Abstract
The French national public health agency (Santé publique France) has used data from the national health insurance reimbursement system (SNDS) to identify medicalised acute gastroenteritis (mAGE) for more than 10 years. This paper presents the method developed to evaluate this system: performance and characteristics of the discriminatory algorithm, portability in mainland and overseas French departments, and verification of the mAGE database updating process. Pharmacy surveys with certified mAGE from 2012 to 2015 were used to characterise mAGE and to estimate the sensitivity and predictive positive value (PPV) of the algorithm. Prescription characteristics from these pharmacy surveys and from 2014 SNDS prescriptions in six mainland and overseas departments were compared. The sensitivity (0.90) and PPV (0.82) did not vary according to the age of the population or year. Prescription characteristics were similar within all studied departments. This confirms that the algorithm can be used in all French departments, for both paediatric and adult populations, with stability and durability over time. The algorithm can identify mAGE cases at a municipal level. The validated system has been implemented in a national waterborne disease outbreaks surveillance system since 2019 with the aim of improving the prevention of infectious disease risk attributable to localised tap water systems.
1. Introduction
Syndromic surveillance is defined by the Center for Disease Control (CDC) as an investigational approach where an existing automated data acquisition system is used for early outbreak detection or for the monitoring of disease indicators in real time or near real time [1]. Berger et al. described all the data sources that can be used for waterborne surveillance [2]. Drug sales have been used in many countries to detect acute gastroenteritis (AGE) outbreaks. Previous studies were either retrospective or predictive and analysed over-the-counter or prescription drug sales [3,4]. AGE is a very common syndrome typically defined as diarrhoea (three or more loose stools in 24 h) and/or vomiting [5]. AGE is used in epidemiology as a generic indicator for infections arising from faecal pathogens. It also provides good reactivity to environmental triggers (i.e., waterborne infectious disease), as AGE has a short incubation period. France has an extensive medical and administrative information system, which covers since 2016 the entire population living in France [6]. This drug reimbursement database, the “Système national des données de santé” (SNDS), collects information on prescriptions, patients, practitioners, and pharmacies from the National Health Insurance reimbursements database. Natural products or the over-the-counter drugs could be used for AGE treatment [7]; as they are not reimbursed, they are not taken into account in the SNDS database. In France, on average, 33% of AGE cases consult a general practitioner (GP) and almost all patients with medical consultation for AGE (medicalised AGE, mAGE) receive a treatment with reimbursed drugs (91%). Over-the-counter drugs are less used than reimbursed drugs with 8.9% and 30.9% of AGE cases, respectively [8].
Since 2007, the French national public health agency (Santé publique France) has been developing syndromic surveillance of mAGE based on the SNDS. A discriminating algorithm was developed to identify mAGE from the SNDS [9]. Data from the SNDS provide a daily exhaustive count of mAGE at the municipal level. The spatiotemporal characteristics of the mAGE indicator (day and municipality) are particularly suitable for the prevention of localised infectious disease risk attributable to tap water [10,11]. An automated detection system of waterborne disease outbreaks based on data from the SNDS and this algorithm has thus been deployed in France since 2019.
The aim of this paper is (i) to evaluate the mAGE discriminatory algorithm and its characteristics using independent data collected in pharmacy surveys; (ii) to compare the mAGE drug prescriptions obtained by using the algorithm on SNDS data from six French departments (mainland and overseas); and (iii) to describe how the updating process of the mAGE database is routinely checked at a national level for the most critical variables used in the algorithm.
