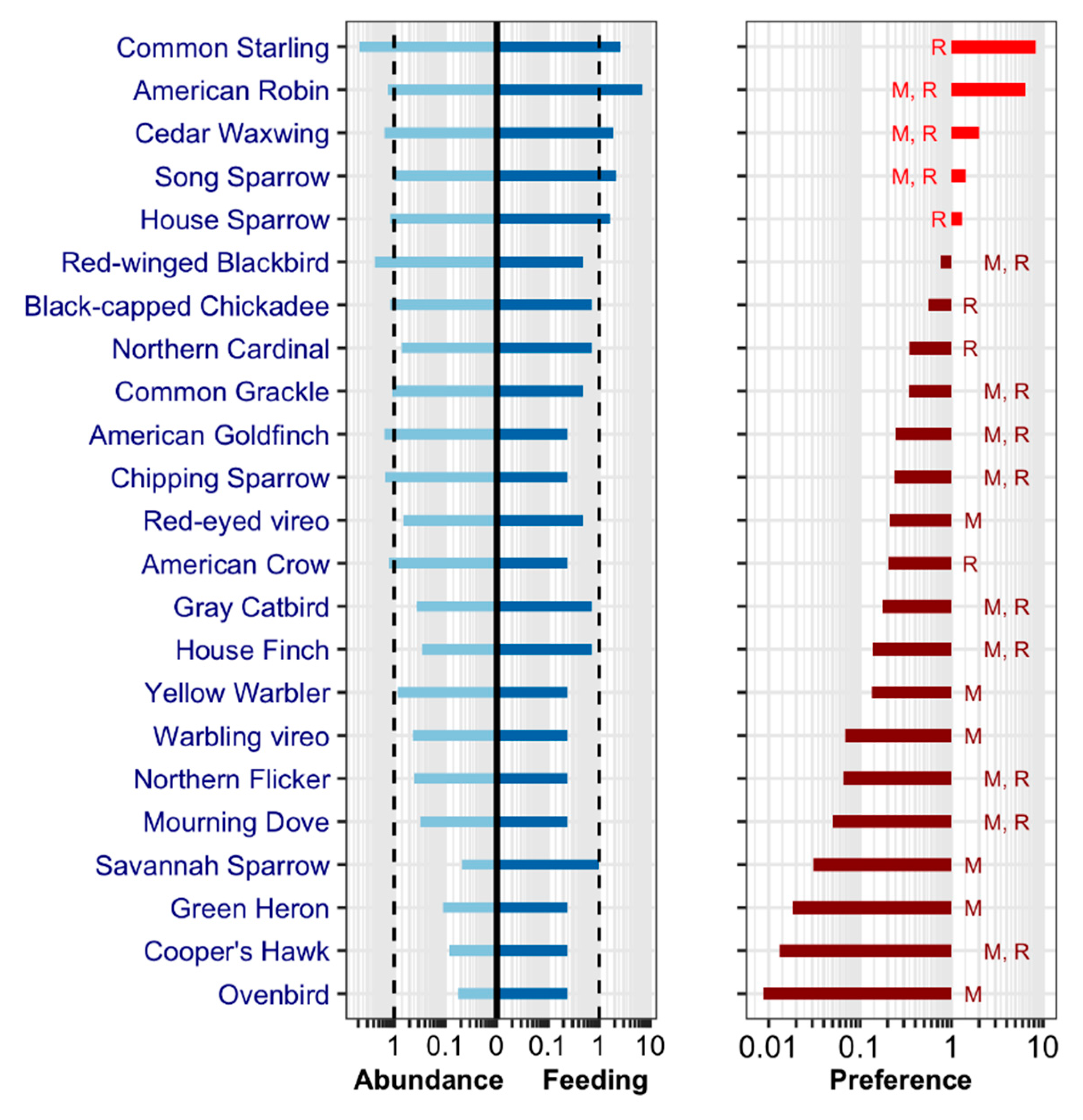
3.2. Blood Meal Analysis: List
Analysis of Seasonal Bird-To-Mammal Feeding Shift of CPR Mosquitoes
3.3. Literature Review: List
3.4. Final List:
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda1. Am. J. Trop. Med. Hyg. 1940, s1–s20, 471–492. [Google Scholar] [CrossRef]
- Work, T.H.; Hurlbut, H.S.; Taylor, R.M. Isolation of West Nile virus from hooded crow and rock pigeon in the Nile delta. Proc. Soc. Exp. Biol. Med. 1953, 84, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.M.; Work, T.H.; Hurlbut, H.S.; Rizk, F. A Study of the Ecology of West Nile Virus in Egypt. Am. J. Trop. Med. Hyg. 1956, 5, 579–620. [Google Scholar] [CrossRef]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, A.E.; Van Kruiningen, H.J.; French, R.A. The West Nile virus: Its recent emergence in North America. Microb. Infect. 2001, 3, 223–229. [Google Scholar] [CrossRef]
- Komar, N.; Panella, N.A.; Boyce, E. Exposure of domestic mammals to West Nile virus during an outbreak of human encephalitis, New York City, 1999. Emerg. Infect. Dis. 2001, 7, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Panella, N.A.; Burns, J.E.; Dusza, S.W.; Mascarenhas, T.M.; Talbot, T.O. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg. Infect. Dis. 2001, 7, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Gancz, A.Y.; Campbell, D.G.; Barker, I.K.; Lindsay, R.; Hunter, B. Detecting West Nile Virus in Owls and Raptors by an Antigen-capture Assay. Emerg. Infect. Dis. 2004, 10, 2204–2206. [Google Scholar] [CrossRef]
- INSPQ. Proposition d’un Programme de Surveillance Entomologique du Virus du Nil Occidental au Québec—Avis Scientifique; Institut National de Santé Publique du Québec: Quebec City, QC, Canada, 2015. [Google Scholar]
- Dauphin, G.; Zientara, S.; Zeller, H.; Murgue, B. West Nile: Worldwide current situation in animals and humans. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 343–355. [Google Scholar] [CrossRef]
- Ripoche, M.; Ludwig, A.; Campagna, C.; Ludwig, A.; Ogden, N.H.; Leighton, P.A. Short-term Forecasting of Daily Abundance of West Nile Virus Vectors Culex pipiens-restuans (Diptera: Culicidae) and Aedes vexans Based on Weather Conditions in Southern Québec (Canada). J. Med. Entomol. 2019, 56, 859–872. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Daszak, P.; Jones, M.J.; Marra, P.P.; Kramer, L.D. Host heterogeneity dominates West Nile virus transmission. Proc. Biol. Sci. 2006, 273, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Kramer, L.D.; Jones, M.J.; Marra, P.P.; Daszak, P. West Nile Virus Epidemics in North America Are Driven by Shifts in Mosquito Feeding Behavior. PLoS Biol. 2006, 4, e82. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, T.G. The contribution of Culex pipiens complex mosquitoes to transmission and persistence of West Nile virus in North America. J. Am. Mosq. Control Assoc. 2012, 28 (Suppl. 4), 137–151. [Google Scholar] [CrossRef] [PubMed]
