Effects of the 5-m Shuttle Run Test on Markers of Muscle Damage, Inflammation, and Fatigue in Healthy Male Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. 5-m Shuttle Run Test
- BD (m) = the greatest distance covered during a 30-s shuttle,
- TD (m) = total distance covered during the six 30-s shuttles,
2.4. Blood Analyses
2.5. Statistical Analysis
3. Results
3.1. Reproducibility of Measurement between Test-Retest
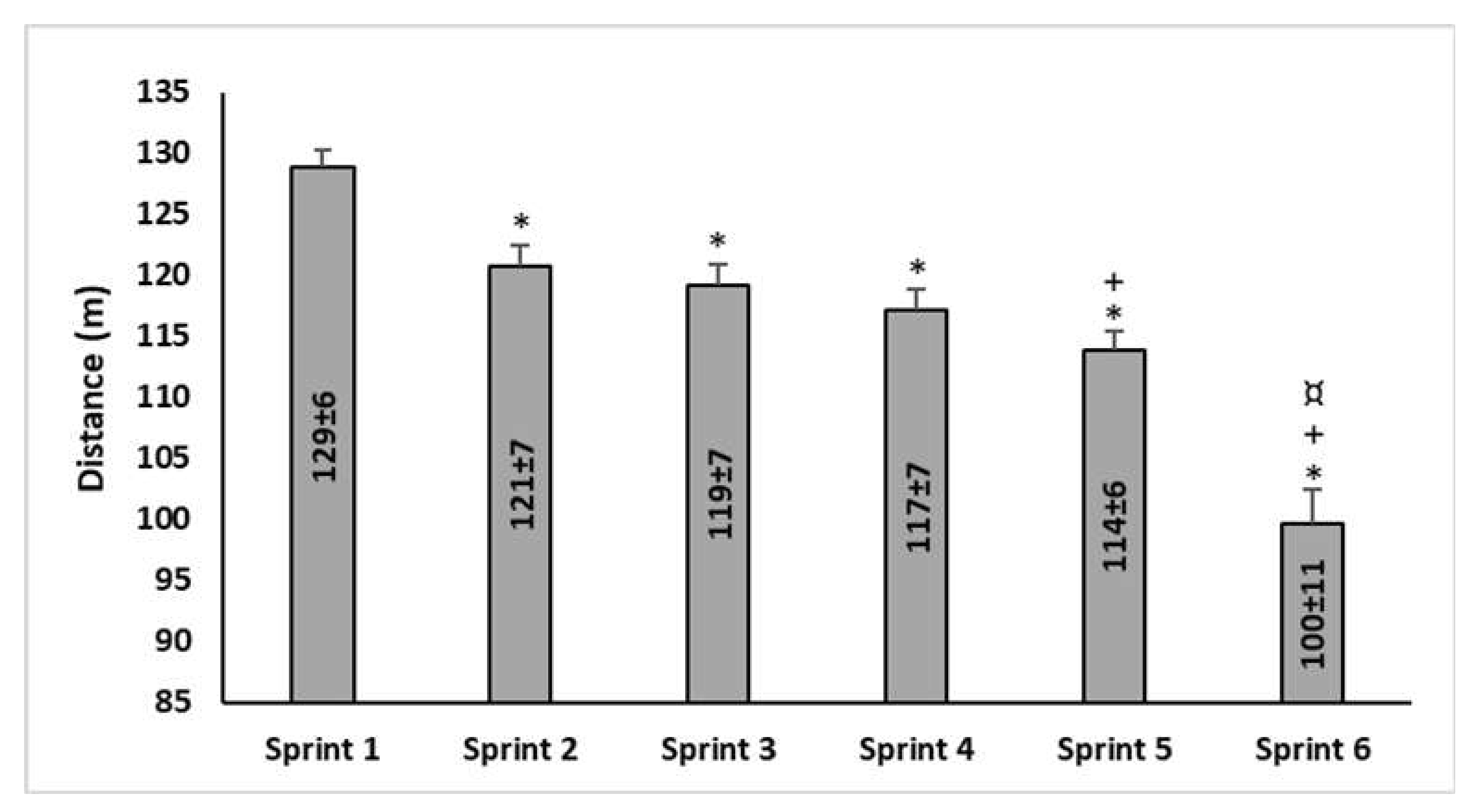
3.2. The 5-m Shuttle Run Test
3.3. Biochemical Parameters
3.4. Delayed Onset Muscle Soreness
3.5. Rating of Perceived Exertion Scale
3.6. Perceived Recovery Status Scale
3.7. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eryılmaz, S.K.; Aslankeser, Z.; Özdemir, Ç.; Özgünen, K.; Kurdak, S. The effect of 30-m repeated sprint exercise on muscle damage indicators, serum insulin-like growth factor-land cortisol. Biomed. Hum. Kinet. 2019, 11, 151–157. [Google Scholar] [CrossRef]
- Bishop, D.J.; Girard, O.; Mendez-Villanueva, A. Repeated-Sprint Ability—Part II. Sports Med. 2011, 41, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, M.H.W. Reliability and Validity of the Welsh Rugby Union Shuttle Run Test. Unpublished BSc Dissertation, University of Wales Institute Cardiff, Cardiff, Wales, UK, 1997. [Google Scholar]
- Boddington, M.K.; Lambert, M.; Gibson, A.S.C.; Noakes, T.D. Reliability of a 5-m multiple shuttle test. J. Sports Sci. 2001, 19, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Boddington, M.K.; I Lambert, M.; Waldeck, M.R. Validity of a 5-meter multiple shuttle run test for assessing fitness of women field hockey players. J. Strength Cond. Res. 2004, 18, 97–100. [Google Scholar]
- Durandt, J.; Tee, J.; Prim, S.K.; Lambert, M. Physical Fitness Components Associated with Performance in a Multiple-Sprint Test. Int. J. Sports Physiol. Perform. 2006, 1, 150–160. [Google Scholar] [CrossRef]
- Reilly, T.; Borrie, A. Physiology Applied to Field Hockey. Sports Med. 1992, 14, 10–26. [Google Scholar] [CrossRef]
- Duthie, G.M.; Pyne, D.; Hooper, S. Applied physiology and game analysis of rugby union. Sports Med. 2003, 33, 973–991. [Google Scholar] [CrossRef]
- Reilly, T.; Gilbourne, D. Science and football: A review of applied research in the football codes. J. Sports Sci. 2003, 21, 693–705. [Google Scholar] [CrossRef]
- Ammar, A.; Chtourou, H.; Trabelsi, K.; Padulo, J.; Turki, M.; El Abed, K.; Hoekelmann, A.; Hakim, A. Temporal specificity of training: Intra-day effects on biochemical responses and Olympic-Weightlifting performances. J. Sports Sci. 2014, 33, 358–368. [Google Scholar] [CrossRef]
- Ammar, A.; Chtourou, H.; Hammouda, O.; Turki, M.; Ayedi, F.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Souissi, N. Relationship between biomarkers of muscle damage and redox status in response to a weightlifting training session: Effect of time-of-day. Acta Physiol. Hung. 2016, 103, 243–261. [Google Scholar] [CrossRef]