2. Materials and Methods
2.1. Description of the mAGE Discriminatory Algorithm
The original algorithm was created based on a sample of GP prescriptions collected in pharmacies in 2000 and 2006, with associated medical diagnosis [9]. This sample was used to define relevant rules to differentiate mAGE prescriptions and non-mAGE prescriptions (intended for patients suffering from another disease). Characteristics included were the lag time between the prescription date and the day of delivery, the occurrence of non-mAGE specific drugs (non-mAGE drugs), mAGE specific drug combinations (mAGE drugs), and the duration of the treatment estimated from the amount of drugs prescribed (Figure 1). The algorithm criteria depend on the number of therapeutic classes for reimbursed mAGE drugs present on the prescription. These classes are intestinal absorbents, antipropulsives, antiemetics and antispasmodics, and rehydration salts. The algorithm is applied on a subset of prescriptions from the SNDS containing at least one of these mAGE drugs. Antispasmodics prescribed alone are excluded as they are widely prescribed. This reduces the volume of the subset of prescriptions without impairing the sensitivity for identifying mAGE. The list of non-mAGE drugs comprises specific drugs to treat other common digestive pathologies and drugs whose side effects include diarrhoea or vomiting [9]. Changes to the lists of mAGE and non-mAGE medications due to, for example, termination of reimbursement and removal from the market, could impact the performance of the algorithm. The impact is low if changes concern a medication inside a therapeutic class with drugs still reimbursed, as the reimbursed drugs will be prescribed instead. In the case of a new medication, the impact will be greater because these prescriptions would not be identified by the algorithm and extracted from SNDS. To address such risks, an update of the medications lists is completed annually, using the French medications database [12]. From 2011 to 2017, no major changes occurred in the mAGE therapeutic classes of drugs.
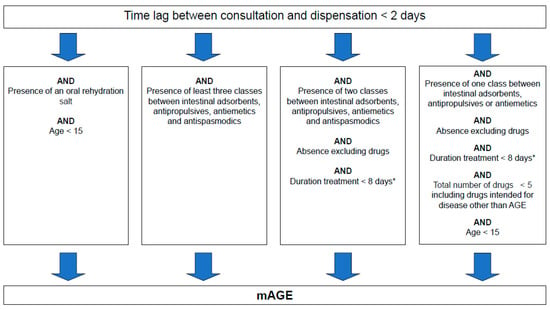
Figure 1.
Algorithm used for medicalised acute gastroenteritis (mAGE) case discrimination based on drug reimbursement data. * Estimated from both the content and the number of the boxes dispensed of any mAGE drugs.
A mAGE case is defined as an individual identified through the SNDS as having a prescription containing at least one reimbursed mAGE drug recognised by the discriminatory algorithm (Figure 1 and Figure 2).
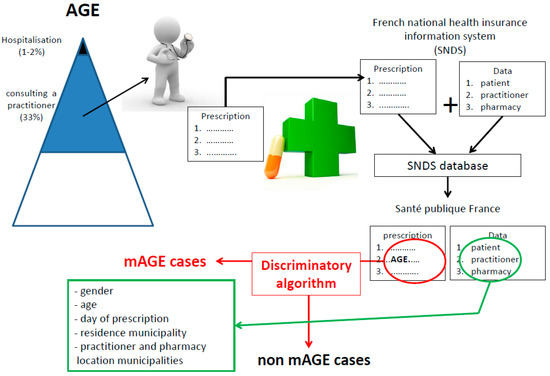
Figure 2.
Process and information collected for mAGE from the SNDS database (French national health insurance reimbursement system).
The algorithm also analyses the location of patient residence, GP, and dispensing pharmacy to distinguish resident from non-resident cases. A patient is considered a non-resident if they consulted a GP located more than 50 km from the place of residence. The municipality of the dispensing pharmacy is used when the information is not available for the GP.
The SNDS reimbursement database is updated monthly and is considered exhaustive at four months after prescription date, as 80% of mAGE prescriptions are identified at one month, 99% at two months, and 99.4% at three months [13]. Santé publique France currently updates its mAGE database every two months and has a 10-year history of mAGE cases.