- Rappole, J.H.; Derrickson, S.R.; Hubalek, Z. Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg. Infect. Dis. 2000, 6, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T.; Vieglais, D.A.; Andreasen, J.K. Migratory birds modeled as critical transport agents for West Nile Virus in North America. Vector-Borne Zoonotic Dis 2003, 3, 27–37. [Google Scholar] [CrossRef]
- Jourdain, E.; Gauthier-Clerc, M.; Bicout, D.J.; Sabatier, P. Bird Migration Routes and Risk for Pathogen Dispersion into Western Mediterranean Wetlands. Emerg. Infect. Dis. 2007, 13, 365–372. [Google Scholar] [CrossRef]
- Dusek, R.J.; McLean, R.G.; Kramer, L.D.; Ubico, S.R.; Dupuis, A.P.; Ebel, G.D.; Guptill, S.C. Prevalence of West Nile virus in migratory birds during spring and fall migration. Am. J. Trop. Med. Hyg. 2009, 81, 1151–1158. [Google Scholar] [CrossRef]
- Owen, J.C.; Nakamura, A.; Coon, C.A.; Martin, L.B. The effect of exogenous corticosterone on West Nile virus infection in Northern Cardinals (Cardinalis cardinalis). Vet. Res. 2012, 43, 34. [Google Scholar] [CrossRef]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.L.; David, B.S.; Bowen, R.A.; Bunning, M.L. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- Marra, P.; Griffing, S.; Caffrey, C.; Kilpatrick, A.; McLean, R.; Brand, C.; Saito, E.; Dupuis, A.; Kramer, L.; Novak, R. West Nile Virus and Wildlife. BioScience 2004, 54, 393–402. [Google Scholar] [CrossRef]
- Thomas-Bachli, A.L.; Pearl, D.L.; Berke, O.; Parmley, E.J.; Barker, I.K. A comparison of West Nile virus surveillance using survival analyses of dead corvid and mosquito pool data in Ontario, 2002–2008. Prev. Vet. Med. 2015, 122, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Veksler, A.; Eidson, M.; Zurbenko, I. Assessment of methods for prediction of human West Nile virus (WNV) disease from WNV-infected dead birds. Emerg. Themes Epidemiol. 2009, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- LaDeau, S.L.; Kilpatrick, A.M.; Marra, P.P. West Nile virus emergence and large-scale declines of North American bird populations. Nature 2007, 447, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Fougères, D.; Macleod, R. Montreal: The History of a North American City; McGill-Queen’s University Press: Montreal, QC, Canada, 2017. [Google Scholar]
- StatisticsCanada. Land Cover and Land Use Montreal. 2016. Available online: http://www.statcan.gc.ca/pub/16-201-x/2016000/c-g/c-g03-29-eng.htm (accessed on 17 June 2019).
- Lepage, D. Avibase—Listes D’oiseaux Mondiales Montérégie: Montréal. 2017. Available online: https://avibase.bsc-eoc.org/checklist.jsp?region=caqc05&list=howardmoore&lang=FR (accessed on 21 May 2020).
- Drebot, M.A.; Lindsay, R.; Barker, I.K.; Buck, P.A.; Fearon, M.; Hunter, F.; Sockett, F.; Artsob, H. West Nile virus surveillance and diagnostics: A Canadian perspective. Can. J. Infect. Dis. 2003, 14, 105–114. [Google Scholar] [CrossRef]
- Lindsay, R.; Barker, I.; Nayar, G.; Drebot, M.; Calvin, S.; Scammell, C.; Sachvie, C.; La Fleur, T.; Dibernardo, A.; Andonova, M.; et al. Rapid Antigen-Capture Assay To Detect West Nile Virus in Dead Corvids. Emerg. Infect. Dis. 2003, 9, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- CWHC. Le Virus du Nil Occidental. 2016. Available online: http://fr.cwhc-rcsf.ca/west_nile_virus.php (accessed on 20 July 2016).
