Maternal but Not Paternal High-Fat Diet (HFD) Exposure at Conception Predisposes for ‘Diabesity’ in Offspring Generations
Abstract
1. Introduction
2. Materials and Methods
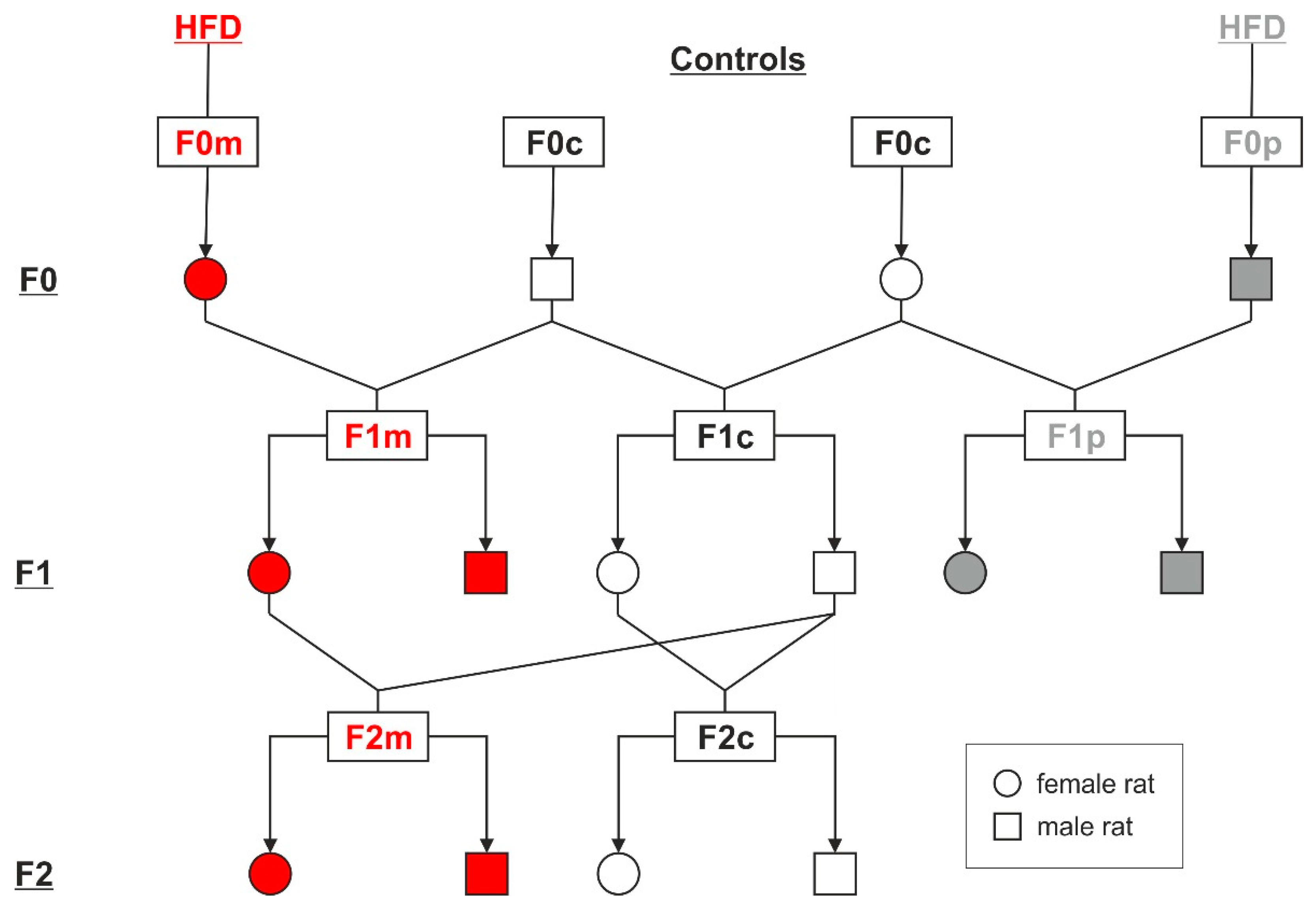
2.1. Animal Model and Study Design
2.2. Body Weight, Food Intake, and Body Composition
2.3. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.4. Metabolic Parameters
2.5. Statistical Analyses
3. Results