2.2. Evaluation of the mAGE Discriminatory Algorithm: Pharmacy Surveys
GP prescription samples were collected annually, using the same method, from January to June, in two pharmacies in 2012 and in one pharmacy from 2013 to 2015 in the same geographical area (Seine Maritime department). Patients were enrolled if their prescription contained one of the defined mAGE drugs, except antispasmodics prescribed alone. All participants gave verbal consent. Participants were asked about their treatments, clinical symptoms, and medical diagnosis. Data collected from the prescription form included dates of medical examination and drug dispensation, drug names, and the number of boxes for each drug. Participants’ reported medical diagnoses were compared to diagnoses obtained from the algorithm applied to the prescription samples.
The performance of the algorithm was evaluated in terms of sensitivity and positive predictive value (PPV). Sensitivity evaluates the ability of the algorithm to correctly detect mAGE cases, and PPV is the probability that identified mAGE are true mAGE cases.
We evaluated the stability of the sensitivity and the PPV over time (2012–2015) and by age group (1–4 years old, 5–15 years old, and more than 15 years old), as well as the temporal stability of prescription characteristics (average number of therapeutic classes). The homogeneity of sensitivity and PPV across time and age classes was tested first by a global test for equality. When rejected (p < 0.05), two by two tests were performed using Holm correction for multi-testing. All analysis was performed on R version 3.4.3.
2.3. Comparison of mAGE Characteristics of Drug Prescription in Six French Departments
To evaluate the portability of the mAGE algorithm to different geographical areas, we reviewed the prescription characteristics in six (of 101) French departments: two mainland departments in the north and the south of France (Seine Maritime and Hérault) and four overseas departments (Martinique, Guadeloupe, Ile de la Réunion, and Guyane). The prescriptions extracted from the SNDS database from these departments were those dispensed in 2014 containing at least one mAGE drug (except antispasmodics). The mAGE algorithm was then applied and the mAGE and non-mAGE characteristics were studied for each department: mean number of drugs per prescription, lag time between the prescription date and the delivery day, percentage of mAGE cases, and the average number and frequency of the therapeutic classes on mAGE prescriptions.
2.4. Routine Check of mAGE Database Updating Process
Sante publique France updates the mAGE database every two months. Checks are performed on different variables to detect discrepancies or suspect changes in the rate of missing data, with a focus on location variables (the prescription is disregarded if the patient’s residence or the location of GP or dispensing pharmacy are not exploitable) and on the age of the patient (which if missing prevents the algorithm from processing the prescription). Patient, GP, and pharmacist locations are essential data for localised cluster detection as waterborne outbreak. Bias can occur in the use of mAGE for epidemiological purposes at a municipality geographical level if we do not distinguish non-resident from resident patients for mAGE. Data for routine checks from 2011 to 2017 at a national level are presented for these critical variables.
3. Results
3.1. Pharmacy Surveys
During the annual pharmacy surveys from 2012 to 2015, 1308 prescriptions were collected, including 728 mAGE (55%) and 580 non-mAGE (Table 1). These prescriptions were prescribed by 330 different GPs in the Seine Maritime department. Non-mAGE prescriptions covered a large set of situations: stockpile of medicines, prevention of drug side effects, gastroenteritic diseases including but not limited to abdominal pain, constipation, gastroesophageal reflux, or non gastroenteritic diseases such as flu, stress, and headache.

Table 1.
Characteristics of Pharmacy survey prescriptions (n = 1308) and sensitivity, positive predictive value of the mAGE algorithm evaluated for stability over time and on age (n = 1178, no data for 2015).
One-drug treatment was used in 8.1% of mAGE cases. Antipropulsives and antiemetics were the most used medications in case of a one-drug treatment. A treatment with two drugs was used in 45.6% of mAGE cases, including mostly antispasmodic, antiemetic, and antipropulsive drugs. A treatment with three drugs was used in 40.8% of mAGE cases, including mostly antiemetics, antispasmodics, and antipropulsives drugs. Intestinal absorbents and rehydration salts were not frequently used in the two-drug or three-drug treatments. A treatment with four drugs was rarely used (5.7%, data not shown). Almost 89% of mAGE cases purchased their drugs the day of consultation vs. 63% for other diagnoses.
