Soil Microbial Community and Its Interaction with Soil Carbon Dynamics Following a Wetland Drying Process in Mu Us Sandy Land
Abstract
1. Introduction
2. Materials and Methods
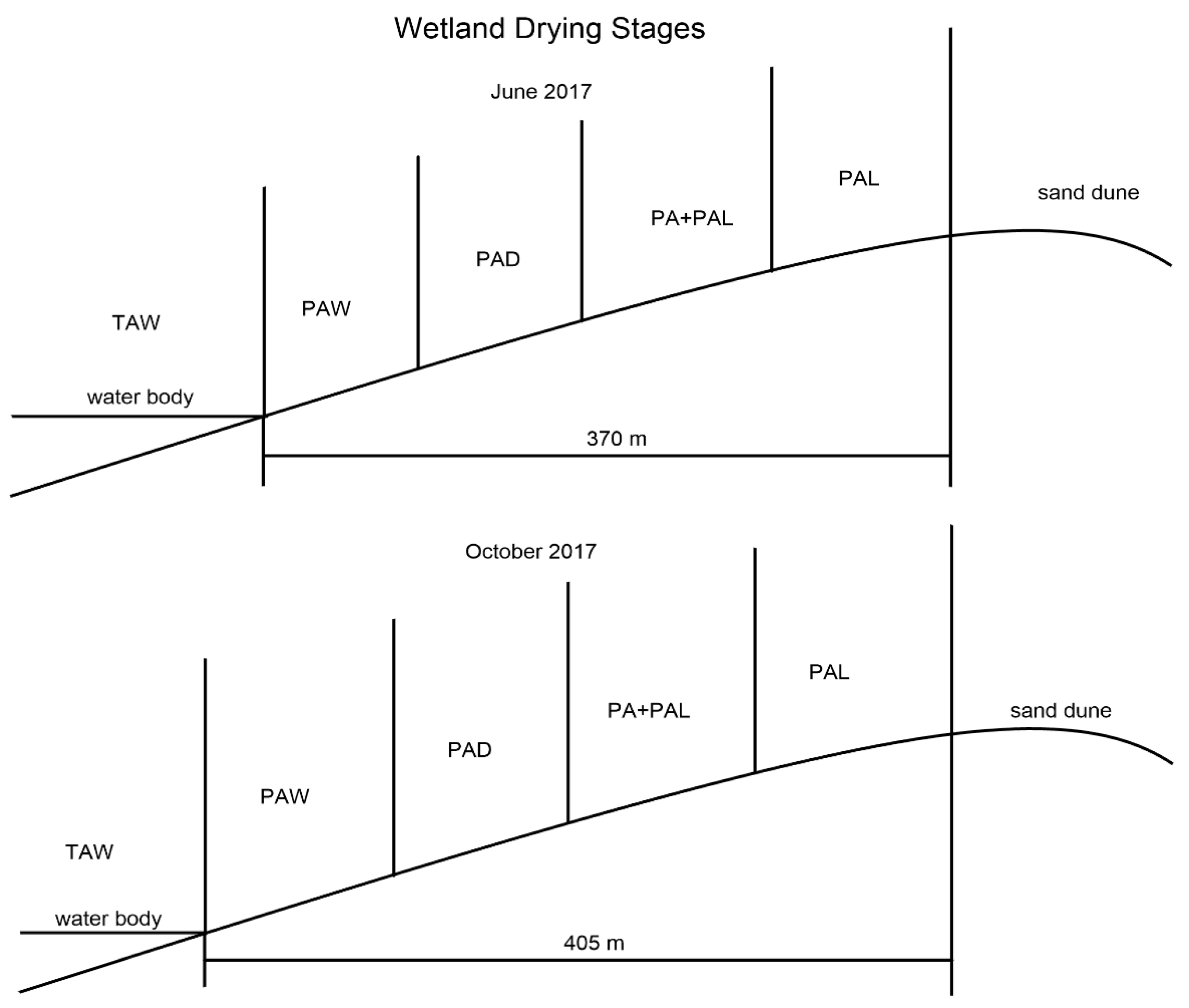
2.1. Study Site and Vegetation Inventory
- Stage TAW means virgin wetland stage. Stages TAW has water depths of 70–120 cm and is characterized by Typha angustifolia, which is a tall hydrophyte with a height higher than 2 m.
- Stage PAW is the area near virgin wetland and is the first stage of dry land area. There is no water submerging in this stage, and soil in PAW is comparatively wetter than PAD. Stage PAW is characterized by Phragmite australis which is higher than stage PAD.
