Interception Systems in Assessment of Dermal Exposure to Pesticides: Laboratory Comparison of Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Patches Preparation
- cellulose filter paper sheets grade 41 (Whatman™ Article No. 28418027), pore size 20–25 μm (Particle retention)
- cellulose filter paper standard grade 1 (Whatman™ Article No. 28413923), pore size 11 μm (Particle retention)
- cotton wipes (TexWipe® Cotton TX304)
- layers of surgical gauze patches (Dynarex)
- polyethylene tissue, a synthetic material used to produce protective overalls (Tyvex® Du Pont)
2.2. Analytical Method
2.3. Experimental
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maroni, M.; Fanetti, A.C.; Metruccio, F. Risk assessment and management of occupational exposure to pesticides in agriculture. Med. Lav. 2006, 97, 430–437. [Google Scholar] [PubMed]
- Damalas, C.A.; Koutroubas, S.D. Farmers’ Exposure to Pesticides: Toxicity Types and Ways of Prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef]
- Semple, S. Dermal exposure to chemicals in the workplace: Just how important is skin absorption? Occup. Environ. Med. 2004, 61, 376–382. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, E.; Carey, R.; Keegel, T.; El-Zaemay, S.; Fritschi, L. Dermal exposure associated with occupational end use of pesticides and the role of protective measures. Saf. Health Work 2013, 4, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Kangas, J.; Laitinen, S.; Jauhiainen, A.; Savolainen, K. Exposure of sprayers and plant handlers to mevinophos in Finnish greenhouses. Am. Ind. Hyg. Assoc. J. 1993, 54, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Cherrie, J.W.; Vermeulen, R.; Kromhout, H. Dermal Exposure Assessment. Ann. Occup. Hyg. 2000, 44, 493–499. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Dermal Exposure Assessment: A Summary of EPA Approaches; EPA 600/R-07/040F; EPA: Washington, DC, USA, 2007. [Google Scholar]
- World Health Organization (WHO). Standard Protocol Ref. VBC/82.1: Field Surveys of Exposure to Pesticides; WHO: Geneva, Switzerland, 1982. [Google Scholar]
- Fenske, R.A. Dermal Exposure assessment technique. Ann. Occup. Hyg. 1993, 37, 687–706. [Google Scholar]
- Soutar, A.; Semple, S.; Aitken, R.J.; Robertson, A. Use of Patches and Whole Body Sampling for the Assessment of Dermal Exposure. Ann. Occup. Hyg. 2000, 44, 511–518. [Google Scholar] [CrossRef]
- Aprea, C. Environmental and biological monitoring in the estimation of adsorbed doses of pesticides. Toxicol. Lett. 2012, 210, 110–118. [Google Scholar] [CrossRef]
- Durham, W.; Wolfe, H.R. Measurement of the exposure of workers to pesticides. Bull. World Health Organ. 1962, 26, 75–91. [Google Scholar]
- Reinert, J.C.; Nielsen, A.P.; Lunchick, C.; Hernandez, O.; Mazzetta, D.M. The United States Environmental Protection Agency’s guidelines for applicator exposure monitoring. Toxicol. Lett. 1986, 33, 183–191. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Pesticide Assessment Guidelines, Subdivision U, Applicator Exposure Monitoring; US Department of Commerce, National Technical Information Service: Springfield, VA, USA, 1986. [Google Scholar]
- Organization for Economic Co-operation and Development (OECD). Environmental Health and Safety Publications Series on Testing and Assessment No 9. Guidance Document for the Conduct of Studies of Occupational Exposure to Pesticides during Agricultural Application; OCDE/GD/(97)148y; OECD: Paris, France, 1997. [Google Scholar]
- Aprea, C.; Lunghini, L.; Banchi, B.; Peruzzi, A.; Centi, L.; Coppi, L.; Bogi, M.; Marianelli, E.; Fantacci, M.; Catalano, P. Evaluation of inhaled and cutaneous doses of Imidacloprid during stapling ornamental plants in tunnels or greenhouses. J. Expo. Sci. Environ. Epidemiol. 2009, 9, 555–569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Health and Safety Executive (HSE). Dermal Exposure to Non-Agricultural Pesticides; EH7/43 ; HSE: London, UK, 1999; ISBN 0717617181. [Google Scholar]
- Popendorf, W.; Selim, M. Exposure while applying commercial disinfectants. Am. Ind. Hyg. Assoc. J. 1995, 56, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Insecticide Resistance Action Committee (IRAC). Insecticide Resistance Action Committee. 2018. Available online: http://www.irac-online.org/modes-of-action/ (accessed on 3 April 2020).