A mAGE case prescription covered an average of 2.4 therapeutic classes of reimbursed drugs used to treat AGE (stable over the study period and for age groups) vs. 1.3 for non-mAGE.
The overall sensitivity and PPV of the mAGE algorithm were estimated to be 0.90 and 0.82, respectively. The annual sensitivity of the algorithm from 2012 to 2015 varied from 0.88 to 0.91, without significant differences. The annual PPV varied from 0.81 to 0.86, also without significant differences over the study period (Table 1). No difference was identified in the characteristics of prescription samples collected each year (number of prescribed drugs, frequency of class prescription, and lag time between prescription and dispensation (data not shown)).
Depending on the age group, the sensitivity varied from 0.87 to 0.96 and the PPV from 0.79 to 0.87. Global equality of the sensitivity according to age was rejected (p < 0.01). The sensitivity was significantly different (p < 0.05) between the 5–15-year-old and the >15-year-old age classes. No significant difference of PPV was identified between the three age groups.
3.2. Characteristics of mAGE and Non-mAGE Case Prescriptions from the SNDS across Six French Departments
More than 1 million prescriptions were extracted from the SNDS in 2014 for the six departments included in the study (Table 2). Around 45% were for mAGE, with an average of 2.2 prescribed drugs. The delay between prescription date and dispensation day was comparable for the two mainland departments, with approximately 92% of mAGE cases filling the prescription on the same day versus 68% (average delay of 3.6 days) for non-mAGE cases. The delays were similar in overseas departments with more than 93% of prescription and delivery on the same day for mAGE versus 75% (average of 2.7 days) for non-mAGE. The average number of therapeutic classes was similar across all departments for mAGE (2.1 to 2.3) and non-mAGE (1.2 to 1.3). These values were close to values obtained from the pharmacy surveys, which identified 55.6% of prescriptions for mAGE with an average of 2.4 prescribed drugs, and 89% of mAGE cases’ prescriptions dispensed on the same day as GP visit (Table 1).

Table 2.
Characteristics of medicalised acute gastroenteritis (mAGE) and non-medicalised AGE (non-mAGE) prescriptions in 2014 (1,058,323 prescriptions from the SNDS).
The most prescribed therapeutic classes were also similar between the SNDS analysis in six departments and the pharmacy surveys with a prescription frequency depending on department. The drug classes most commonly used were the same: antiemetics, with an average prescription frequency of 65.5% and antipropulsives with 59.8%, vs. 73.3% and 77.2%, respectively, in pharmacy surveys. Antispasmodics were found on 55.1% of prescriptions and adsorbents on 30.3% of the prescriptions vs. 54.3% and 25.6%, respectively, in pharmacy surveys. The prescription of rehydration salts varied to a greater degree by department, with the highest use in Guyane (17.6% vs. 10% for others). These similar results across departments seem to confirm the relevance of the algorithm for use in overseas departments.
3.3. Routine Check of mAGE Database Updating Process
Between 2011 and 2017, 13,300,000 to 17,100,000 prescriptions containing at least one drug prescribed for AGE were extracted yearly from the SNDS for all 101 French departments (Table 3). The algorithm was run on almost all the extracted prescriptions, as the prescription and dispensation dates were always completed in the SNDS, and the proportion of missing data for patient age decreased from 0.2% to 0 over the study period. The proportion of prescriptions for mAGE ranged from 36% to 45%, depending on the year.

Table 3.
Number of prescriptions extracted from the reimbursement database (SNDS) and classified as related to medicalised acute gastroenteritis (mAGE) cases. 2011–2017, all French departments.