- Compared with stage PAW, stage PAD has smaller P. australis and drier soils.
- Stage PA + PAL is characterized by mixed dominant species of P. australis and P. arundinacea L. The plants of P. australis in stage PA + PAL are much smaller than stages PAW and PAD.
- Stage PAL is characterized by the plants of Phalaris arundinacea L. and much drier soil. The sand content in this stage is much higher, and stage PAL is the last stage in wetland drying process.
2.2. Field Sampling and Laboratory Analysis
2.3. Statistic Analysis
3. Results
3.1. Litter Biological Traits and Soil Physicochemical Properties in Wetland Drying Process
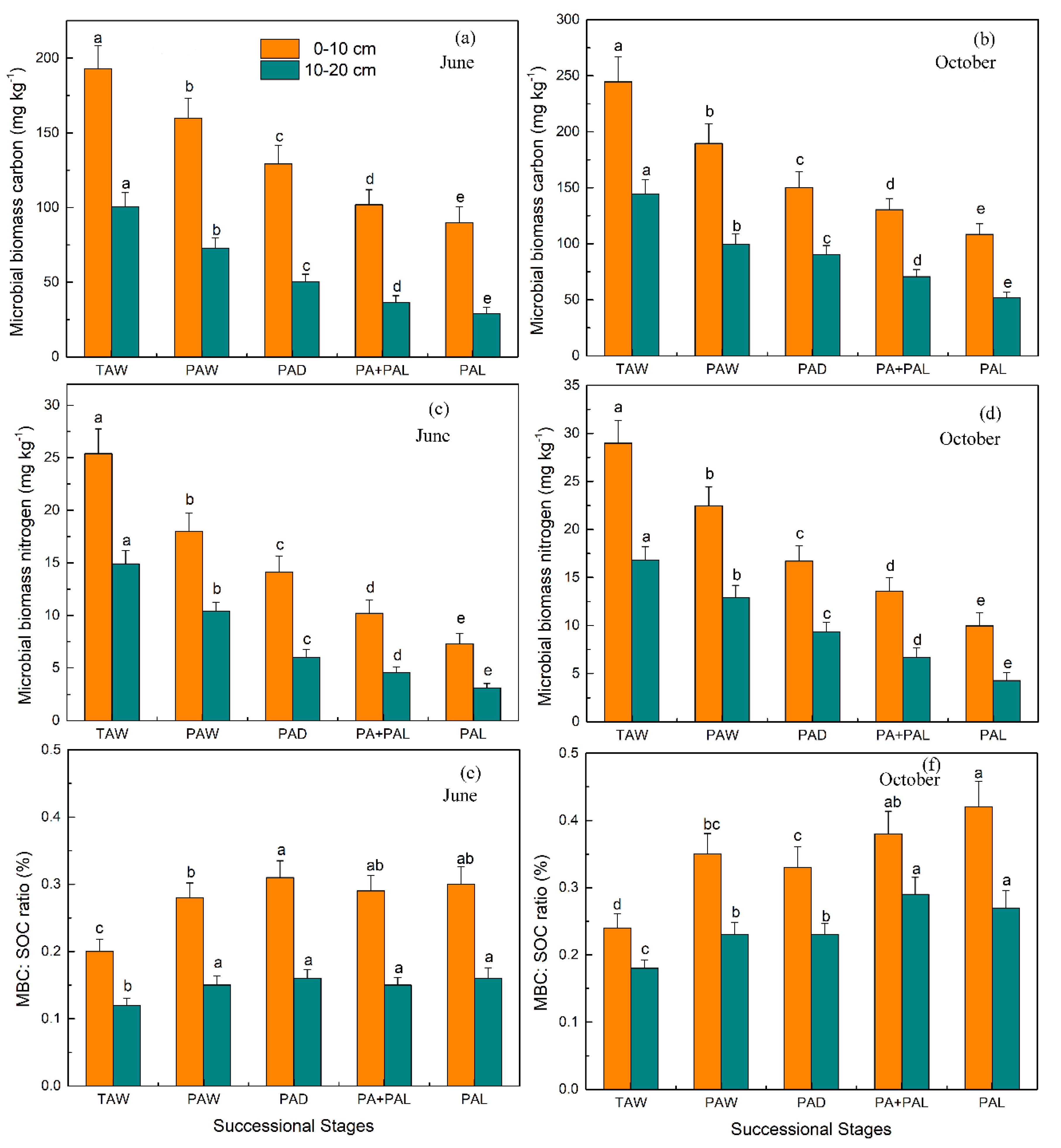
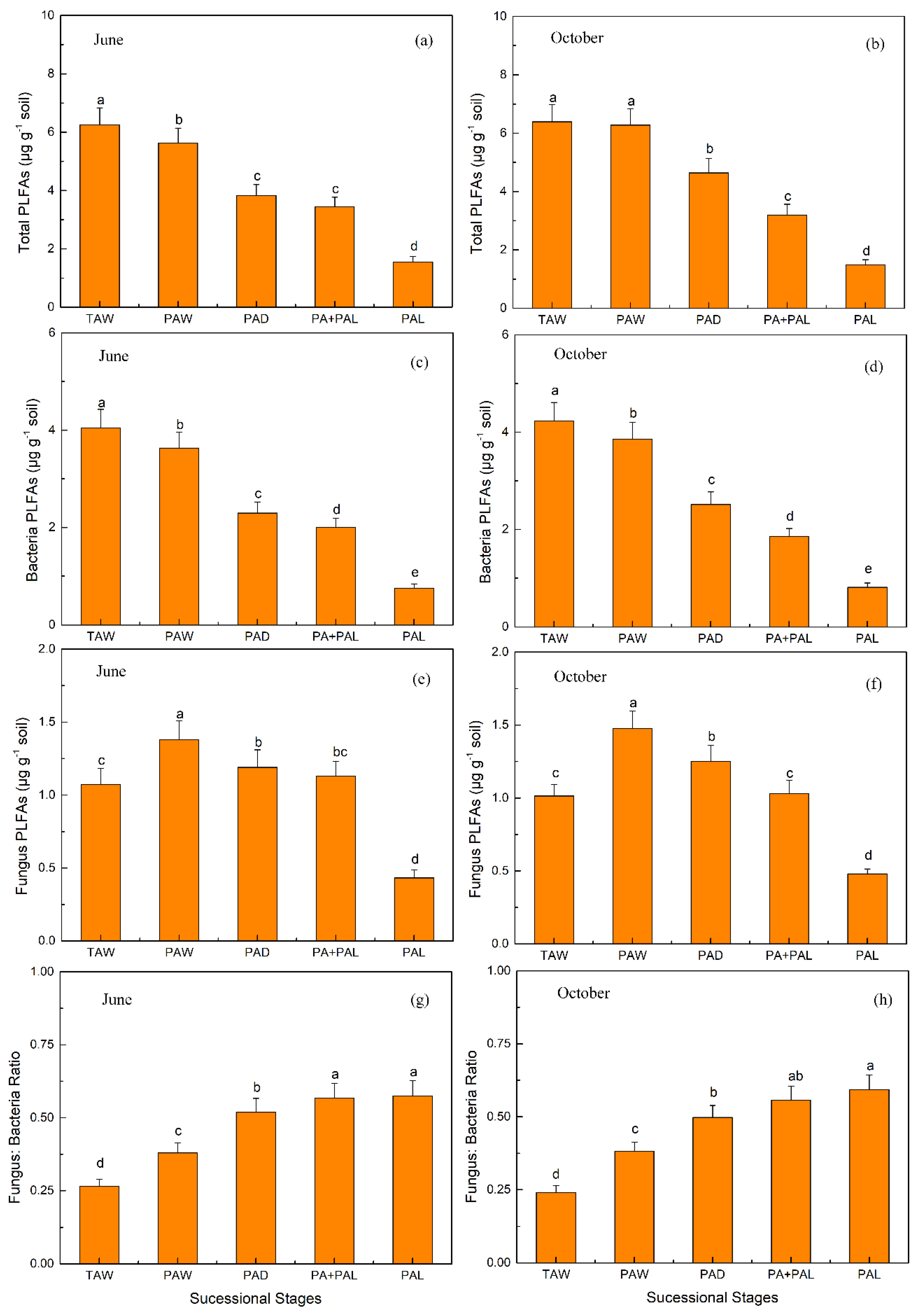
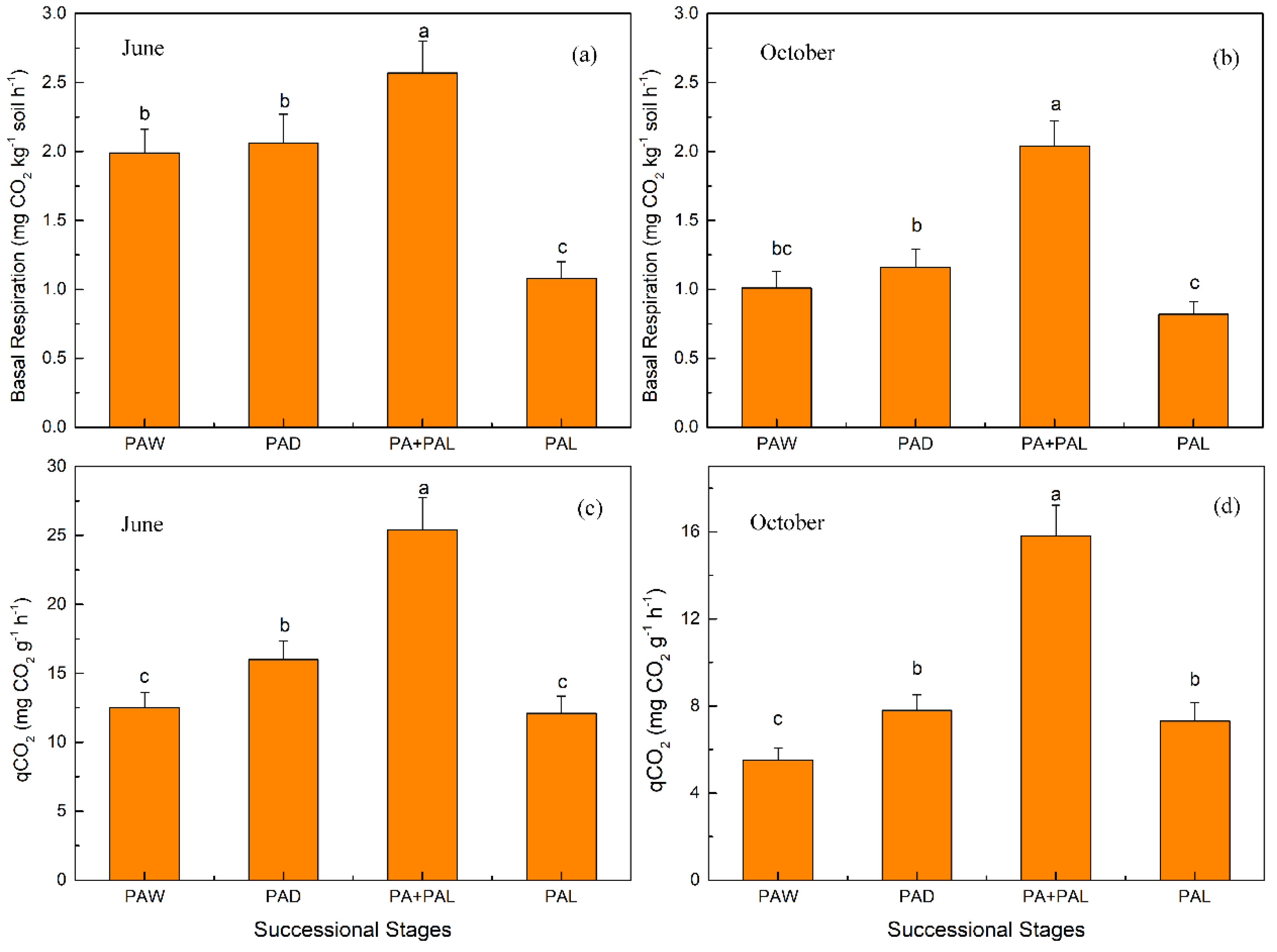
3.2. Microbial Properties in Different Stages of Wetland Drying Process
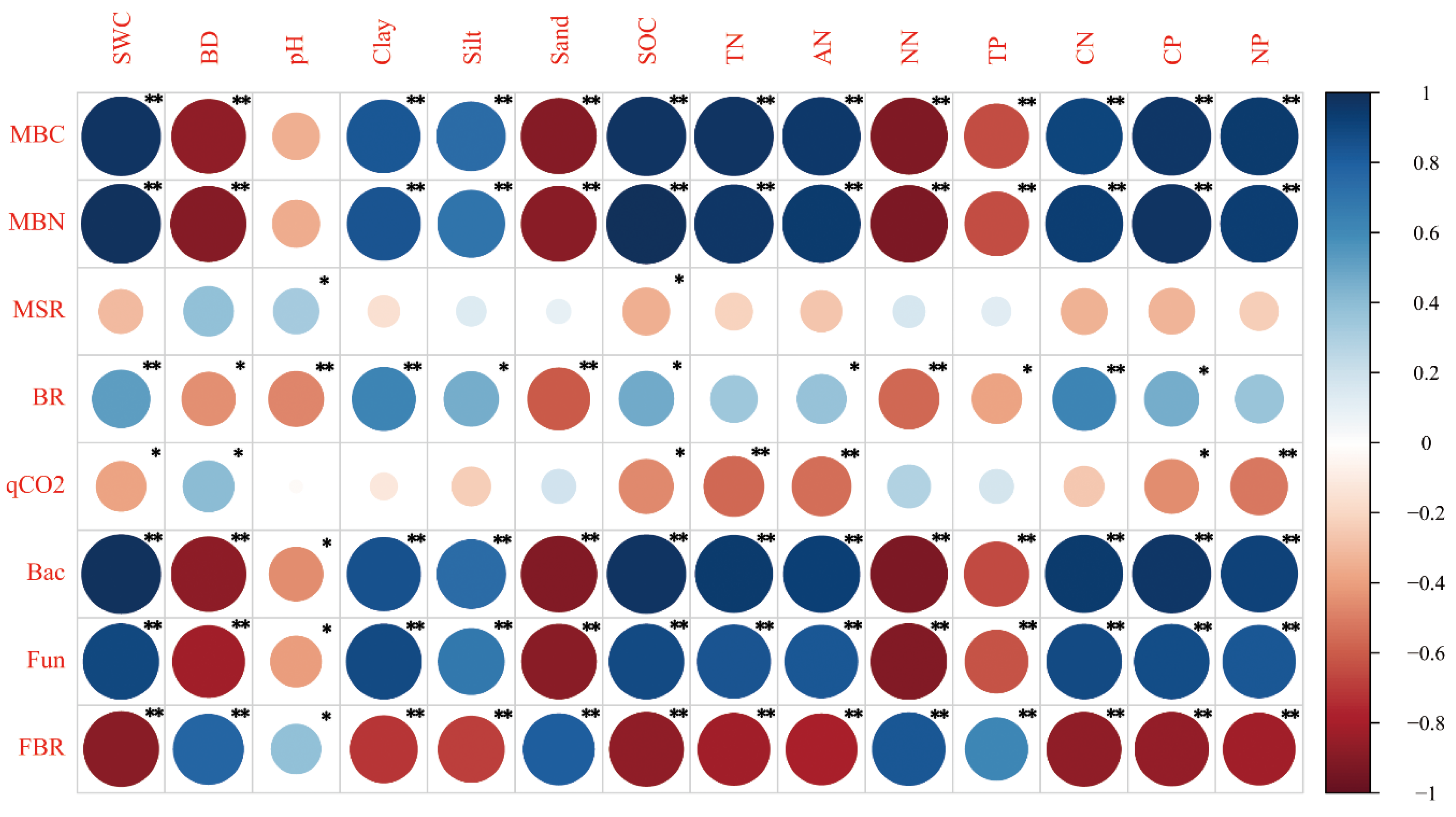
3.3. Correlation between Soil Physicochemical Properties and Soil Microbial Properties
3.4. Direct and Indirect Effects of SWC on Microbial Properties by Influencing Soil Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gao, X.; Giorgi, F. Increased aridity in the Mediterranean region under greenhouse gas forcing estimated from high resolution simulations with a regional climate model. Glob. Planet. Chang. 2008, 62, 195–209. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Feng, S.; Fu, Q. Expansion of global drylands under a warming climate. Atmos. Chem. Phys. 2013, 13, 10081–10094. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.; Gallardo, A.; Bowker, M.; Wallenstein, M.; Quero, J.; Ochoa, V.; Gozalo, B.; García-Gómez, M.; Soliveres, S.; et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 2013, 502, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Blankinship, J.C.; Niklaus, P.A.; Hungate, B.A. A meta-analysis of responses of soil biota to global change. Oecologia 2011, 165, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Singh, B.K. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xia, G.T.; Yang, W.J.; Zhu, Y.P.; Wang, G.D.; Shen, W.B. Response of soil C:N:P stoichiometry, organic carbon stock, and release to wetland grasslandification in Mu Us Desert. J. Soil Sediment 2019, 19, 3954–3968. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Cao, R.; Xi, X.Q.; Yang, Y.H.S.; Wei, X.; Wu, X.W.; Sun, S.C. The effect of water table declining on soil CO2 emission of Zoige peatland on eastern Tibetan Plateau: A four-year in situ experimental drainage. Appl. Soil Ecol. 2017, 120, 55–61. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Agren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.H.; Domsch, K.H. Soil microbial biomass: The eco-physiological approach. Soil Biol. Biochem. 2010, 42, 2039–2043. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; Brussaard, L. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Sistla, S.A.; Schimel, J.P. Stoichiometric flexibility as a regulator of carbon and nutrient cycling in terrestrial ecosystems under change. New Phytol. 2012, 196, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, K.B.; Li, J.P.; Zhao, G.W.; Shangguan, Z.P. Effect of soil moisture and atmospheric humidity on both plant productivity and diversity of native grasslands across the Loess Plateau, China. Ecol. Eng. 2016, 94, 525–531. [Google Scholar] [CrossRef]
- Aponte, C.; Marañón, T.; García, L.V. Microbial C, N and P in soils of Mediterranean oak forests: Influence of season, canopy cover and soil depth. Biogeochemistry 2010, 101, 77–92. [Google Scholar] [CrossRef]
- Serna-Chavez, H.M.; Fierer, N.; van Bodegom, P.M. Global drivers and patterns of microbial abundance in soil. Glob. Ecol. Biogeogr. 2013, 22, 1162–1172. [Google Scholar] [CrossRef]
- Frazo, L.A.; Piccolo, M.D.C.; Feigl, B.J.; Cerri, C.C.; Cerri, C.E.P. Inorganic nitrogen, microbial biomass and microbial activity of a sandy Brazilian Cerrado soil under different land uses. Agric. Ecosyst. Environ. 2010, 135, 161–167. [Google Scholar] [CrossRef]