- Ammar, A.; Chtourou, H.; Souissi, N. Effect of Time-of-Day on Biochemical Markers in Response to Physical Exercise. J. Strength Cond. Res. 2017, 31, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med Bull. 2007, 81, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Maffulli, N.; Buonauro, R.; Limongelli, F.M. Serum Enzyme Monitoring in Sports Medicine. Clin. Sports Med. 2008, 27, 1–18. [Google Scholar] [CrossRef]
- Baker, J.S.; Bailey, D.M.; Hullin, D.; Young, I.; Davies, B. Metabolic implications of resistive force selection for oxidative stress and markers of muscle damage during 30 s of high-intensity exercise. Eur. J. Appl. Physiol. 2004, 92, 321–327. [Google Scholar] [CrossRef]
- Hammouda, O.; Chtourou, H.; Chahed, H.; Ferchichi, S.; Chaouachi, A.; Kallel, C.; Miled, A.; Chamari, K.; Souissi, N. High intensity exercise affects diurnal variation of some biological markers in trained subjects. Int. J. Sports Med. 2012, 33, 886–891. [Google Scholar] [CrossRef]
- Hammouda, O.; Chtourou, H.; Chaouachi, A.; Chahed, H.; Ferchichi, S.; Kallel, C.; Chamari, K.; Souissi, N. Effect of Short-Term Maximal Exercise on Biochemical Markers of Muscle Damage, Total Antioxidant Status, and Homocysteine Levels in Football Players. Asian J. Sports Med. 2012, 3, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Twist, C.; Eston, R. The effects of exercise-induced muscle damage on maximal intensity intermittent exercise performance. Eur. J. Appl. Physiol. 2005, 94, 652–658. [Google Scholar] [CrossRef]
- Howatson, G.; Milak, A. Exercise-Induced Muscle Damage Following a Bout of Sport Specific Repeated Sprints. J. Strength Cond. Res. 2009, 23, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, O.; Chtourou, H.; Chahed, H.; Ferchichi, S.; Kallel, C.; Miled, A.; Chamari, K.; Souissi, N. Diurnal Variations of Plasma Homocysteine, Total Antioxidant Status, and Biological Markers of Muscle Injury During Repeated Sprint: Effect on Performance and Muscle Fatigue—A Pilot Study. Chrono- Int. 2011, 28, 958–967. [Google Scholar] [CrossRef]
- Hammouda, O.; Chtourou, H.; Chaouachi, A.; Chahed, H.; Zarrouk, N.; Miled, A.; Chamari, K.; Souissi, N. Biochemical Responses to Level-1 Yo-Yo Intermittent Recovery Test in Young Tunisian Football Players. Asian J. Sports Med. 2012, 4, 23–28. [Google Scholar] [CrossRef]
- Woolley, B.P.; Jakeman, J.R.; Faulkner, J.A. Multiple Sprint Exercise with a Short Deceleration Induces Muscle Damage and Performance Impairment in Young, Physically Active Males. J. Athl. Enhanc. 2014, 3, 2. [Google Scholar] [CrossRef]
- Keane, K.M.; Salicki, R.; Goodall, S.; Thomas, K.; Howatson, G. Muscle Damage Response in Female Collegiate Athletes After Repeated Sprint Activity. J. Strength Cond. Res. 2015, 29, 2802–2807. [Google Scholar] [CrossRef]
- Cheikh, M.; Makhlouf, K.; Ghattassi, K.; Graja, A.; Ferchichi, S.; Kallel, C.; Houda, M.; Souissi, N.; Hammouda, O. Melatonin ingestion after exhaustive late-evening exercise attenuate muscle damage, oxidative stress, and inflammation during intense short term effort in the following day in teenage athletes. Chronobiol. Int. 2020, 37, 236–247. [Google Scholar] [CrossRef] [PubMed]