Municipality codes are essential for the geolocation of the mAGE case and their categorisation as a local resident or non-resident (defined as patients who consulted the doctor farther than 50 km from home). “Fully located case” means that both practitioner’s and patient’s residence location are available. This condition makes it possible to distinguish “resident” patients, with the hypothesis that they are exposed in their area of residence, from “non-resident” patient, for whose exposure likely occurred away from home. The pharmacy location was used when the GP’s municipality was missing. A sharp decrease in missing geolocation data was observed from 2011 to 2017, as a result of a decrease of the missing data for the patient. Since 2012, 96% of mAGE cases can be classified as a resident or as a non-resident, and in 2016 this proportion increased to 98%.
4. Discussion
4.1. Main Results
The evaluation presented in this study based on data collected in pharmacy surveys demonstrated that our discriminatory algorithm was able to effectively distinguish mAGE from prescriptions for other pathologies. The sensitivity and PPV of the algorithm are high, around 0.90 and 0.82, respectively. This is a robust indicator for surveillance of AGE, as it remains stable over both time and in different geographical areas. The evaluation using data from the SNDS, demonstrated that most of the parameters remained stable across French departments: the percentage of mAGE cases with prescriptions containing at least one drug to treat AGE (between 40% and 50%), mean number of therapeutic classes for mAGE cases (2.2), percentage of mAGE prescriptions prescribed and dispensed within 24 h (over 90%). Only therapeutic class proportion on mAGE prescriptions seems department dependent. As the mAGE discriminating algorithm is based on syndromic treatment, it is not possible to estimate whether differences are due to specific pathogen distribution and/or GP prescription practices between departments.
4.2. Surveillance System Representativeness
Regarding the use of data from the SNDS, only mAGE cases that have consulted and filled prescriptions for AGE reimbursed drugs are detected. Van Cauteren et al. [8] showed, using a population-based retrospective cross-sectional telephone survey, that 33% of AGE cases consulted a GP, and 31% bought the prescribed drugs. However, GP consultation is dependent on age [14], the nature of the pathogen and the duration and severity of the symptoms [15], and access to health services [16]. Cohort investigations of waterborne outbreaks have shown that the proportion of patients consulting for AGE varies greatly: from 80% in an area where drinking water was highly contaminated by Cryptosporidium oocysts [17], to 10% in a campground serviced by drinking water with probable viral contamination [18].
The SNDS covers 99% of the population living in France [7]. However, caution is required when studying certain subpopulations, such as the elderly or students. Elderly persons living in retirement homes may not appear in the SNDS if drugs are provided by a hospital pharmacy. Their proportion increases with age, especially after 85 years old. Students are mobile and often prefer to report to administrations at their parent’s address instead of their own. They may therefore be classified as non-resident when they in fact consult in their current (but unregistered) municipality of residence.
4.3. Limitations
There are several limitations to this work. First, possible representativeness bias could be introduced in pharmacy surveys, as data were all collected from January to June. However, this bias is probably limited, as this period covers both the winter AGE epidemic periods and non-epidemic periods. Second, regarding the algorithm, certain populations are not fully covered. For example, infants under one year of age are not taken into account. The prescriptions for mAGE in infants frequently contain rehydration salts. This medication can also be systematically prescribed upon discharge from the maternity ward to promote its future use to cope with the risk of mAGE-associated dehydration. The sensitivity of the algorithm for the elderly is lower, as their prescriptions often contain several drugs for various chronic diseases, and thus, may be wrongly rejected by the algorithm.