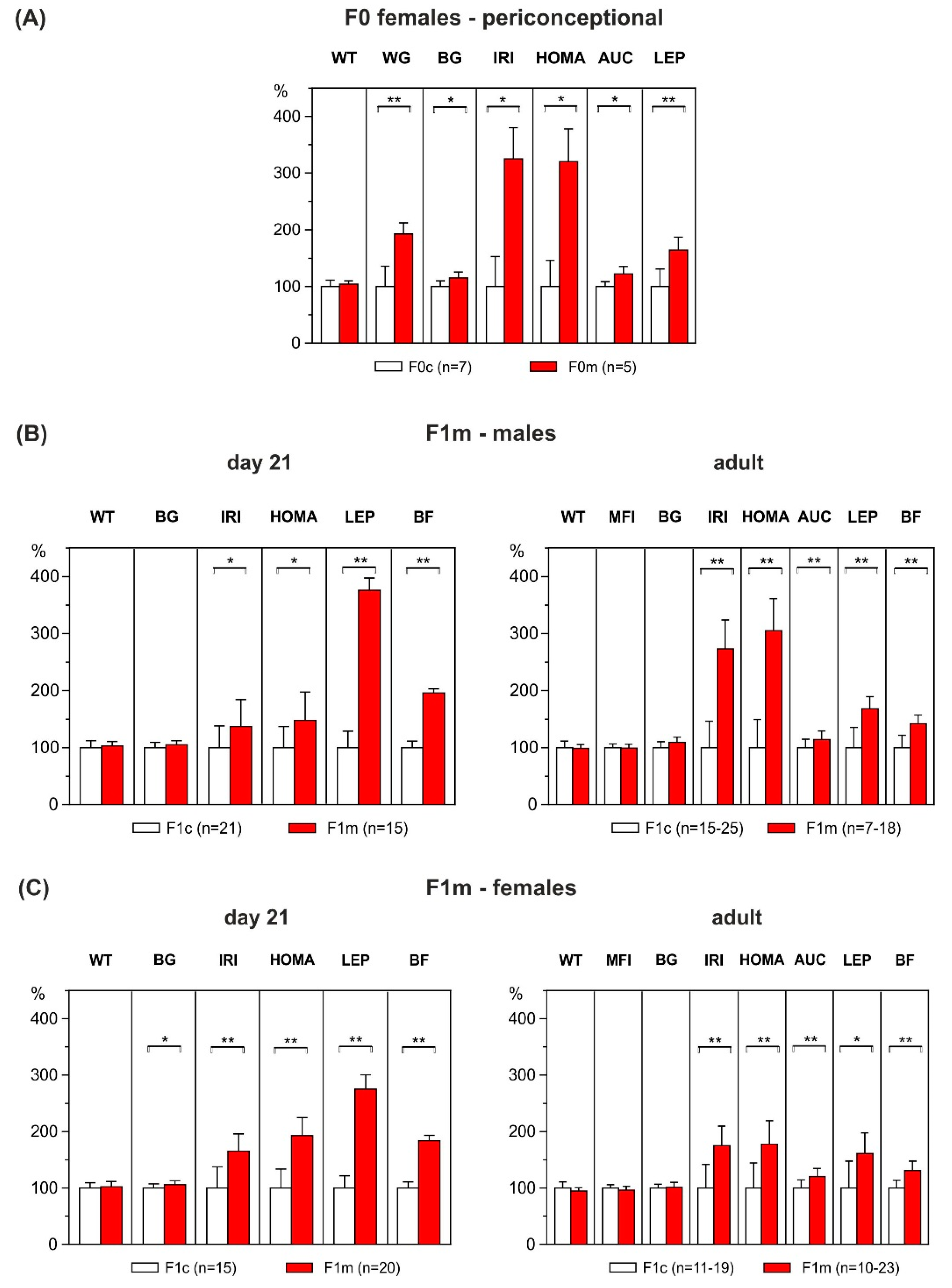
3.1. Metabolic Profile of F0 Dams
3.2. Effect of Maternal HFD-Overfeeding on F1 Offspring
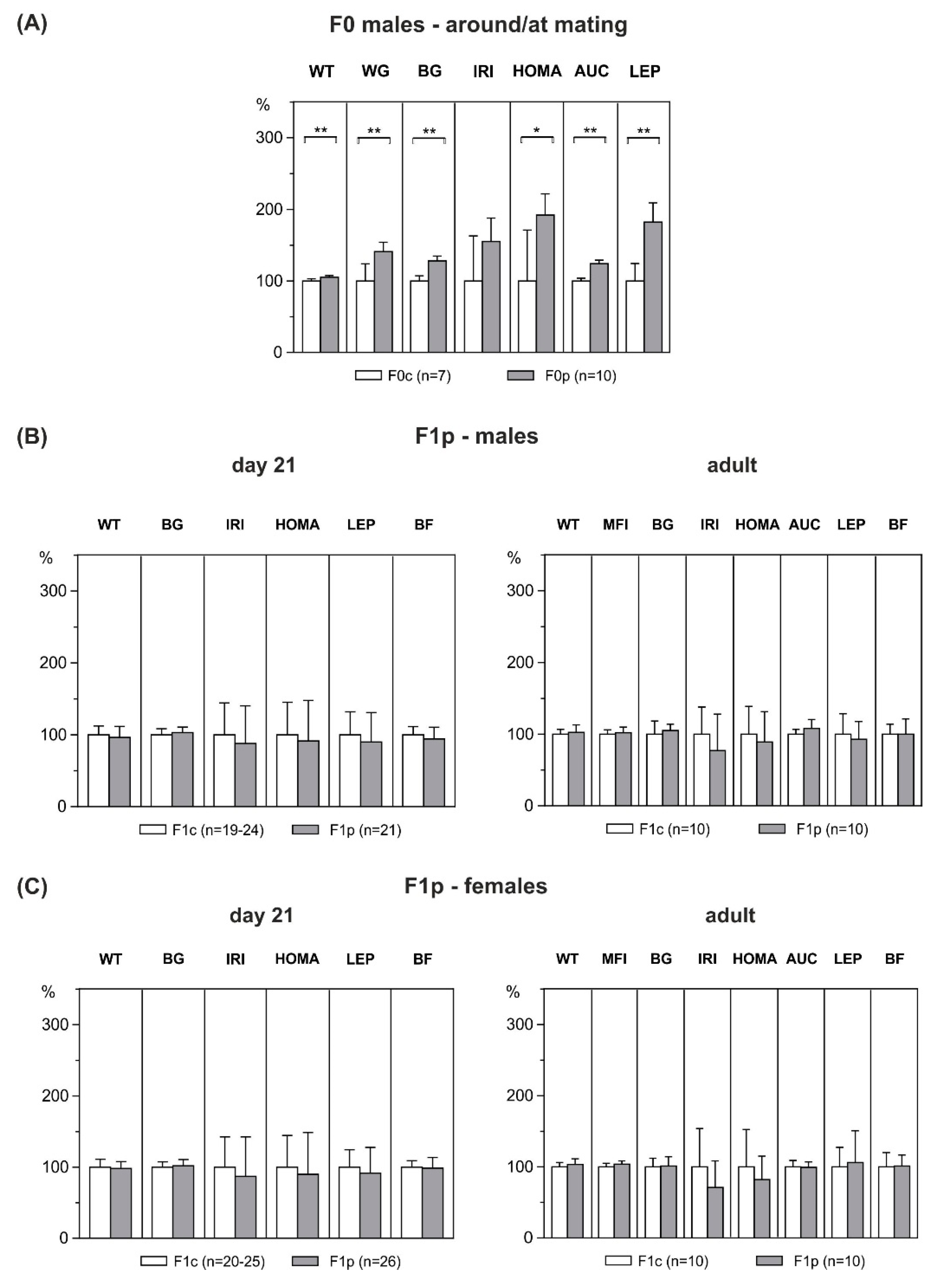
3.3. Metabolic Profile of F0 Male Founders
3.4. Effect of Paternal HFD-Overfeeding on F1 Offspring
3.5. Metabolic Profile of F1 Dams
3.6. Effect of Maternal HFD-Overfeeding on F2 Offspring
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Political Declaration of the High-Level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases. WHO. 2012. Available online: https://www.who.int/nmh/events/un_ncd_summit2011/political_declaration_en.pdf (accessed on 9 June 2020).
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity among Adults and Youth: United States, 2015–2016; NCHS Data Brief, no 288; National Center for Health Statistics: Hyattsville, MD, USA, 2017.