- De Hoyo, M.; Cohen, D.D.; Sañudo, B.; Carrasco, L.; Álvarez-Mesa, A.; Del Ojo, J.J.; Dominguez-Cobo, S.; Manas, V.; Otero-Esquina, C. Influence of football match time–motion parameters on recovery time course of muscle damage and jump ability. J. Sports Sci. 2016, 34, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Oxendale, C.L.; Twist, C.; Daniels, M.; Highton, J. The relationship between match-play characteristics of elite rugby league and indirect markers of muscle damage. Int. J. Sports Physiol. Perform. 2016, 11, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Fransson, D.; Vigh-Larsen, J.F.; Fatouros, I.G.; Krustrup, P.; Mohr, M. Fatigue Responses in Various Muscle Groups in Well-Trained Competitive Male Players after a Simulated Soccer Game. J. Hum. Kinet. 2018, 61, 85–97. [Google Scholar] [CrossRef]
- Tomazin, K.; Morin, J.B.; Millet, G.Y. Neuromuscular fatigue etiology after repeated sprints depends on exercise modality. Int J. Sports Physiol. Perform. 2016, 5, 1–28. [Google Scholar] [CrossRef]
- Bogdanis, G.C.; E Nevill, M.; Boobis, L.H.; Lakomy, H.K. Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J. Appl. Physiol. 1996, 80, 876–884. [Google Scholar] [CrossRef]
- Bogdanis, G.C.; Nevill, M.E.; Lakomy, H.K.A.; Boobis, L.H. Power output and muscle metabolism during and following recovery from 10 and 20 s of maximal sprint exercise in humans. Acta Physiol. Scand. 1998, 163, 261–272. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal Muscle Fatigue: Cellular Mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef]
- Stewart, D.; Farina, D.; Shen, C.; Macaluso, A. Muscle fibre conduction velocity during a 30-s Wingate anaerobic test. J. Electromyogr. Kinesiol. 2011, 21, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Ascensão, A.; Rebelo, A.; Oliveira, E.; Marques, F.; Pereira, L.; Magalhães, J. Biochemical impact of a soccer match—analysis of oxidative stress and muscle damage markers throughout recovery. Clin. Biochem. 2008, 41, 841–851. [Google Scholar] [CrossRef]
- McLellan, C.P.; Lovell, D.I.; Gass, G.C. Markers of Postmatch Fatigue in Professional Rugby League Players. J. Strength Cond. Res. 2011, 25, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.D.; Gabbett, T.J.; Jenkins, D.G. Influence of an intensified competition on fatigue and match performance in junior rugby league players. J. Sci. Med. Sport 2013, 16, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Glaister, M.; Howatson, G.; Pattison, J.R.; McInnes, G. The Reliability and Validity of Fatigue Measures During Multiple-Sprint Work: An Issue Revisited. J. Strength Cond. Res. 2008, 22, 1597–1601. [Google Scholar] [CrossRef]
- Boukhris, O.; Abdessalem, R.; Ammar, A.; Hsouna, H.; Trabelsi, K.; Engel, F.A.; Sperlich, B.; Hill, D.W.; Chtourou, H. Nap Opportunity During the Daytime Affects Performance and Perceived Exertion in 5-m Shuttle Run Test. Front. Physiol. 2019, 10, 779. [Google Scholar] [CrossRef]