- Tang, Y.S.; Wang, L.; Jia, J.W.; Fu, X.H.; Le, Y.Q.; Chen, X.Z.; Sun, Y. Response of soil microbial community in Jiuduansha wetland to different successional stages and its implications for soil microbial respiration and carbon turnover. Soil Biol. Biochem. 2011, 43, 638–646. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, B.; Jin, C.; Wang, F. Soil aggregate stratification of nematodes and microbial communities affects the metabolic quotient in an acid soil. Soil Biol. Biochem. 2013, 60, 1–9. [Google Scholar] [CrossRef]
- Yuste, J.C.; Penuelas, J.; Estiarte, M.; Garcia-Mas, J.; Mattana, S.; Ogaya, R.; Pujol, M.; Sardans, J. Drought-resistant fungi control soil organic matter decomposition and its response to temperature. Glob. Chang. Biol. 2011, 17, 1475–1486. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Piao, S.; Janssens, I.A.; Tang, J.; Liu, W.; Chi, Y.; Wang, J.; Xu, S. Soil respiration under climate warming: Differential response of heterotrophic and autotrophic respiration. Glob. Chang. Biol. 2014, 20, 3229–3237. [Google Scholar] [CrossRef]
- Spohn, M.; Chodak, M. Microbial respiration per unit biomass increases with carbon-to-nutrient ratios in forest soils. Soil Biol. Biochem. 2015, 81, 128–133. [Google Scholar] [CrossRef]
- Barba, J.; Cueva, A.; Bahn, M.; Barron-Gafford, G.A.; Bond-Lamberty, B.; Hanson, P.J.; Jaimes, A.; Kulmala, L.; Pumpanen, J.; Scott, R.L. Comparing ecosystem and soil respiration: Review and key challenges of tower-based and soil measurements. Agric. For. Meteorol. 2017, 249, 434–443. [Google Scholar] [CrossRef]
- Pabst, H.; Gerschlauer, F.; Kiese, R.; Kuzyakov, Y. Land use and precipitation affect organic and microbial carbon stocks and the specific mmetabolic quotient in soils of eleven ecosystems of Mt. Kilimanjaro, Tanzania. Land Degrad. Dev. 2016, 27, 592–602. [Google Scholar] [CrossRef]
- Wardle, D.A.; Ghani, A. A tale of two theories, a chronosequence and a bioindicator of soil quality. Soil Biol. Biochem. 2018, 121, A3–A7. [Google Scholar] [CrossRef]
- Spohn, M. Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeosciences 2015, 12, 817–823. [Google Scholar] [CrossRef]