- Sacks, D.A.; Hadden, D.R.; Maresh, M.; Deerochanawong, C.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Coustan, D.R.; Hod, M.; Oats, J.J.N.; et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: The hyperglycemia and adverse pregnancy outcome (HAPO) study. Diabetes Care 2012, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Melchior, H.; Kurch-Bek, D.; Mund, M. Population-based analysis of a nationwide screening program. Dtsch. Arztebl. Int. 2017, 114, 412–418. [Google Scholar] [PubMed]
- Fernandez-Twinn, D.S.; Hjort, L.; Novakovic, B.; Ozanne, S.E.; Saffery, R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia 2019, 62, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Oken, E.; Dabelea, D. Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetologia 2019, 62, 1779–1788. [Google Scholar] [CrossRef]
- Plagemann, A.; Harder, T.; Schellong, K.; Schulz, S.; Stupin, J.H. Early postnatal life as a critical time window for determination of long-term metabolic health. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 641–653. [Google Scholar] [CrossRef]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef]
- Kawasaki, M.; Arata, N.; Miyazaki, C.; Mori, R.; Kikuchi, T.; Ogawa, Y.; Ota, E. Obesity and abnormal glucose tolerance in offspring of diabetic mothers: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0190676. [Google Scholar] [CrossRef]
- Plagemann, A. Maternal diabetes and perinatal programming. Early Hum. Dev. 2011, 87, 743–747. [Google Scholar] [CrossRef]
- Plagemann, A. A matter of insulin: Developmental programming of body weight regulation. J. Matern. Fetal Neonatal. Med. 2008, 21, 143–148. [Google Scholar] [CrossRef]
- Van Assche, F.A.; Holemans, K.; Aerts, L. Long-term consequences for offspring of diabetes during pregnancy. Br. Med. Bull. 2001, 60, 173–182. [Google Scholar] [CrossRef][Green Version]
- Fullston, T.; Ohlsson Teague, E.M.C.; Palmer, N.O.; DeBlasio, M.J.; Mitchell, M.; Corbett, M.; Print, C.G.; Owens, J.A. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013, 27, 4226–4243. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.F.; Lin, R.C.Y.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic high-fat diet in fathers programs ß-cell dysfunction in female rat offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, H.; Mitsui, T.; Eguchi, T.; Tamada, S.; Hiramatsu, Y. The effects of paternal high-fat diet exposure on offspring metabolism with epigenetic changes in the mouse adiponectin and leptin gene promoters. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E236–E245. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Bale, T.L. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 2009, 150, 4999–5009. [Google Scholar] [CrossRef]
- Graus-Nunes, F.; Dalla Corte Frantz, E.; Lannes, W.R.; da Silva Menezes, M.C.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Pregestational maternal obesity impairs endocrine pancreas in male F1 and F2 progeny. Nutrition 2015, 31, 380–387. [Google Scholar] [CrossRef]
- Huang, Y.H.; Ye, T.T.; Liu, C.X.; Wang, L.; Chen, Y.W.; Dong, Y. Maternal high-fat diet impairs glucose metabolism, ß-cell function and proliferation in the second generation of offspring rats. Nutr. Metab. 2017, 14, 67. [Google Scholar] [CrossRef]
- Aiken, C.E.; Ozanne, S.E. Transgenerational developmental programming. Hum. Reprod. Update 2014, 20, 63–75. [Google Scholar] [CrossRef]
- Su, L.; Patti, M.E. Paternal nongenetic intergenerational transmission of metabolic disease risk. Curr. Diab. Rep. 2019, 19, 38. [Google Scholar] [CrossRef]
- Sales, V.M.; Ferguson-Smith, A.C.; Patti, M.E. Epigenetic Mechanisms of Transmission of Metabolic Disease across Generations. Cell Metab. 2017, 25, 559–571. [Google Scholar] [CrossRef]
- Dunford, A.R.; Sangster, J.M. Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: A systematic review. Diabetes Metab. Syndr. 2017, 11, S655–S662. [Google Scholar] [CrossRef]
- Levin, B.E.; Hogan, S.; Sullivan, A.C. Initiation and perpetuation of obesity and obesity resistance in rats. Am. J. Physiol. 1989, 256, R766–R771. [Google Scholar] [CrossRef] [PubMed]