- Boukhris, O.; Hsouna, H.; Chtourou, L.; Abdesalem, R.; BenSalem, S.; Tahri, N.; Trabelsi, K.; Stannard, S.R.; Chtourou, H. Effect of Ramadan fasting on feelings, dietary intake, rating of perceived exertion and repeated high intensity short-term maximal performance. Chronobiol. Int. 2019, 36, 1–10. [Google Scholar] [CrossRef]
- Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Lichstein, K.L.; Morin, C.M. Recommendations for a standard research assessment of insomnia. Sleep 2006, 29, 1155–1173. [Google Scholar] [CrossRef]
- Bougard, C.; Moussay, S.; Gauthier, A.; Espie, S.; Davenne, D. Effects of waking time and breakfast intake prior to evaluation of psychomotor performance in the early morning. Chronobiol. Int. 2009, 26, 324–336. [Google Scholar] [CrossRef]
- Thorpe, R.; Sunderland, C. Muscle Damage, Endocrine, and Immune Marker Response to a Soccer Match. J. Strength Cond. Res. 2012, 26, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.M.; Green, J.M.; A Bishop, P.; Sjökvist, J.; E Schumacker, R.; Richardson, M.T.; Curtner-Smith, M. A Practical Approach to Monitoring Recovery: Development of a Perceived Recovery Status Scale. J. Strength Cond. Res. 2011, 25, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 2. [Google Scholar]
- Grazioli, R.; Lopez, P.; Machado, C.L.F.; Farinha, J.B.; Fagundes, A.D.O.; Voser, R.; Reischak-Oliveira, Á.; Setuain, I.; Izquierdo, M.; Pinto, R.S.; et al. Moderate volume of sprint bouts does not induce muscle damage in well-trained athletes. J. Bodyw. Mov. Ther. 2019, 24, 206–211. [Google Scholar] [CrossRef]
- Kingsley, M.I.; Wadsworth, D.; Kilduff, L.P.; McEneny, J.; Benton, D. Effects of Phosphatidylserine on Oxidative Stress following Intermittent Running. Med. Sci. Sports Exerc. 2005, 37, 1300–1306. [Google Scholar] [CrossRef]
- Deminice, R.; Trindade, C.S.; DeGiovanni, G.C.; Garlip, M.R.; Portari, G.V.; Teixeira, M.; A Jordao, A. Oxidative stress biomarkers response to high intensity interval training and relation to performance in competitive swimmers. J. sports Med. Phys. Fit. 2010, 50, 356. [Google Scholar]
- Semple, S.J. C-reactive protein-biological functions, cardiovascular disease and physical exercise. S. Afr. J. Sports Med. 2006, 18, 24–28. [Google Scholar] [CrossRef]
- Chatzinikolaou, A.; Fatouros, I.G.; Gourgoulis, V.; Avloniti, A.; Jamurtas, A.Z.; Nikolaidis, M.G.; Douroudos, I.; Michailidis, Y.; Beneka, A.; Malliou, P.; et al. Time Course of Changes in Performance and Inflammatory Responses After Acute Plyometric Exercise. J. Strength Cond. Res. 2010, 24, 1389–1398. [Google Scholar] [CrossRef]
- Mendham, A.E.; Donges, C.E.; Liberts, E.A.; Duffield, R. Effects of mode and intensity on the acute exercise-induced IL-6 and CRP responses in a sedentary, overweight population. Eur. J. Appl. Physiol. 2011, 111, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Nicholas, C.W.; Williams, C. Muscular soreness following prolonged intermittent high-intensity shuttle running. J. Sports Sci. 1999, 17, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Chang, W.-Y.; Chan, K.