- Molaei, G.; Thomas, M.C.; Muller, T.; Medlock, J.; Shepard, J.J.; Armstrong, P.M.; Andreadis, T.G. Dynamics of Vector-Host Interactions in Avian Communities in Four Eastern Equine Encephalitis Virus Foci in the Northeastern U.S. PLoS Negl. Trop. Dis. 2016, 10, e0004347. [Google Scholar] [CrossRef]
- Rizzoli, A.; Bolzoni, L.; Chadwick, E.A.; Capelli, G.; Montarsi, F.; Grisenti, M.; de la Puente, J.M.; Munoz, J.; Figuerola, J.; Soriguer, R.; et al. Understanding West Nile virus ecology in Europe: Culex pipiens host feeding preference in a hotspot of virus emergence. Parasit. Vectors 2015, 8, 213. [Google Scholar] [CrossRef]
- Balenghien, T.; Fouque, F.; Sabatier, P.; Bicout, D.J. Theoretical Formulation for Mosquito Host-Feeding Patterns: Application to a West Nile Virus Focus of Southern France. J. Med. Entomol. 2011, 48, 1076–1090. [Google Scholar] [CrossRef]
- Bicout, D.J. Le virus du Nil occidental; Éditions Quæ: Versailles, France, 2013; p. 239. [Google Scholar]
- Regroupement Québec Oiseaux. Etude des Populations d’oiseaux du Québec. 2018. Available online: https://www.oiseauxqc.org/epoq.jsp (accessed on 15 October 2018).
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, A.M. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evolut. 2011, 11, 1577–1585. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. Available online: http://www.R-project.org (accessed on 21 May 2020).
- Peterson, R.T.; Peterson, V.M. Les Oiseaux du Québec et de l’Est de l’Amérique du Nord, 5th ed.; Broquet: Saint-Constant, QC, Canada, 2004. [Google Scholar]
- Komar, N.; Dohm, D.J.; Turell, M.J.; Spielman, A. Eastern equine encephalitis virus in birds: Relative competence of European starlings (Sturnus vulgaris). Am. J. Trop. Med. Hyg. 1999, 60, 387–391. [Google Scholar] [CrossRef]
- Ringia, A.M.; Blitvich, B.J.; Koo, H.Y.; Van de Wyngaerde, M.; Brawn, J.D.; Novak, R.J. Antibody prevalence of West Nile virus in birds, Illinois, 2002. Emerg. Infect. Dis. 2004, 10, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Godsey, M.S., Jr.; Blackmore, M.S.; Panella, N.A.; Burkhalter, K.; Gottfried, K.; Halsey, L.A.; Rutledge, R.; Langevin, S.A.; Gates, R. West Nile virus epizootiology in the southeastern United States, 2001. Vector Borne Zoonotic Dis. 2005, 5, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Panella, N.A.; Langevin, S.A.; Brault, A.C.; Amador, M.; Edwards, E.; Owen, J.C. Avian hosts for West Nile virus in St. Tammany Parish, Louisiana, 2002. Am. J. Trop. Med. Hyg. 2005, 73, 1031–1037. [Google Scholar] [CrossRef]
- Gibbs, S.E.; Allison, A.B.; Yabsley, M.J.; Mead, D.G.; Wilcox, B.R.; Stallknecht, D.E. West Nile virus antibodies in avian species of Georgia, USA: 2000–2004. Vector Borne Zoonotic Dis. 2006, 6, 57–72. [Google Scholar] [CrossRef]
- Loss, S.R.; Hamer, G.L.; Walker, E.D.; Ruiz, M.O.; Goldberg, T.L.; Kitron, U.D.; Brawn, J.D. Avian host community structure and prevalence of West Nile virus in Chicago, Illinois. Oecologia 2009, 159, 415–424. [Google Scholar] [CrossRef]
- Dubé, M.C.; Bird, D.M.; Dibernardo, A.; Lindsay, L.R.; Charest, H. Prevalence of West Nile virus in wild American Kestrels (Falco sparverius) of southern Quebec, Canada. J. Wildl. Dis. 2010, 46, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.W.; Knepper, R.G.; Stanuszek, W.W.; Walker, E.D.; Wilson, M.L. Temporal and spatial patterns of West Nile virus transmission in Saginaw County, Michigan, 2003–2006. J. Med. Entomol. 2011, 48, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Peters, R.J.; Dupuis, A.P., 2nd; Jones, M.J.; Marra, P.P.; Kramer, L.D. Predicted and observed mortality from vector-borne disease in small songbirds. Biol. Conserv. 2013, 165, 79–85. [Google Scholar] [CrossRef]