4.4. External Comparison
Comparisons between the results of this work and previous studies related to AGE demonstrate similar findings. A population-based survey estimated an annual incidence rate of 0.33 AGE cases/person-year in the French mainland population between May 2009 and April 2010, with 31% of AGE cases filling a prescription from a GP [8]. The 2010 average incidence of mAGE (0.10 cases/person-year) drawn from SNDS data is consistent with the survey results (0.33 × 0.31 = 0.10). An algorithm derived from our algorithm was also tested on a commercial SNDS-like database, known as the Longitudinal Treatment Dynamics™ (LTD) database, directly fed by 30% of French pharmacists. For the latter study, drugs for peptic ulcer and gastro-oesophageal reflux disease were excluded as they were too frequently prescribed. This difference reduces the specificity (PPV) in comparison of our algorithm. The authors have found a strong agreement in the dynamic of mAGE activity in winter, between the estimate derived from the LTD data and the reporting of a primary care surveillance system (GP Sentinelles network) with a correlation coefficient between 0.84 and 0.94 on the mAGE weekly rate [19]. The work of Vilcu et al. covers an estimation of regional or national mAGE rate during winter seasons with an exclusion of overseas departments and is not suitable for a local mAGE surveillance.
4.5. Perspectives for Waterborne Infectious Disease Surveillance
mAGE syndromic surveillance based on SNDS data is particularly relevant for studying and preventing waterborne infectious risk of faecal origin [11]. Data from previously investigated AGE outbreaks provided the opportunity to retrospectively test the SNDS database for outbreak descriptions [14,20,21]. Mouly et al. compared data from the SNDS and data from cohort studies obtained during two waterborne infections and demonstrated that the temporal distribution of cases, the day of the peak, and the duration of the epidemic were similar [14].
The SNDS approach has also been used to evaluate outcomes of water safety plans (WSPs) implemented in two large drinking water systems in France [22], and to evaluate the endemic risk of AGE based on drinking water conditions in French urban areas in time series studies [23].
In 2019, a waterborne outbreak surveillance system based on the presented algorithm was implemented in France. The objectives of this system are to facilitate the identification and management of drinking water systems that need to be secured to protect consumers’ health and to improve the prevention of waterborne disease outbreak. This surveillance system is the result of evaluation of different methods of detection of mAGE clusters with a possible waterborne origin [10,24,25], followed by a pilot study in seven French departments to test the feasibility of such a surveillance system before any national implementation [26]. It should increase 10 to 100-fold the number of waterborne outbreaks reported to health authorities [24,26]. The current surveillance system uses a web-application also developed by Santé publique France (EpiGEH) (article in process) for data presentation and is based on the localisation and the characterisation of clusters of mAGE cases sharing the same water distribution system [10]. The system includes local environmental investigations to identify water distribution system contaminations, failures, or vulnerability and aims to aid in prevention strategies and WSPs for water distribution systems.
5. Conclusions
Santé publique France has developed a novel syndromic surveillance system of mAGE based on a discriminatory algorithm that exploits data from the SNDS medication database. SNDS data are routinely and automatically available and cover the whole French population. The algorithm used to identify prescriptions for mAGE has been evaluated and validated on data from different mainland and overseas French departments and for both paediatric and adult populations. The implementation of the algorithm to develop a nationwide surveillance system for retrospective waterborne outbreak surveillance highlights the utility/benefit of using the SNDS database for syndromic surveillance of mAGE.
Author Contributions
Conceptualisation, F.B., D.M., C.G., and P.B.; methodology, M.B. and C.G.; validation, D.M. and C.G.; formal analysis, F.B., J.C., M.L.-S., and C.G.; investigation, M.B., F.B., M.S., and M.L.-S.; writing—original draft preparation, F.B. and C.G.; writing—review and editing, D.M., P.B., J.C., G.J., M.L.-S., and M.S.; project administration, D.M. and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the pharmacist students, Alice Lahure, Paul Morel, Karim Hamdy, Pascale Penit, and Marion Zahaf for their valuable contribution to this work; Christophe Bonaldi and Yann Le Strat for their time and help to review this article; Andréa Guajardo for her time and help to review English.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Henning, K.J. Overview of Syndromic Surveillance. What is Syndromic Surveillance? MMWR 2004, 53, 5–11. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/su5301a3.htm (accessed on 19 May 2020).