- Schellong, K.; Neumann, U.; Rancourt, R.C.; Plagemann, A. Increase of long-term ‘diabesity’ risk, hyperphagia, and altered hypothalamic neuropeptide expression in neonatally overnourished ‘small-for-gestational-age’ (SGA) rats. PLoS ONE 2013, 8, e78799. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.; Schoelch, C.; Ziska, T.; Schneider, D.; Simon, E.; Plagemann, A. Interaction of genetic and environmental programming of the leptin system and of obesity disposition. Physiol. Genomics 2000, 3, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A.; Harder, T.; Rake, A.; Voits, M.; Fink, H.; Rohde, W.; Dörner, G. Perinatal increase of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome X-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999, 836, 146–155. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Schaalan, M.; El-Abhar, H.S.; Barakat, M.; El-Denshary, E.S. Westernized-like-diet-fed rats: Effect on glucose homeostasis, lipid profile, and adipocyte hormones and their modulation by rosiglitazone and glimepiride. J. Diabetes Complications 2009, 23, 199–208. [Google Scholar] [CrossRef]
- Dias-Rocha, C.P.; Almeida, M.M.; Santana, E.M.; Costa, J.C.B.; Franco, J.G.; Pazos-Moura, C.C.; Trevenzoli, I.H. Maternal high-fat diet induces sex-specific endocannabinoid system changes in newborn rats and programs adiposity, energy expenditure and food preference in adulthood. J. Nutr. Biochem. 2018, 51, 56–68. [Google Scholar] [CrossRef]
- Nivoit, P.; Morens, C.; Van Assche, F.A.; Jansen, E.; Poston, L.; Remacle, C.; Reusens, B. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia 2009, 52, 1133–1142. [Google Scholar] [CrossRef]
- Schellong, K.; Melchior, K.; Ziska, T.; Ott, R.; Henrich, W.; Rancourt, R.C.; Plagemann, A. Hypothalamic insulin receptor expression and DNA promoter methylation are sex-specifically altered in adult offspring of high-fat diet (HFD)-overfed mother rats. J. Nutr. Biochem. 2019, 67, 28–35. [Google Scholar] [CrossRef]
- Schellong, K.; Melchior, K.; Ziska, T.; Henrich, W.; Rancourt, R.C.; Plagemann, A. Sex-specific epigenetic alterations of the hypothalamic Agrp-Pomc system do not explain ‘diabesity’ in offspring of high-fat diet (HFD) overfed mother rats. J. Nutr. Biochem. 2020, 75, 108257. [Google Scholar] [CrossRef]
- Sun, B.; Purcell, R.H.; Terrillion, C.E.; Yan, J.; Moran, T.H.; Tamashiro, K.L.K. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 2012, 61, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.C.; Paeger, L.; Hess, S.; Steculorum, S.M.; Awazawa, M.; Hampel, B.; Neupert, S.; Nicholls, H.T.; Mauer, J.; Hausen, A.C.; et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 2014, 156, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.G.; Morgan, M.D.; Heger, A.H.; Sharpe, R.M.; Drake, A.J. High-fat diet disrupts metabolism in two generations of rats in a parent-of-origin specific manner. Sci. Rep. 2016, 6, 31857. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Holemans, K.; Van Assche, F.A. Maternal diabetes during pregnancy: Consequences for the offspring. Diabetes Metab. Rev. 1990, 6, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Harder, T.; Aerts, L.; Melchior, K.; Fahrenkrog, S.; Rodekamp, E.; Ziska, T.; Van Assche, F.A.; Dudenhausen, J.W.; Plagemann, A. ’Programming’ of orexigenic and anorexigenic hypothalamic neurons in offspring of treated and untreated diabetic mother rats. Brain Res. 2005, 1031, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A.; Harder, T.; Kohlhoff, R.; Rohde, W.; Dörner, G. Glucose tolerance and insulin secretion in children of mothers with pregestational insulin-dependent diabetes mellitus or gestational diabetes. Diabetologia 1997, 40, 1094–1100. [Google Scholar] [CrossRef]
- Reece, E.A. The fetal and maternal consequences of gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2010, 23, 199–203. [Google Scholar] [CrossRef]