- Komar, N.; Panella, N.A.; Young, G.R.; Brault, A.C.; Levy, C.E. Avian hosts of West Nile virus in Arizona. Am. J. Trop. Med. Hyg. 2013, 89, 474–481. [Google Scholar] [CrossRef]
- Randall, N.J.; Blitvich, B.J.; Blanchong, J.A. Association between agricultural land use and West Nile virus antibody prevalence in Iowa birds. J. Wildl. Dis. 2013, 49, 869–878. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; LaDeau, S.L.; Marra, P.P. Ecology of West Nile Virus Transmission and its Impact on Birds in the Western Hemisphere. The Auk 2007, 124, 1121–1136. [Google Scholar] [CrossRef]
- Wheeler, S.S.; Barker, C.M.; Fang, Y.; Armijos, M.V.; Carroll, B.D.; Husted, S.; Johnson, W.O.; Reisen, W.K. Differential impact of West Nile Virus on California birds. The Condor 2009, 111, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Bigras-Poulin, M.; Michel, P.; Belanger, D. Risk factors associated with West Nile virus mortality in American Crow populations in Southern Quebec. J. Wildl. Dis. 2010, 46, 195–208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foppa, I.M.; Beard, R.H.; Mendenhall, I.H. The impact of West Nile virus on the abundance of selected North American birds. BMC Vet. Res. 2011, 7, 43. [Google Scholar] [CrossRef]
- Thomas-Bachli, A.L.; Pearl, D.L.; Parmley, E.J.; Berke, O. The Influence of Sociodemographic Factors on the Engagement of Citizens in the Detection of Dead Corvids during the Emergence of West Nile Virus in Ontario, Canada. Front. Vet. Sci. 2020, 6, 483. [Google Scholar] [CrossRef] [PubMed]
- Worwa, G.; Hutton, A.A.; Brault, A.C.; Reisen, W.K. Comparative fitness of West Nile virus isolated during California epidemics. PLoS Negl. Trop. Dis. 2019, 13, e0007135. [Google Scholar] [CrossRef]
- Hamer, G.L.; Kitron, U.D.; Goldberg, T.L.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Hayes, D.B.; Walker, E.D. Host selection by Culex pipiens mosquitoes and west nile virus amplification. Am. J. Trop. Med. Hyg. 2009, 80, 268–278. [Google Scholar] [CrossRef]
- Andreadis, T.G.; Anderson, J.F.; Vossbrinck, C.R.; Main, A.J. Epidemiology of West Nile virus in Connecticut: A five-year analysis of mosquito data 1999–2003. Vector Borne Zoonotic Dis. 2004, 4, 360–378. [Google Scholar] [CrossRef]
- Giordano, B.V.; Turner, K.W.; Hunter, F.F. Geospatial Analysis and Seasonal Distribution of West Nile Virus Vectors (Diptera: Culicidae) in Southern Ontario, Canada. Int. J. Env. Res. Pub. Health 2018, 15. [Google Scholar] [CrossRef]
- Silver, J.B. CHAP2: Sampling the Egg Population. In Mosquito Ecology—Field Sampling Methods, 3rd ed.; Springer: Dordrecht, The Netherlands, 2018; pp. 25–136. [Google Scholar]
- Kilpatrick, A.M.; Kramer, L.D.; Campbell, S.R.; Alleyne, E.O.; Dobson, A.P.; Daszak, P. West Nile virus risk assessment and the bridge vector paradigm. Emerg. Infect. Dis. 2005, 11, 425–429. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Kramer, L.D.; Campbell, S.R.; Alleyne, E.O.; Dobson, A.P.; Daszak, P. Supersuppression: Reservoir Competency and Timing of Mosquito Host Shifts Combine to Reduce Spillover of West Nile Virus. Am. J. Trop. Med. Hyg. 2016, 95, 1174–1184. [Google Scholar] [CrossRef]
- West Nile virus and other mosquito-borne diseases national surveillance report. Available online: https://www.canada.ca/en/public-health/services/diseases/west-nile-virus/west-nile-virus-other-mosquito-borne-disease.html (accessed on 21 May 2020).