- Berger, M.; Rita Shiau, R.; Weintraub, J.M. Review of syndromic surveillance: Implications for waterborne disease detection. J. Epidemiol. Community Health 2006, 60, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Pivette, M.; Mueller, J.E.; Crepey, P.; Bar-Hen, A. Drug sales data analysis for outbreak detection of infectious diseases: A systematic literature review. BMC Infect. Dis. 2014, 14, 604. [Google Scholar] [CrossRef]
- Pivette, M.; Mueller, J.E.; Crépey, P.; Bar-Hen, A. Surveillance of gastrointestinal disease in France using drug sales data. Epidemics 2014, 8, 1–8. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Hall, G.; Scallan, E.; Adak, G.K.; Gauci, C.; Jones, T.F.; O’Brien, S.; Henao, O.; Sockett, P.N. A common, symptom-based case definition for gastroenteritis. Epidemiol. Infect. 2008, 136, 886–894. [Google Scholar] [CrossRef]
- Tuppin, P.; Rudant, J.; Constantinou, P.; Gastaldi-Menager, C.; Rachas, A.; de Roquefeuil, L.; Maura, G.; Caillol, H.; Tajahmady, A.; Coste, J.; et al. Value of a national administrative database to guide public decisions: From the systeme national d’information interregimes de l’Assurance Maladie (SNIIRAM) to the systeme national des données de sante (SNDS) in France. Rev. Epidemiol. Sante Publique 2017, 65 (Suppl. 4), S149–S167. [Google Scholar] [CrossRef] [PubMed]
- Khiveh, A.; Hashempur, M.H.; Shakiba, M.; Lotfi, M.H.; Shakeri, A.; Kazemeini, S.; Mousavi, Z.; Jabbari, M.; Kamalinejad, M.; Emtiazy, M. Effects of rhubarb (Rheum ribes L.) syrup on dysenteric diarrhea in children: A randomized, double-blind, placebo-controlled trial. J. Integr. Med. 2017, 15, 365–372. [Google Scholar] [CrossRef]
- Van Cauteren, D.; de Valk, H.; Vaux, S.; Le Strat, Y.; Vaillant, V. Burden of acute gastroenteritis and healthcare-seeking behaviour in France: A population-based study. Epidemiol. Infect. 2012, 140, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Bounoure, F.; Beaudeau, P.; Mouly, D.; Skiba, M.; Lahiani-Skiba, M. Syndromic surveillance of acute gastroenteritis based on drug consumption. Epidemiol. Infect. 2011, 139, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Coly, S.; Vincent, N.; Vaissiere, E.; Charras-Garrido, M.; Gallay, A.; Ducrot, C.; Mouly, D. Waterborne disease outbreak detection: An integrated approach using health administrative databases. J. Water Health 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Beaudeau, P. Syndromic Surveillance of Acute Gastroenteritis: An Opportunity for the Prevention of the Infectious Risk Attributable to Tap Water. Ph.D Thesis, Université de Rennes, Rennes, France, 2012. [Google Scholar]
- Theriaque.org. Available online: http://www.theriaque.org (accessed on 19 May 2020).