-H.; Tsai, W.-Y.; Lin, C.-L.; Hsu, M.-C. Blood Lipid Peroxides and Muscle Damage Increased following Intensive Resistance Training of Female Weightlifters. Ann. New York Acad. Sci. 2005, 1042, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.; Hindorf, U.; Persson, P.; Bengtsson, T.; Malmqvist, U.; Werkström, V.; Ekelund, M. Muscular exercise can cause highly pathological liver function tests in healthy men. Br. J. Clin. Pharmacol. 2008, 65, 253–259. [Google Scholar] [CrossRef]
- Cuevas, M.J.; Almar, M.; García-Glez, J.C.; García-López, D.; De Paz, J.A.; Alvear-Órdenes, I.; González-Gallego, J. Changes in oxidative stress markers and NF-κB activation induced by sprint exercise. Free Radic. Res. 2005, 39, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Chiboub, J.; Turki, M.; Abdelkarim, O.; El Abed, K.; Ben Ali, M.; Hoekelmann, A.; et al. Acute and delayed responses of C-reactive protein, malondialdehyde and antioxidant markers after resistance training session in elite weightlifters: Effect of time of day. Chronobiol. Int. 2015, 32, 1211–1222. [Google Scholar] [CrossRef]
- Glaister, M.; Stone, M.H.; Stewart, A.M.; Hughes, M.; Moir, G.L. The reliability and validity of fatigue measures during short-duration maximal-intensity intermittent cycling. J. Strength. Cond. Res. 2004, 18, 459–462. [Google Scholar]

| Parameters | before | 5 Min after | 72 h after | % of Increase between after and before | % of Decrease between 5 Min after and 72 h after the 5mSRT | % of Decrease between before and 72 h after the 5mSRT | ANOVA | p Value | Effect Size |
|---|---|---|---|---|---|---|---|---|---|
| CK (IU/L) | 190.6 ± 109.1 | 234.6 ± 113.7 # | 125.3 ± 80.5 #¤ | 21.3% | −46.1% | −29.3% | Test = 28.13 | <0.0005 | 0.93 |
| LDH (IU/L) | 163.6 ± 35.1 | 209.9 ± 50.8 # | 143.9 ± 36.6 #¤ | 21.0% | −30.9% | −12.2% | F = 47.13 | <0.0005 | 0.77 |
| ASAT (IU/L) | 18.0 ± 4.4 | 21.7 ± 6.2 # | 15.0 ± 4.7 #¤ | 16.2% | −30.4% | −16.4% | F = 44.46 | <0.0005 | 0.76 |
| ALAT (IU/L) | 10.2 ± 3.4 | 12.7 ± 3.8 # | 8.6 ± 2.4 #¤ | 19.4% | −30.9% | −13.0% | F = 36.09 | <0.0005 | 0.72 |
| CRP (mg/L) | 2.1 ± 2.5 | 2.8 ± 3.3 # | 1.4 ± 2.3 #¤ | 25.8% | −46.8% | −27.9% | Test = 26.77 | <0.0005 | 0.89 |
| Parameters | Before the 5mSRT | 5 min after the 5mSRT | 72 h after the 5mSRT |
|---|---|---|---|
| DOMS (AU) | 2.4 ± 1.0 | 6.7 ± 1.1 # | 1.9 ± 0.7 #¤ |
| End RPE (AU) | 2.1 ± 0.6 | 8.1 ± 0.6 # | 1.5 ± 0.6 #¤ |
| Mean RPE(AU) | 2.1 ± 0.6 | 5.6 ± 0.6 # | 1.5 ± 0.6 #¤ |
| PRS (AU) | 6 ± 1 | 3 ± 1 # | 7 ± 1 #¤ |
| Acute Responses | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FI (%) | TD (m) | BD (m) | ASAT (%) | ALAT (%) | CK (%) | LDH (%) | CRP (%) | DOMS (%) | PRS (%) | RPE (%) | ||
| PD (%) | r | 0.92 | 0.82 | 0.78 | 0.74 | 0.73 | 0.71 | 0.73 | 1 | 0.84 | 0.87 | 0.76 |
| p | p < 0.0005 | p < 0.0005 | p = 0.001 | p = 0.002 | p = 0.002 | p = 0.003 | p = 0.002 | p < 0.0005 | p < 0.0005 | p < 0.0005 | p = 0.001 | |
| FI (%) | r | - | 0.77 | 0.77 | 0.67 | 0.73 | 0.78 | 0.69 | 0.93 | 0.85 | 0.86 | 0.86 |