- Ogden, N.H.; Ludwig, A.; Morse, A.P.; Zheng, H.; Zhu, H. Weather-based forecasting of mosquito-borne disease outbreaks in Canada. Can Commun. Dis. Rep. 2019, 45, 127–132. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.; Martin, S.W.; Landry, K.; Gould, C.V.; Lehman, J.; Fischer, M.; Lindsey, N.P. West Nile Virus and Other Domestic Nationally Notifiable Arboviral Diseases—United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 673–678. [Google Scholar] [CrossRef]
- Ronca, S.E.; Murray, K.O.; Nolan, M.S. Cumulative Incidence of West Nile Virus Infection, Continental United States, 1999–2016. Emerg. Infect. Dis. 2019, 25, 325–327. [Google Scholar] [CrossRef]
- Mermel, L.A. Association of Human Eastern Equine Encephalitis with Precipitation Levels in Massachusetts. JAMA Netw. Open 2020, 3, e1920261. [Google Scholar] [CrossRef] [PubMed]

| Question | Description | Answer | |
|---|---|---|---|
| No | Yes/Cannot Tell | ||
| Level 1: Language | |||
| Q1 | Is the paper written in English or French? | 0 | 1 |
| L1 = Q1; eligible for L1 = 1 | |||
| Level 2: Title | |||
| Q1 | Does the title mention West Nile terms? | 0 | 1 |
| Q2 | Does the title mention bird terms? | 0 | 1 |
| Q3 | Does the title mention a region of study that is concerned (East Coast USA and Canada)? | 0 | 1 |
| Q4 | Does the title mention terms relate to sero-prevalence, prevalence, competence, capacity or transmission? | 0 | 1 |
| L2 = Q1 × Q2 (1 + Q3 + Q4); eligible for L2 ≥ 1 | |||
| Level 3: Abstract | |||
| Q1 | Does the abstract describe search results rather than a method? | 0 | 1 |
| Q2 | Do the abstract mention terms related to bird mortality? | 0 | 1 |
| Q3 | Do the abstract mention terms related to prevalence or sero-prevalence? | 0 | 1 |
| Q4 | Does the abstract mention terms relate to host competence 1? | 0 | 1 |
| Q5 | Does the abstract mention terms relate to host capacity 2? | 0 | 1 |
| Q6 | Does the abstract specify the regions of study: states of the Eastern migratory corridor for the USA and Canada? | 0 | 1 |
| L3 = Q1 × Q6 × (1 + Q2 + Q3 + Q4 + Q5); eligible for L3 ≥ 1 | |||
| Score = L1 × L2 × L3; eligible for score ≥ 1 | |||
| Species | Family | Order | n | Birds (%) (n = 97) | Mammals (%) (n = 14) | Total (%) (n = 111) * |
|---|---|---|---|---|---|---|
| Birds | ||||||
| American robin (Turdus migratorius) | Turdidae | Passeriformes | 30 | 30.9 | - | 27.0 |
| Common starling (Sturnus vulgaris) | Sturnidae | Passeriformes | 11 | 11.3 | - | 9.9 |
| Song sparrow (Melospiza melodia) | Emberizidae | Passeriformes | 9 | 9.3 | - | 8.1 |
| Cedar waxwing (Bombycilla cedrorum) | Bombycillidae | Passeriformes | 8 | 8.2 | - | 7.2 |
| House sparrow (Passer domesticus) | Passeridae | Passeriformes | 7 | 7.2 | - | 6.3 |
| Savannah sparrow (Passerculus sandwichensis) | Emberizidae | Passeriformes | 4 | 4.1 | - | 3.6 |
| Northern cardinal (Cardinalis cardinalis) | Cardinalidae | Passeriformes | 3 | 3.1 | - | 2.7 |
| Gray catbird (Dumetella carolinensis) | Mimidae | Passeriformes | 3 | 3.1 | - | 2.7 |
| House finch (Haemorhous mexicanus) | Fringillidae | Passeriformes | 3 | 3.1 | - | 2.7 |
| Black-capped chickadee (Poecile atricapilla) | Paridae | Passeriformes | 3 | 3.1 | - | 2.7 |
| Red-winged blackbird (Agelaius phoeniceus) | Icteridae | Passeriformes | 2 | 2.1 | - | 1.8 |
| Common grackle (Quiscalus quiscula) | Icteridae | Passeriformes | 2 | 2.1 | - | 1.8 |