- Beaudeau, P.; Bentayeb, M.; Corso, M.; Rambaud, L.; Galey, C. Les Données de L’entrepôt de Cas de Gastro-Entérite Médicalisés Issues du SNIIRAM: Description, Qualité et Utilisation; Santé Publique France: Saint-Maurice, France, 2017. [Google Scholar]
- Mouly, D.; Van Cauteren, D.; Vincent, N.; Vaissiere, E.; Beaudeau, P.; Ducrot, C.; Gallay, A. Description of two waterborne disease outbreaks in France: A comparative study with data from cohort studies and from health administrative databases. Epidemiol. Infect. 2016, 144, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.G.; Sethi, D.; Cowden, J.M.; Wall, P.G.; Rodrigues, L.C.; Tompkins, D.S.; Hudson, M.J.; Roderick, P.J. Study of infectious intestinal disease in England: Rates in the community, presenting to general practice, and reported to national surveillance. Br. Med. J. 1999, 318, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Pirard, P.; Goria, S.; Nguengang Wakap, S.; Galey, C.; Motreff, Y.; Guillet, A.; Le Tertre, A.; Corso, M.; Beaudeau, P. No increase in drug dispensing for acute gastroenteritis after Storm Klaus, France 2009. J. Water Health 2015, 13, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, M.; Carbonel, S.; Beaudeau, P.; Checlair, E.; Gallay, A. Epidémie de Gastro-Entérites à Cryptospridium, Dracy-le-Fort, Saône et Loire, Septembre 2001; Rapport de la Drass de Bourgogne, de la Cirre Dijon et de l’Institut de Veille Sanitaire; Santé Publique France: Saint-Maurice, France, 2003. [Google Scholar]
- Galey, C.; L’Azou, M.; Duchen, C.; Beaudeau, P. Epidémie de gastro-entérites aiguës dans un camping, Ardèche, France, août 2008. Bull. Epidemiol. Hebd. 2012, 33, 379–382. [Google Scholar]
- Vilcu, A.M.; Blanchon, T.; Sabatte, L.; Souty, C.; Maravic, M.; Hanslik, T.; Steichen, O. Cross-validation of an algorithm detecting acute gastroenteritis episodes from prescribed drug dispensing data in France: Comparison with clinical data reported in a primary care surveillance system, winter seasons 2014/15 to 2016/17. BMC Med. Res. Methodol. 2019, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Mansotte, F.; Dejean, G.; Coquet, S.; Gault, G.; Beaudeau, P.; Galey, C. Retour d’expérience sur une épidémie de gastro-entérites aiguës d’origine hydrique en Gironde, juillet 2010. Tech. Sci. Méthodes 2017, 4, 28–41. [Google Scholar] [CrossRef]
- Six, C.; Giron, S.; Galey, C. Investigation D’une Epidémie de Gastro-Entérites Virales Survenues Après une Course à Obstacles Alpes-Maritimes, June 2015; Santé Publique France: Saint-Maurice, France, 2016. [Google Scholar]
- Setty, K.E.; Kayser, G.L.; Bowling, M.; Enault, J.; Loret, J.F.; Serra, C.P.; Alonso, J.M.; Mateu, A.P.; Bartram, J. Water quality, compliance, and health outcomes among utilities implementing Water Safety Plans in France and Spain. Int. J. Hyg. Environ. Health 2017, 220, 513–530. [Google Scholar] [CrossRef]
- Beaudeau, P. A systematic review of the time series studies addressing the endemic risk of acute gastroenteritis according to drinking water operation conditions in urban areas of developed countries. Int J. Environ. Res. Public Health 2018, 15, 867. [Google Scholar] [CrossRef] [PubMed]
- Rambaud, L.; Galey, C.; Beaudeau, P. Automated detection of case clusters of waterborne acute gastroenteritis from health insurance data—Pilot study in three French districts. J. Water Health 2016, 14, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Mouly, D.; Goria, S.; Mounié, M.; Beaudeau, P.; Galey, C.; Gallay, A.; Ducrot, C.; Le Strat, Y. Waterborne disease outbreak detection: A simulation-based study. Int. J. Environ. Res Public Health 2018, 15, 1505. [Google Scholar] [CrossRef] [PubMed]
- Galey, C.; Pouey, J.; Guillet, A.; Goria, S.; Mouly, D. Détection d’épidémies de gastro-entérite aigue médicalisée d’origine hydrique. Etude pilote concernant 7 départements de 7 régions françaises. In Detection of Outbreaks of Acute Medicalized Waterborne Gastroenteritis—Pilot Study Concerning 7 Districts in 7 French Regions; Santé Publique France: Saint-Maurice, France, 2018; pp. 1–73. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).