- Jeschke, P.; Nauen, R. Neonicotinoids–from zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- World Health Organization; International Programme on Chemical Safety. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009; World Health Organization: Geneva, Switzerland, 2010; Available online: https://apps.who.int/iris/handle/10665/44271 (accessed on 10 June 2020).
- US EPA. Pesticide fate database. In Environmental Fate and Effects Division of the Office of Pesticide Programs; US EPA: Washington, DC, USA, 2005. [Google Scholar]
- EFSA. Assesses Potential Link between Two Neonicotinoids and Developmental Neurotoxicity. Available online: https://www.efsa.europa.eu/en/press/news/131217 (accessed on 17 April 2020).
- Agarwal, R.; Srinivas, R. Severe neuropsychiatric manifestations and rhabdomyolysis in a patient with Imidacloprid poisoning. Am. J. Emerg. Med. 2007, 25, 844–845. [Google Scholar] [CrossRef]
- The European Commission. Commission Implementing Regulation (EU) No 783/2018 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance Imidacloprid. Off. J. Eur. Union 2018, 132, 31–34. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R0783&from=EN (accessed on 26 February 2020).
- US EPA. Memorandum of Third Peer Review of Hexythiazox (Savey): Report of the Cancer Assessment Review Committee; US EPA: Washington, DC, USA, 2009. [Google Scholar]
- US EPA. Office of Pesticide Programs, Health Effects Division, Science Information Management Branch: “Chemicals Evaluated for Carcinogenic Potential”; US EPA: Washington, DC, USA, 2006. [Google Scholar]
- Product Safety Assessment: Myclobutanil. Available online: http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_08d6/0901b803808d60fd.pdf?filepath=productsafety/pdfs/noreg/233-01023.pdf&fromPage=GetDoc (accessed on 26 February 2020).
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance Myclobutanil. EFSA J. 2010, 8, 1682. [Google Scholar] [CrossRef]
- Sekiyama, M.; Tanaka, M.; Gunawan, B.; Abdoellah, O.; Watanabe, C. Pesticide usage and its association with health symptoms among farmers in rural villages in West Java, Indonesia. Environ. Sci. 2007, 14, 23–33. [Google Scholar]
- U.S. EPA. Residential Exposure Test Guidelines. Office of Prevention, Pesticides and Toxic Substance (OPPTS) 875.1100- Dermal Exposure—Outdoor; U.S. EPA: Washington, DC, USA, 1996. [Google Scholar]
- US EPA. Organochlorine Pesticides by Gas Chromatography (Method 8081B), the Scope and Application. 2007. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/8081b.pdf (accessed on 10 April 2020).