- Dias, A.T.C.; Ruijven, J.; Berendse, F. Plant species richness regulates soil respiration through changes in productivity. Oecologia 2010, 163, 805–813. [Google Scholar] [CrossRef]
- Deng, Q.; Cheng, X.L.; Hui, D.F.; Zhang, Q.; Li, M.; Zhang, Q.F. Soil microbial community and its interaction with soil carbon and nitrogen dynamics following afforestation in central China. Sci. Total Environ. 2016, 541, 230–237. [Google Scholar] [CrossRef]
- Li, B.; Li, S.H.; Sun, J.M. Isochron dating of sand-loess-soil deposits from the the Mu Us Desert margin, central China. Quat. Geochronol. 2011, 6, 556–563. [Google Scholar] [CrossRef]
- Sun, Y.L.; Li, X.Y.; Xu, H.Y.; Yang, Z.P.; Tang, J.; Zhang, X.Y. Effect of soil crust on evaporation and dew deposition in Mu Us sandy land, China. Front. Environ. Sci. Eng. 2008, 2, 480–486. [Google Scholar] [CrossRef]
- Liu, Y.S.; Zhang, X.P.; Li, X.W.; Jay, G. Mechanism and regulation of land degradation in Yulin district. J. Geogr. Sci. 2003, 13, 217–224. [Google Scholar]
- Sun, J.M. Origin of eolian sand mobilization during the past 2300 years in the Mu Us desert, China. Quat. Res. 2000, 53, 78–88. [Google Scholar] [CrossRef]
- Li, C.P.; Xiao, C.W. Above- and belowground biomass of Artemisia ordosica communities in three contrasting habitats of the Mu Us Desert, northern China. J. Arid Environ. 2007, 70, 195–207. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.H.; Dong, Z.; Xia, D. Evolution of the southern Mu Us Desert in north China over the past 50 years: An analysis using proxies of human activity and climate parameters. Land Degrad. Dev. 2010, 16, 351–366. [Google Scholar] [CrossRef]
- Lyseng, M.P.; Bork, E.W.; Hewins, D.B.; Alexander, M.J.; Carlyle, C.N.; Chang, S.X.; Willms, W.D. Long-term grazing impacts on vegetation diversity, composition, and exotic species presence across an aridity gradient in northern temperate grasslands. Plant Soil 2018, 219, 649–663. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Z.Q.; Tian, F.P.; Wang, D.; Wu, G.L. Soil organic carbon and inorganic carbon accumulation along a 30-year grassland restoration chronosequence in semi-arid regions (China). Land Degrad. Dev. 2017, 28, 189–198. [Google Scholar] [CrossRef]