- Ott, R.; Stupin, J.H.; Loui, A.; Eilers, E.; Melchior, K.; Rancourt, R.C.; Schellong, K.; Ziska, T.; Dudenhausen, J.W.; Henrich, W.; et al. Maternal overweight is not an independent risk factor for increased birth weight, leptin and insulin in newborns of gestational diabetic women: Observations from the prospective ‘EaCH’ cohort study. BMC Pregnancy Childbirth 2018, 18, 250. [Google Scholar] [CrossRef]
- Ornellas, F.; Carapeto, P.V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Obese fathers lead to an altered metabolism and obesity in their children in adulthood: Review of experimental and human studies. J. Pediatr. 2017, 93, 551–559. [Google Scholar] [CrossRef]
- Sharp, G.C.; Lawlor, D.A. Paternal impact on the life course development of obesity and type 2 diabetes in the offspring. Diabetologia 2019, 62, 1802–1810. [Google Scholar] [CrossRef]
- de Castro Barbosa, T.; Ingerslev, L.R.; Alm, P.S.; Versteyhe, S.; Massart, J.; Rasmussen, M.; Donkin, I.; Sjögren, R.; Mudry, J.M.; Vetterli, L.; et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol. Metab. 2016, 5, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, V.; Maloney, C.A.; Wang, K.W.; Morris, M.J. Effects of paternal obesity on growth and adiposity of male rat offspring. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E117–E125. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Han, G.; Ross, M.G. Programmed hyperphagia in offspring of obese dams: Altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite 2016, 99, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Marco, A.; Kisliouk, T.; Tabachnik, T.; Meiri, N.; Weller, A. Overweight and CpG methylation of the Pomc promoter on offspring of high-fat-diet-fed dams are not “reprogrammed” by regular chow diets in rats. FASEB J. 2014, 28, 4148–4157. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, T.G.; Allen, T.J.; Davies, A.; Harno, E.; Sefton, C.; Murgatroyd, C.; White, A. Maternal overnutrition programs epigenetic changes in the regulatory regions of hypothalamic Pomc in the offspring of rats. Int. J. Obes. 2018, 42, 1431–1444. [Google Scholar] [CrossRef]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef]
- Kaati, G.; Bygren, L.O.; Edvinsson, S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur. J. Hum. Genet. 2002, 10, 682–688. [Google Scholar] [CrossRef]
- Kaati, G.; Bygren, L.O.; Pembrey, M.; Sjöström, M. Transgenerational response to nutrition, early life circumstances and longevity. Eur. J. Hum. Genet. 2007, 15, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Portha, B.; Grandjean, V.; Movassat, J. Mother or father: Who is in the front line? Mechanisms underlying the non-genomic transmission of obesity/diabetes via the maternal or the paternal line. Nutrients 2019, 11, 233. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schellong, K.; Melchior, K.; Ziska, T.; Rancourt, R.C.; Henrich, W.; Plagemann, A. Maternal but Not Paternal High-Fat Diet (HFD) Exposure at Conception Predisposes for ‘Diabesity’ in Offspring Generations. Int. J. Environ. Res. Public Health 2020, 17, 4229. https://doi.org/10.3390/ijerph17124229
Schellong K, Melchior K, Ziska T, Rancourt RC, Henrich W, Plagemann A. Maternal but Not Paternal High-Fat Diet (HFD) Exposure at Conception Predisposes for ‘Diabesity’ in Offspring Generations. International Journal of Environmental Research and Public Health. 2020; 17(12):4229. https://doi.org/10.3390/ijerph17124229
Chicago/Turabian StyleSchellong, Karen, Kerstin Melchior, Thomas Ziska, Rebecca C. Rancourt, Wolfgang Henrich, and Andreas Plagemann. 2020. "Maternal but Not Paternal High-Fat Diet (HFD) Exposure at Conception Predisposes for ‘Diabesity’ in Offspring Generations" International Journal of Environmental Research and Public Health 17, no. 12: 4229. https://doi.org/10.3390/ijerph17124229
APA StyleSchellong, K., Melchior, K., Ziska, T., Rancourt, R. C., Henrich, W., & Plagemann, A. (2020). Maternal but Not Paternal High-Fat Diet (HFD) Exposure at Conception Predisposes for ‘Diabesity’ in Offspring Generations. International Journal of Environmental Research and Public Health, 17(12), 4229. https://doi.org/10.3390/ijerph17124229




