Unusual Domestic Source of Lead Poisoning
Abstract
1. Introduction
2. Material and Methods
2.1. Lead Assay
2.2. δ-Aminolevulinic Acid (ALA) and Porphobilinogen (PBG) Quantitative Assay
2.3. Free Erythrocyte protoporhyrins (FEP) Quantitative Assay
2.4. δ-Aminolevulinic Acid Dehydratase (ALAD) Activity Assay
2.5. Environmental Sampling
2.6. Migration Testing in Kitchenware
2.7. Treatment of Pills and Eyewash
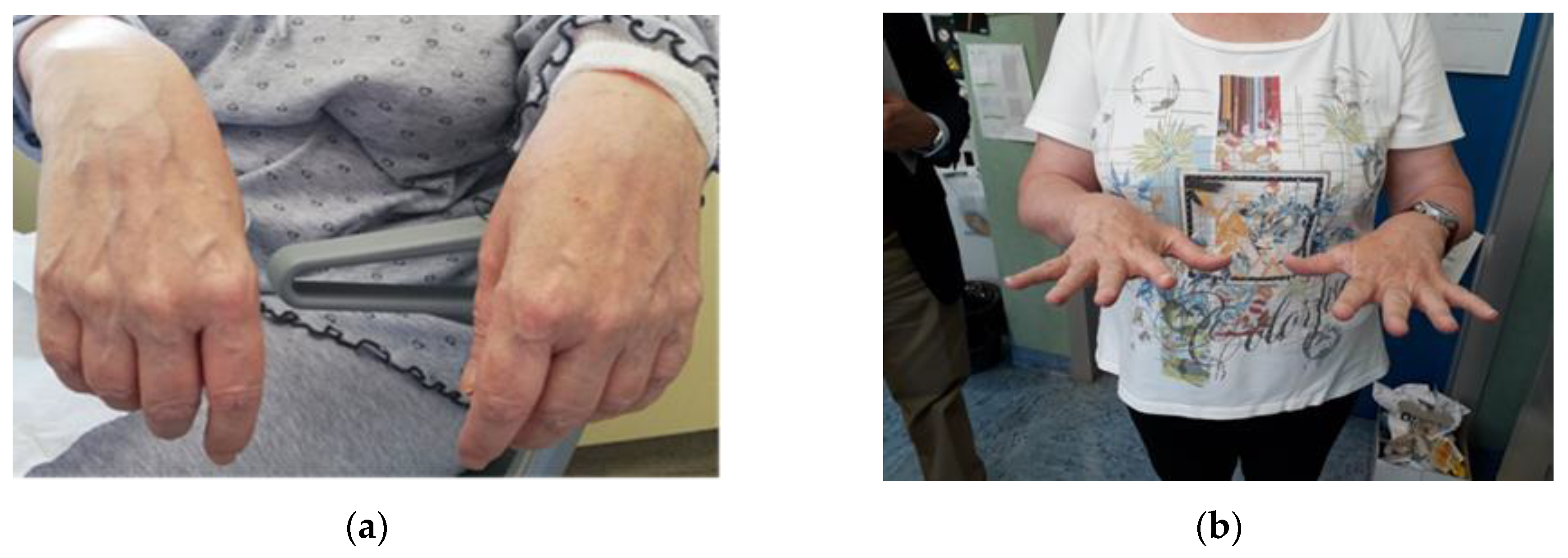
3. Case Report
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dapul, H.; Laraque, D. Lead poisoning in children. Adv. Pediatr. 2014, 61, 313–333. [Google Scholar] [CrossRef] [PubMed]
- WHO. Safety Evaluation of Certain Food Additives and Contaminants in Food: Seventy-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 2011; pp. 381–497. [Google Scholar]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Petracca, M.; Scafa, F.; Boeri, R.; Flachi, D.; Candura, S. Imported occupational lead poisoning: Report of four cases. Med. Lav. 2014, 104, 428–433. [Google Scholar]
- Jonasson, M.; Afshari, R. Historical documentation of lead toxicity prior to the 20th century in English literature. Hum. Exp. Toxicol. 2017, 37, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Hellström-Lindberg, E.; Björklund, A.; Karlson-Stiber, C.; Harper, P.; Seldén, A.I. Lead poisoning from souvenir earthenware. Int. Arch. Occup. Environ. Heal. 2005, 79, 165–168. [Google Scholar] [CrossRef]
- Coon, T.; Miller, M.; Shirazi, F.; Sullivan, J. Lead Toxicity in a 14-Year-Old Female with Retained Bullet Fragments. Pediatrics 2006, 117, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Karri, S.K.; Saper, R.; Kales, S.N. Lead encephalopathy due to traditional medicines. Curr. Drug Saf. 2008, 3, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Shadnia, S.; Soltaninejad, K. Lead poisoning in opium abuser in Iran: A systematic review. Int. J. Prev. Med. 2018, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.; Fung, C.; Leung, A.K. Childhood lead poisoning: An overview. Hong Kong Med. J. 2017, 23, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.R.; Andelman, S.L. Urinary delta-aminolevulinic acid (ALA) levels in lead poisoning. I. A modified method for the rapid determination of urinary delta-aminolevulinic acid using disposable ion-exchange chromatography columns. Arch. Environ. Heal. Int. J. 1967, 15, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, S. Free erythrocyte porphyrins in the detection of undue absorption of Pb and of Fe deficiency. Clin. Chem. 1977, 23, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Schaller, K.H. European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Z. Klin. Chem. Klin. Biochem. 1974, 12, 389–390. [Google Scholar] [PubMed]
- Wang, J.; El-Fahmawi, A.; Yan, C.-H.; Liu, J. Childhood lead poisoning from domestic products in China: A case study with implications for practice, education, and policy. Public Heal. Nurs. 2019, 36, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Njati, S.Y.; Maguta, M.M. Lead-based paints and children’s PVC toys are potential sources of domestic lead poisoning—A review. Environ. Pollut. 2019, 249, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Gorospe, E.C.; Gerstenberger, S.L. Atypical sources of childhood lead poisoning in the United States: A systematic review from 1966–2006. Clin. Toxicol. 2008, 46, 728–737. [Google Scholar] [CrossRef] [PubMed]