- Wang, W.Q.; Wang, C.; Sardans, J.; Tong, C.; Jia, R.X.; Zeng, C.S.; Penuelas, J. Flood regime affects soil stoichiometry and the distribution of the invasive plants in subtropical estuarine wetlands in China. Catena 2015, 128, 144–154. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M.; Gunapala, N.; Graham, K.J. Determination of soil microbial communities: Effects of agricultural management, season, and soil type on phospho-lipid fatty acid profiles. Microb. Ecol. 1998, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Parker, K.M.; Luo, Y.Q.; Wan, S.Q.; Wallac, L.L.; Hu, S. Soil microbial responses to experimental warming and clipping in a tallgrass prairie. Glob. Chang. Biol. 2005, 11, 266–277. [Google Scholar] [CrossRef]
- Moscatelli, M.C.; Di Tizio, A.; Marinari, S.; Grego, S. Microbial indicators related to soil carbon in Mediterranean land use system. Soil Tillage Res. 2007, 97, 51–59. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Application of eco-physiological quotients (qCO2 and qD) on microbial biomasses from soils of different cropping histories. Soil Biol. Biochem. 1990, 22, 251–255. [Google Scholar] [CrossRef]
- Ma, M.J.; Baskin, C.C.; Yu, K.L.; Ma, Z.; Du, G.Z. Wetland drying indirectly influences plant community and seed bank diversity through soil pH. Ecol. Indic. 2017, 80, 186–195. [Google Scholar] [CrossRef]
- Bachar, A.; AL-Ashhab, A.; Soares, M.I.M.; Sklarz, M.Y.; Angel, R.; Ungar, E.D.; Gillor, O. Soil microbial abundance and diversity along a low precipitation gradient. Microb. Ecol. 2010, 60, 453–461. [Google Scholar] [CrossRef]
- Or, D.; Smets, B.F.; Wraith, J.M.; Dechesne, A.; Friedman, S.P. Physical constraints affecting bacterial habitats and activity in unsaturated porous media—A review. Adv. Water Resour. 2007, 30, 1505–1527. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Physical vs. physiological controls on water-stress response in soil microbial communities. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Xu, X.; Schimel, J.P.; Thornton, P.E.; Song, X.; Yuan, F.; Goswami, S.; Bardgett, R. Substrate and environmental controls on microbial assimilation of soil organic carbon: A framework for earth system models. Ecol. Lett. 2014, 17, 547–555. [Google Scholar] [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Soil microbial community response to drying and rewetting stress: Does historical precipitation regime matter? Biogeochemistry 2012, 109, 101–116. [Google Scholar] [CrossRef]
- Gordon, H.; Haygarth, P.M.; Bardgett, R.D. Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biol. Biochem. 2008, 40, 302–311. [Google Scholar] [CrossRef]
- de Vries, F.T.; Liiri, M.E.; Bjornlund, L.; Bowker, M.A.; Chrisensen, S.; Setala, H.M.; Bardgett, R.D. Land use alters the resistance and resilience of soil food webs to drought. Nat. Clim. Chang. 2012, 2, 276–280. [Google Scholar] [CrossRef]
- Barnard, R.L.; Osborne, C.A.; Firestone, M.K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013, 7, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, M.; Chen, H.; Liu, L.; Wu, N.; Zhu, D.; Tian, J.; Peng, C.; Zhu, Q.; He, Y. Responses of CO2 emission and pore water DOC concentration to soil warming and water table drawdown in Zoige Peatlands. Atmos. Environ. 2017, 152, 323–329. [Google Scholar] [CrossRef]
- Laganiere, J.; Pare, D.; Bergeron, Y.; Chen, H.Y.H. The effect of boreal forest composition on soil respiration is mediated through variations in soil temperature and C quality. Soil Biol. Biochem. 2012, 53, 18–27. [Google Scholar] [CrossRef]
- Chivers, M.R.; Turetsky, M.R.; Waddington, J.M.; Harden, J.W.; Mcguire, A.D. Effects of experimetal water table and temperature manipulations on ecosystems CO2 fluxes in an Alaskan rich fen. Ecosystems 2009, 12, 1329–1342. [Google Scholar] [CrossRef]
- Makiranta, P.; Laiho, R.; Fritze, H.; Hytonen, J.; Laine, J.; Minkkinen, K. Indirect regulation of heterotrophic peat soil respiration by water level via microbial community structure and temperature sensitivity. Soil Biol. Biochem. 2009, 41, 695–703. [Google Scholar] [CrossRef]