- Castro Cano, M.L.; Martìnez Vidal, J.L.; Gonzalez, E.; Martinez, M. Gas Chromatographic Method for Assessing the Dermal Exposure of Greenhouse Applicators to Dimethoate and Malathion. J. Chromatogr. Sci. 2001, 39, 345–350. [Google Scholar] [CrossRef][Green Version]
- Machera, K.; Goumenou, M.; Kapetanakis, E.; Kalamarakis, A.; Glass, C.R. Determination of Potential Dermal and Inhalation Operator Exposure to Malathion in Greenhouses with the Whole Body Dosimetry Method. Ann. Occup. Hyg. 2003, 47, 61–70. [Google Scholar] [CrossRef]
- Sherman, M. Medical Device Packaging Handbook, Revised and Expanded, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Delhomme, O.; Raeppel, C.; Briand, O.; Millet, M. Analytical method for assessing potential dermal exposure to pesticides of a non-agricultural occupationally exposed population. Anal. Bioanal. Chem. 2011, 399, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.E. Procedure for dermal and inhalation studies to assess occupational exposure to pesticides. In Determination and Assessment of Pesticide Exposure; Siewierski, M., Ed.; Elsevier: New York, NY, USA, 1984; pp. 123–131. [Google Scholar]
- Cao, L.; Zhang, H.; Li, F.; Zhou, Z.; Wang, W.; Ma, D.; Yang, L.; Zhou, P.; Huang, Q. Potential dermal and inhalation exposure to imidacloprid and risk assessment among applicators during treatment in cotton field in China. Sci. Total Environ. 2018, 624, 1195–1201. [Google Scholar] [CrossRef] [PubMed]


| Active Substance | Molecular Structure | Molecular Mass (g mol−1) | Vapour Pressure at 25 °C (mPa) | Acetone Solubility g/L | Water Solubility mg/L | Field Sprayed Concentration mg/L |
|---|---|---|---|---|---|---|
| Hexythiazox |  | 352.88 | 1.33 × 10−3 | 160 | 142 | 64 |
| Imidacloprid | 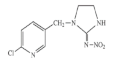 | 255.66 | 4.0 × 10−7 | 50 | 610 | 86 |
| Boscalid | 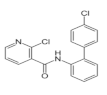 | 343.21 | 0.00072 | 160–200 | 4.6 | 214 |
| Myclobutanil | 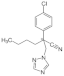 | 288.78 | 0.198 | 50 to 100 | 0.5 | 200 |
| Active Substance | Cotton | Gauze | Polyethylene | W41 | W1 |
|---|---|---|---|---|---|
| Myclobutanil | 95.1 (0.14) | 90.1 (0.07) | 89.2 (0.07) | 89.7 (0.01) | 89.1 (0.11) |
| Imidacloprid | 89.5 (0.21) | 95.6 (0.39 | 90.2 (0.28) | 89.5 (0.21) | 90.5 (0.07) |
| Boscalid | 89.7 (0.01) | 91.1 (0.92) | 94.5 (0.11) | 90.6 (0.92) | 89.2 (0.14) |