| Red-eyed vireo (Vireo olivaceus) | Vireonidae | Passeriformes | 2 | 2.1 | - | 1.8 |
| Cooper’s hawk (Accipiter cooperii) | Accipitridae | Accipitriformes | 1 | 1.0 | - | 0.9 |
| Green heron (Butorides virescens) | Ardeidae | Pelecaniformes | 1 | 1.0 | - | 0.9 |
| Northern flicker (Colaptes auratus) | Picidae | Piciformes | 1 | 1.0 | - | 0.9 |
| American crow (Corvus brachyrhynchos) | Corvidae | Passeriformes | 1 | 1.0 | - | 0.9 |
| American yellow warbler (Dendroica petechia) | Parulidae | Passeriformes | 1 | 1.0 | - | 0.9 |
| Ovenbird (Seiurus aurocapilla) | Parulidae | Passeriformes | 1 | 1.0 | - | 0.9 |
| American goldfinch (Spinus tristis) | Fringillidae | Passeriformes | 1 | 1.0 | - | 0.9 |
| Chipping sparrow (Spizella passerina) | Emberizidae | Passeriformes | 1 | 1.0 | - | 0.9 |
| Warbling vireo (Vireo gilvus) | Vireonidae | Passeriformes | 1 | 1.0 | - | 0.9 |
| Mourning dove (Zenaida macroura) | Columbidae | Passeriformes | 1 | 1.0 | - | 0.9 |
| Mammals | ||||||
| White-tailed deer (Odocoileus virginianus) | Cervidae | Artiodactyla | 7 | - | 50.0 | 6.3 |
| Bovine (Bos taurus) | Bovidae | Artiodactyla | 2 | - | 14.3 | 1.8 |
| Cat (Felis catus) | Felidae | Carnivora | 2 | - | 14.3 | 1.8 |
| Human (Homo sapiens) | Hominidae | Primates | 2 | - | 14.3 | 1.8 |
| Mule deer (Odocoileus hemionus) | Cervidae | Artiodactyla | 1 | - | 7.1 | 0.9 |
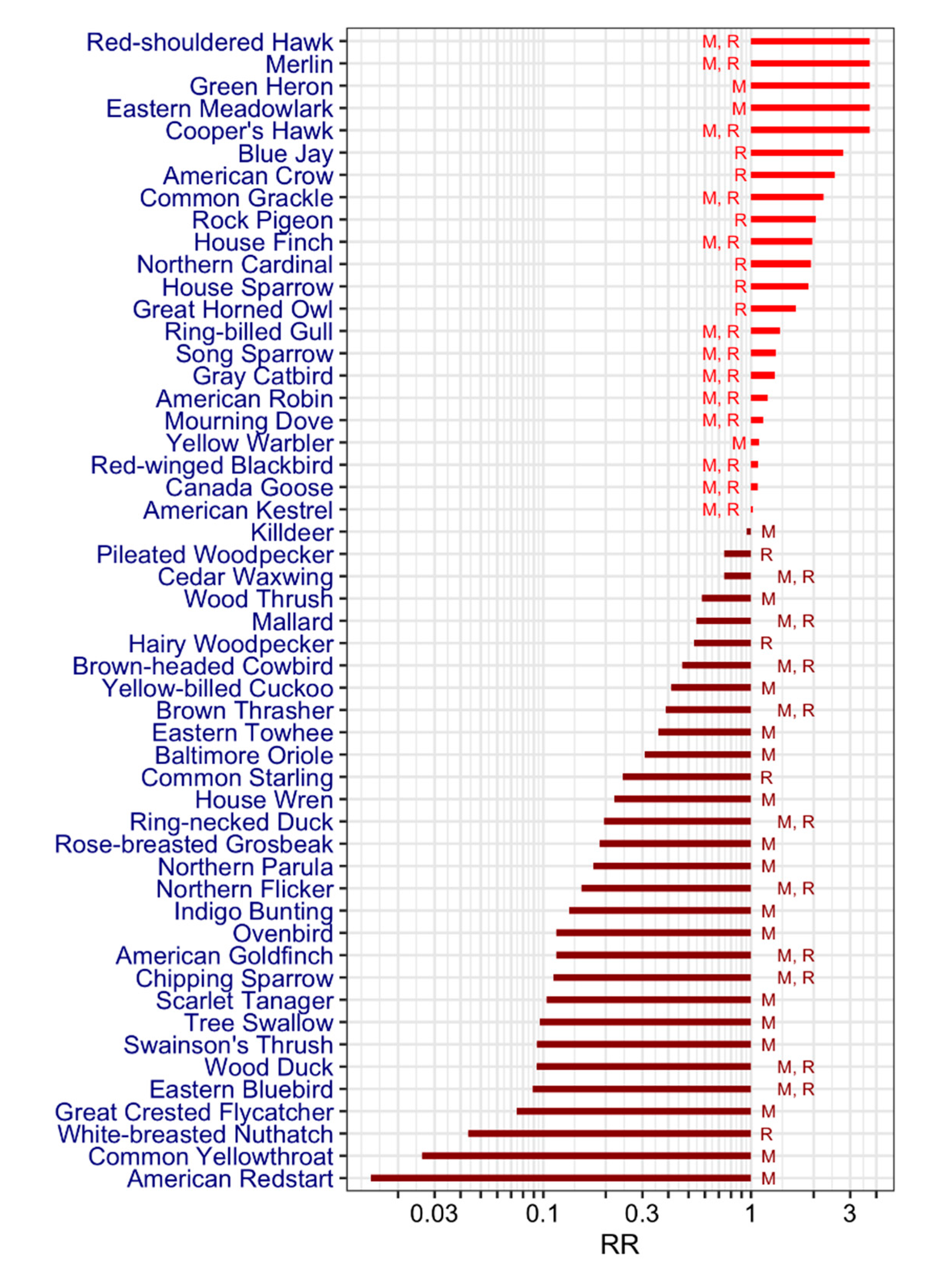
| Family | Species (English Name) | Species (Latin Name) | Literature | Mortality, % (n) 3 | Status 6 | Wintering Area7 | Breeding Area 7 | Spring Migration | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seroprevalence (%) 1 Min (n)–Max (n) | Host Competence 2 Min-Max | 2001–2008 | 2009–2017 | ||||||||||
| Anatidae | Canada Goose | Branta canadensis | 0.8 (1038)–29 (7) | 0.03–0.05 | M, R | S | QC, N | March | |||||
| Anatidae | Wood Duck | Aix sponsa | 0 (1)–2.5 (12) | 0 (1) | M, R | S | QC, N, S | March | |||||
| Anatidae | Mallard † | Anas platyrhynchos | 8 (13)–10.6 (66) | 0.48–0.5 | 50 (4) | M, R | S | QC, N, S | March | ||||
| Anatidae | Ring-necked Duck | Aythya collaris | 5.3 (19) | M, R | S | QC, N | March | ||||||
| Ardeidae | Green Heron | Butorides virescens | 100 (1) | 0.0049 | 0.0103 | M | S | QC, N, S | April | ||||
| Accipitridae | Northern Harrier† | Circus cyaneus | 100 (1) | M | QC, N, S | QC, N | March | ||||||
| Accipitridae | Sharp-shinned Hawk† | Accipiter striatus | 86.4 (22) | M, R | QC, N, S | QC, N, S | March | ||||||
| Accipitridae | Cooper’s Hawk† | Accipiter cooperii | 100 (1) | 65.4 (26) | 0.0036 | 0.0103 | M, R | QC, N, S | QC, N, S | March | |||
| Accipitridae | Northern Goshawk† | Accipiter gentilis | 85.7 (7) | M, R | QC, N | QC, N | March | ||||||
| Accipitridae | Bald Eagle† | Haliaeetus leucophalus | 25 (4) | M | N, S | QC, N, S | March | ||||||
| Accipitridae | Red-shouldered Hawk† | Buteo lineatus | 100 (1) | 100 (5) | M, R | QC, N, S | QC, N, S | March | |||||
| Accipitridae | Broad-winged Hawk† | Buteo platypterus | 70 (10) | M | S | QC, N, S | April | ||||||
| Accipitridae | Red-tailed Hawk† | Buteo jamaicensis | 76.9 (13) | 0.0013 | M, R | QC, N, S | QC, N, S | March | |||||
| Charadriidae | Killdeer | Charadrius vociferus | 0.84–0.87 | M | N, S | QC, N, S | March | ||||||
| Laridae | Ring-billed Gull | Larus delawarensis | 1.18–1.26 | 0 (3) | M, R | QC, N, S | QC, N | February | |||||
| Columbidae | Rock Pigeon | Columba livia | 4.3 (23)–55 (20) | 0 | R | QC, N, S | QC, N, S | ||||||