| Admission | EDTA | Control * | EDTA | CONTROL † | EDTA | Control ‡ | |
|---|---|---|---|---|---|---|---|
| 07/24/2018 | 07/31/2018 | 08/07/2018 | 08/16/2018 | 09/26/2018 | 11/07/2018 | 02/06/2019 | |
| Hemoglobin (g/L) | 88 | 91 | 105 | 118 | 108 | n.d. | n.d. |
| Hematocrit (%) | 30 | 28 | 34 | 28 | 36 | 36 | 40 |
| Pb-B (µg/L) | 885 | 540 | 938 | 400 | 815 | 525 | 425 |
| Pb-U (µg/L) | 285 | 185 | 521 | 196 | 317 | 178 | 59 |
| ALA (mg/g creat) | 45.9 | 26.1 | 91.4 | 5.7 | 54.2 | n.d. | 14.3 |
| PBG (mg/g creat) | 4.2 | 2.5 | 4.2 | 0.5 | 1.7 | n.d. | 1.4 |
| ALAD (U/L) | 15.7 | 11.9 | 1.8 | 2.9 | n.d. | n.d. | 3.5 |
| FEP (µg/L) | 1488 | 856 | 1256 | 506 | 619 | 2459 | 1352 |
| pot # | Inner Material | Lead µg/L | Lead µg/cm2 |
|---|---|---|---|
| 1 | teflon | 187.20 | 2.163 |
| 2 | teflon | 303.05 | 2.681 |
| 3 | teflon | 35.07 | 0.338 |
| 4 | teflon | 22.52 | 0.112 |
| 5 | teflon | 45.96 | 0.278 |
| 6 | teflon | 18.18 | 0.080 |
| 7 | teflon | 6.67 | 0.026 |
| 8 | teflon | 2.05 | 0.014 |
| 9 | teflon | 2.10 | 0.009 |
| 10 | teflon | 0.00 | 0.000 |
| 11 | teflon | 1.66 | 0.004 |
| 12 | pottery | 25.82 | 0.146 |
| 13 | pottery | 4.74 | 0.027 |
| 14 | pottery | 71.21 | 0.431 |
| 15 | pottery | 12.20 | 0.043 |
| 16 | stainless steel | 17.28 | 0.121 |
| Sample | Lead µg/L |
|---|---|
| Kitchen water tap | <0.8 |
| Bathroom water tap | <0.8 |
| Shower water tap | <0.8 |
| Valerian pills | <0.8 |
| Melatonin pills | <0.8 |
| Eyewash | <0.8 |
| Room | Lead mg/m3 |
|---|---|
| Kitchen | <0.0000141 |
| Living room | <0.0000141 |
| Bedroom 1 | <0.0000141 |
| Bedroom 2 | <0.0000141 |
| Bathroom | <0.0000141 |
| Landing | <0.0000141 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolli, A.; Mina, G.G.; De Nuzzo, D.; Bortoletti, I.; Gambalunga, A.; Martinelli, A.; Pasqualato, F.; Cacciavillani, M.; Carrieri, M.; Trevisan, A. Unusual Domestic Source of Lead Poisoning. Int. J. Environ. Res. Public Health 2020, 17, 4374. https://doi.org/10.3390/ijerph17124374
Nicolli A, Mina GG, De Nuzzo D, Bortoletti I, Gambalunga A, Martinelli A, Pasqualato F, Cacciavillani M, Carrieri M, Trevisan A. Unusual Domestic Source of Lead Poisoning. International Journal of Environmental Research and Public Health. 2020; 17(12):4374. https://doi.org/10.3390/ijerph17124374
Chicago/Turabian StyleNicolli, Annamaria, Grazia Genga Mina, Davide De Nuzzo, Isabella Bortoletti, Alberto Gambalunga, Andrea Martinelli, Fabiola Pasqualato, Mario Cacciavillani, Mariella Carrieri, and Andrea Trevisan. 2020. "Unusual Domestic Source of Lead Poisoning" International Journal of Environmental Research and Public Health 17, no. 12: 4374. https://doi.org/10.3390/ijerph17124374
APA StyleNicolli, A., Mina, G. G., De Nuzzo, D., Bortoletti, I., Gambalunga, A., Martinelli, A., Pasqualato, F., Cacciavillani, M., Carrieri, M., & Trevisan, A. (2020). Unusual Domestic Source of Lead Poisoning. International Journal of Environmental Research and Public Health, 17(12), 4374. https://doi.org/10.3390/ijerph17124374