| Hexythiazox | 89.3 (0.28) | 93.1 (0.07) | 92.1 (1.91) | 89.1 (0.11) | 89 (0.57) |
| Mean Values in µg/pad (SD)—n = 4 | |||||||
|---|---|---|---|---|---|---|---|
| Matrix | Sprayed Amount (µg) | Cotton | Gauze | Polyethylene | W41 | W1 | |
| Myclobutanil | 640 | 26.83 (2.59) | 15.48 (3.31) | 10.26 (0.89) | 17.15 (7.90) | 10.55 (9.09) | |
| Imidacloprid | 860 | 5.41 (0.97) | 130.28 (2.80) | 34.8 (0.98) | 6.0 (0.03) | 14.82 (4.38) | |
| Boscalid | 2140 | 39.45 (7.0) | 106.68 (5.66) | 306.6 (15.63) | 672 (29.70) | 160.5 (23.26) | |
| Hexythiazox | 2000 | 43 (7.07) | 195.92 (15.26) | 211.65 (8.56) | 6.45 (0.66) | 16.82 (2.97) | |
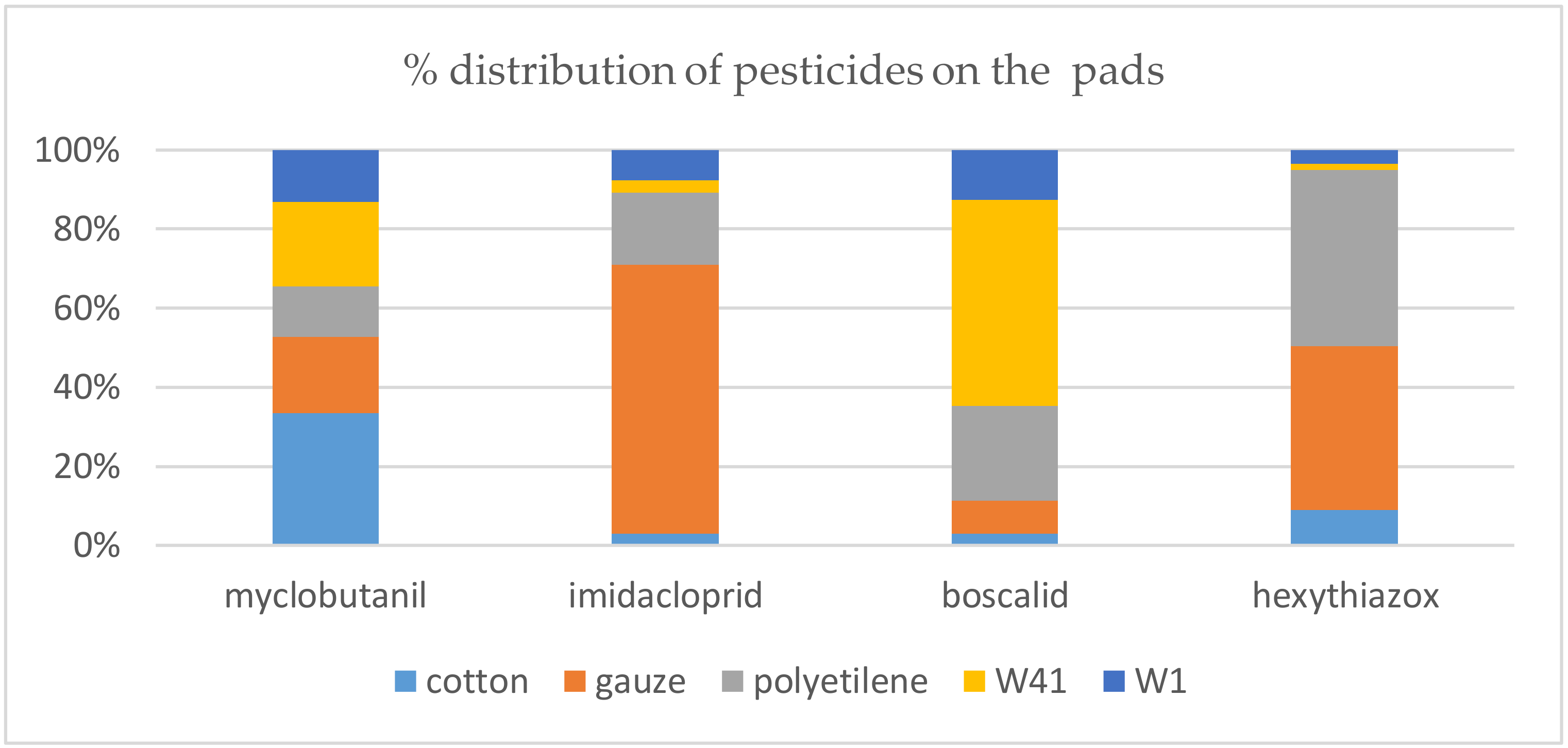
| Patch Material | Myclobutanil% Recovery | Normalized Recovery | Imidacloprid% Recovery | Normalized Recovery | Boscalid% Recovery | Normalized Recovery | Hexythiazox% Recovery | Normalized Recovery |
|---|---|---|---|---|---|---|---|---|
| Cotton | 4.19 | 33.41 | 0.63 | 2.83 | 0.28 | 0.48 | 2.15 | 9.08 |
| Gauze | 2.42 | 19.30 | 15.15 | 68.09 | 4.99 | 8.53 | 9.79 | 41.34 |
| Polyethylene | 1.60 | 12.76 | 4.05 | 18.20 | 14.33 | 24.50 | 10.58 | 44.68 |
| W41 | 2.68 | 21.37 | 0.70 | 3.15 | 31.40 | 53.68 | 0.32 | 1.35 |
| W1 | 1.65 | 13.16 | 1.72 | 7.73 | 7.50 | 12.82 | 0.84 | 3.55 |
| Total | 12.54 | 100.00 | 22.25 | 100.00 | 58.50 | 100.00 | 23.68 | 100.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrese, E.; Tranfo, G.; Marramao, A.; Scarpelli, M. Interception Systems in Assessment of Dermal Exposure to Pesticides: Laboratory Comparison of Media. Int. J. Environ. Res. Public Health 2020, 17, 4389. https://doi.org/10.3390/ijerph17124389
Barrese E, Tranfo G, Marramao A, Scarpelli M. Interception Systems in Assessment of Dermal Exposure to Pesticides: Laboratory Comparison of Media. International Journal of Environmental Research and Public Health. 2020; 17(12):4389. https://doi.org/10.3390/ijerph17124389
Chicago/Turabian StyleBarrese, Elena, Giovanna Tranfo, Antonella Marramao, and Marialuisa Scarpelli. 2020. "Interception Systems in Assessment of Dermal Exposure to Pesticides: Laboratory Comparison of Media" International Journal of Environmental Research and Public Health 17, no. 12: 4389. https://doi.org/10.3390/ijerph17124389
APA StyleBarrese, E., Tranfo, G., Marramao, A., & Scarpelli, M. (2020). Interception Systems in Assessment of Dermal Exposure to Pesticides: Laboratory Comparison of Media. International Journal of Environmental Research and Public Health, 17(12), 4389. https://doi.org/10.3390/ijerph17124389