| Columbidae | Mourning Dove | Zenaida macroura | 3.8 (26)–57.69 (26) | 0–0.19 | 0 (2) | 0.0137 | 0.0103 | M, R | QC, N, S | QC, N, S | April | ||
| Cuculidae | Yellow-billed Cuckoo | Coccyzus americanus | 5.9 (17)–100 (1) | M | QC, N, S | Mai | |||||||
| Strigidae | Great Horned Owl† | Bubo virginianus | 44.4 (9) | 0.68–0.9 | 50 (6) | R | QC, N, S | QC, N, S | |||||
| Strigidae | Snowy Owl† | Bubo scandiacus | 100 (2) | M | QC, N | March | |||||||
| Strigidae | Barred Owl† | Strix varia | 66.7 (3) | R | QC, N, S | QC, N, S | |||||||
| Strigidae | Northern Saw-whet Owl† | Aegolius acadicus | 50 (8) | R | QC, N | QC, N | |||||||
| Picidae | Hairy Woodpecker | Picoides villosus | 0 (14)–14.3 (7) | R | QC, N, S | QC, N, S | |||||||
| Picidae | Northern Flicker | Colaptes auratus | 0.06–0.14 | 0.0178 | 0.0103 | M, R | N, S | QC, N, S | April | ||||
| Picidae | Pileated Woodpecker | Dryocopus pileatus | 20 (5) | R | QC, N, S | QC, N, S | |||||||
| Falconidae | American Kestrel | Falco sparverius | 16 (152)–100 (1) | 0.79–0.93 | 0 (1) | 0 (5) | M, R | QC, N, S | QC, N, S | April | |||
| Falconidae | Merlin† | Falco columbarius | 100 (1) | 65.5 (55) | M, R | QC, N, S | QC, N | March | |||||
| Falconidae | Gyrfalcon† | Falco rusticolus | 100 (1) | M, R | QC | ||||||||
| Tyrannidae | Great Crested Flycatcher | Myiarchus crinitus | 2 (50) | M | QC, N, S | May | |||||||
| Vireonidae | Warbling vireo | Vireo gilvus | 0.0189 | 0.0103 | M | QC, N, S | May | ||||||
| Vireonidae | Red-eyed vireo | Vireo olivaceus | 0.0288 | 0.0206 | M | QC, N, S | May | ||||||
| Corvidae | Blue Jay† | Cyanocitta cristata | 0.8 (121)–35.8 (134) | 2.39–2.55 | 23.6 (886) | 66.7 (6) | 0.0093 | R | QC, N, S | QC, N, S | |||
| Corvidae | American Crow† | Corvus brachyrhynchos | 3.2 (157)–68.3 (183) | 1.04–1.62 | 40.1 (1418) | 73.9 (46) | 0.0549 | 0.0103 | R | QC, N, S | QC, N, S | ||
| Hirundinidae | Tree Swallow | Tachycineta bicolor | 2.6 (156) | M | S | QC, N, S | March | ||||||
| Paridae | Black-capped Chickadee | Poecile atricapillus | 0 (107) | 0.0511 | 0.0309 | R | QC, N | QC, N | |||||
| Sittidae | White-breasted Nuthatch | Sitta carolinensis | 0 (40)–2.9 (35) | R | QC, N, S | QC, N, S | |||||||
| Troglodytidae | House Wren | Troglodytes aedon | 5.9 (17) | M | S | QC, N, S | April | ||||||
| Turdidae | Eastern Bluebird | Sialia sialis | 2.4 (126) | M, R | N. S | QC, N, S | March | ||||||
| Turdidae | Swainson’s Thrush | Catharus ustulatus | 2.13 (47)–3.1 (32) | M | QC, N | May | |||||||
| Turdidae | Wood Thrush | Hylocichla mustelina | 1 (101)–15.6 (32) | M | QC, N, S | May | |||||||
| Turdidae | American Robin | Turdus migratorius | 2.6 (76)–10.11 (366) | 1.04–1.1 | 0.0578 | 0.3093 | M, R | QC, N, S | QC, N, S | April | |||
| Mimidae | Gray Catbird | Dumetella carolinensis | 3.5 (2706)–35 (17) | 0.07–0.1 | 0.0158 | 0.0309 | M, R | N, S | QC, N, S | May | |||
| Mimidae | Brown Thrasher | Toxostoma rufum | 3.7 (643)–10.5 (19) | M, R | N, S | QC, N, S | April | ||||||
| Sturnidae | Common Starling | Sturnus vulgaris | 0.16–0.22 | 0.2045 | 0.1134 | R | QC, N, S | QC, N, S | |||||
| Bombycillidae | Cedar Waxwing | Bombycilla cedrorum | 20 (5) | 0.0671 | 0.0825 | M, R | QC, N, S | QC, N, S | March | ||||
| Parulidae | Ovenbird | Seiurus aurocapilla | 0.9 (115)–3.1 (32) | 0.0024 | 0.0103 | M | S | QC, N, S | May | ||||
| Parulidae | Common Yellowthroat | Geothlypis trichas | 0.7 (299) | M | S | QC, N, S | May | ||||||
| Parulidae | American Redstart | Setophaga ruticilla | 0.4 (280) | M | S | QC, N, S | May | ||||||