| p | - | p = 0.001 | p = 0.001 | p = 0.006 | p = 0.002 | p = 0.001 | p = 0.004 | p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | |
| TD (m) | r | - | - | 0.95 | 0.94 | 0.97 | 0.91 | 0.95 | 0.90 | 0.97 | 0.87 | 0.78 |
| p | - | - | p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | p = 0.001 | |
| BD (m) | r | - | - | - | 0.96 | 0.95 | 0.85 | 0.97 | 0.88 | 0.92 | 0.91 | 0.81 |
| p | - | - | - | p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | |
| Delayed Responses | ||||||||||||
| FI (%) | TD (m) | BD (m) | ASAT (%) | ALAT (%) | CK (%) | LDH (%) | CRP (%) | DOMS (%) | PRS (%) | RPE (%) | ||
| PD (%) | r | - | - | - | 0.67 | 0.57 | 0.96 | 0.64 | 0.75 | 0.90 | 0.69 | 0.61 |
| p | - | - | - | p = 0.005 | p = 0.02 | p < 0.0005 | p = 0.009 | p = 0.001 | p < 0.0005 | p = 0.004 | p = 0.01 | |
| FI (%) | r | - | - | - | 0.71 | 0.57 | 0.81 | 0.64 | 0.58 | 0.61 | 0.64 | 0.66 |
| p | - | - | - | p = 0.003 | p = 0.02 | p < 0.0005 | p = 0.009 | p = 0.02 | p = 0.01 | p = 0.009 | p = 0.007 | |
| TD (m) | r | - | - | - | 0.65 | 0.63 | 0.97 | 0.67 | 0.61 | 0.62 | 0.57 | 0.70 |
| p | - | - | - | p = 0.008 | p = 0.01 | p < 0.0005 | p = 0.006 | p = 0.01 | p = 0.01 | p = 0.02 | p = 0.003 | |
| BD (m) | r | - | - | - | 0.74 | 0.66 | 0.90 | 0.85 | 0.71 | 0.67 | 0.63 | 0.70 |
| p | - | - | - | p = 0.001 | p = 0.007 | p < 0.0005 | p < 0.0005 | p = 0.003 | p = 0.006 | p = 0.01 | p = 0.003 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukhris, O.; Trabelsi, K.; Abdessalem, R.; Hsouna, H.; Ammar, A.; Glenn, J.M.; Bott, N.; Irandoust, K.; Taheri, M.; Turki, M.; et al. Effects of the 5-m Shuttle Run Test on Markers of Muscle Damage, Inflammation, and Fatigue in Healthy Male Athletes. Int. J. Environ. Res. Public Health 2020, 17, 4375. https://doi.org/10.3390/ijerph17124375
Boukhris O, Trabelsi K, Abdessalem R, Hsouna H, Ammar A, Glenn JM, Bott N, Irandoust K, Taheri M, Turki M, et al. Effects of the 5-m Shuttle Run Test on Markers of Muscle Damage, Inflammation, and Fatigue in Healthy Male Athletes. International Journal of Environmental Research and Public Health. 2020; 17(12):4375. https://doi.org/10.3390/ijerph17124375
Chicago/Turabian StyleBoukhris, Omar, Khaled Trabelsi, Raouf Abdessalem, Hsen Hsouna, Achraf Ammar, Jordan M. Glenn, Nick Bott, Khadijah Irandoust, Morteza Taheri, Mouna Turki, and et al. 2020. "Effects of the 5-m Shuttle Run Test on Markers of Muscle Damage, Inflammation, and Fatigue in Healthy Male Athletes" International Journal of Environmental Research and Public Health 17, no. 12: 4375. https://doi.org/10.3390/ijerph17124375
APA StyleBoukhris, O., Trabelsi, K., Abdessalem, R., Hsouna, H., Ammar, A., Glenn, J. M., Bott, N., Irandoust, K., Taheri, M., Turki, M., Ayadi, F., Bragazzi, N. L., Engel, F. A., & Chtourou, H. (2020). Effects of the 5-m Shuttle Run Test on Markers of Muscle Damage, Inflammation, and Fatigue in Healthy Male Athletes. International Journal of Environmental Research and Public Health, 17(12), 4375. https://doi.org/10.3390/ijerph17124375