| Parulidae | Cape May Warbler † | Setophaga tigrina | 100 (1) | M | QC, N | May | |||||||
| Parulidae | Northern Parula | Setophaga americana | 4.7 (43) | M | S | QC, N, S | May | ||||||
| Parulidae | Yellow Warbler | Setophaga petechia | 1 | 0.0368 | 0.0103 | M | QC, N, S | April | |||||
| Passerellidae | Chipping Sparrow | Spizella passerina | 3 (59) | 0.0652 | 0.0103 | M, R | S | QC, N, S | April | ||||
| Passerellidae | Savannah Sparrow | Passerculus sandwicheris | 0.0021 | 0.0412 | M | S | QC, N | April | |||||
| Passerellidae | Song Sparrow | Melospiza melodia | 0 (13)–3.4 (88) | 1.2 | 0.0431 | 0.0928 | M, R | QC, N, S | QC, N, S | April | |||
| Passerellidae | Eastern Towhee | Pipilo erythrophthalmus | 0.7 (144)–9.6 (197) | M | N, S | QC, N, S | April | ||||||
| Cardinalidae | Scarlet Tanager | Piranga olivacea | 2.8 (71) | M | QC, N, S | April | |||||||
| Cardinalidae | Northern Cardinal | Cardinalis cardinalis | 6.2 (503)–52.2 (115) | 0.38 | 0.0313 | 0.0309 | R | QC, N, S | QC, N, S | ||||
| Cardinalidae | Rose-breasted Grosbeak | Pheucticus ludovicianus | 1 (98)–5 (22) | M | QC, N | April | |||||||
| Cardinalidae | Indigo Bunting | Passerina cyanea | 3.6 (28)–2.2 (223) | M | S | QC, N, S | April | ||||||
| Icteridae | Eastern Meadowlark | Sturnella magna | 100 (1) | M | N, S | QC, N, S | March | ||||||
| Icteridae | Baltimore Oriole | Icterus galbula | 8.3 (12) | M | S | QC, N, S | May | ||||||
| Icteridae | Red-winged Blackbird | Agelaius phoeniceus | 0 (63)–10.5 (67) | 0.9–0.99 | 0.1036 | 0.0206 | M, R | QC, N, S | QC, N, S | March | |||
| Icteridae | Brown-headed Cowbird | Molothrus ater | 1.8 (494)–12.5 (24) | 0 | M, R | QC, N, S | QC, N | April | |||||
| Icteridae | Common Grackle | Quiscalus quiscula | 0 (106)–15.4 (13) | 1.39–2.04 | 0.0462 | 0.0206 | M, R | QC, N, S | QC, N, S | March | |||
| Fringillidae | House Finch | Haemorhous mexicanus | 2 (927)–100 (5) | 1.29–1.8 | 0.0125 | 0.0309 | M, R | QC, N, S | QC, N, S | March | |||
| Fringillidae | American Goldfinch | Spinus tristis | 0.3 (337)–3.1 (128) | 0.0666 | 0.0103 | M, R | QC, N, S | QC, N, S | February | ||||
| Passeridae | House Sparrow | Passer domesticus | 1.6 (1042)–51 (107) | 1.25–1.6 | 0 (1) | 0.0508 | 0.0722 | R | QC, N, S | QC, N, S | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taieb, L.; Ludwig, A.; Ogden, N.H.; Lindsay, R.L.; Iranpour, M.; Gagnon, C.A.; Bicout, D.J. Bird Species Involved in West Nile Virus Epidemiological Cycle in Southern Québec. Int. J. Environ. Res. Public Health 2020, 17, 4517. https://doi.org/10.3390/ijerph17124517
Taieb L, Ludwig A, Ogden NH, Lindsay RL, Iranpour M, Gagnon CA, Bicout DJ. Bird Species Involved in West Nile Virus Epidemiological Cycle in Southern Québec. International Journal of Environmental Research and Public Health. 2020; 17(12):4517. https://doi.org/10.3390/ijerph17124517
Chicago/Turabian StyleTaieb, Ludivine, Antoinette Ludwig, Nick H. Ogden, Robbin L. Lindsay, Mahmood Iranpour, Carl A. Gagnon, and Dominique J. Bicout. 2020. "Bird Species Involved in West Nile Virus Epidemiological Cycle in Southern Québec" International Journal of Environmental Research and Public Health 17, no. 12: 4517. https://doi.org/10.3390/ijerph17124517
APA StyleTaieb, L., Ludwig, A., Ogden, N. H., Lindsay, R. L., Iranpour, M., Gagnon, C. A., & Bicout, D. J. (2020). Bird Species Involved in West Nile Virus Epidemiological Cycle in Southern Québec. International Journal of Environmental Research and Public Health, 17(12), 4517. https://doi.org/10.3390/ijerph17124517