- De Boeck, H.J.; Lemmens, C.M.H.M.; Vicca, S.; Berge, J.V.D.; Dongen, S.V.; Janssens, I.A.; Ceulemans, R.; Nijs, I. How do climate warming and species richness affect CO2 fluxes in experimental grasslands? New Phytol. 2007, 175, 512–522. [Google Scholar] [CrossRef]
- Traore, S.; Thiombiano, L.; Millogo, J.R.; Guinko, S. Carbon and nitrogen enhancement in Cambisols and Vertisols by Acacia spp. in eastern Burkina Faso: Relation to soil respiration and microbial biomass. Appl. Soil Ecol. 2007, 35, 660–669. [Google Scholar] [CrossRef]
- Manzoni, S.; Trofymow, J.A.; Jackson, R.B.; Porporato, A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 2010, 80, 89–106. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Baath, E. Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiol. Ecol. 2011, 78, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Waring, B.G.; Averill, C.; Hawkes, C.V. Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: Insights from meta-analysis and theoretical models. Ecol. Lett. 2013, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Zhang, F.S.; Gao, Q.; Mao, R.Z.; Liu, X.J. Impact of land-use types on soil nitrogen net mineralization in the sandstorm and water source area of Beijing, China. Catena 2010, 10, 15–22. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Liu, Y.; Hu, Y.; Zhang, M.; Wang, G.; Shen, W. Soil Microbial Community and Its Interaction with Soil Carbon Dynamics Following a Wetland Drying Process in Mu Us Sandy Land. Int. J. Environ. Res. Public Health 2020, 17, 4199. https://doi.org/10.3390/ijerph17124199
He H, Liu Y, Hu Y, Zhang M, Wang G, Shen W. Soil Microbial Community and Its Interaction with Soil Carbon Dynamics Following a Wetland Drying Process in Mu Us Sandy Land. International Journal of Environmental Research and Public Health. 2020; 17(12):4199. https://doi.org/10.3390/ijerph17124199
Chicago/Turabian StyleHe, Huan, Yixuan Liu, Yue Hu, Mengqi Zhang, Guodong Wang, and Weibo Shen. 2020. "Soil Microbial Community and Its Interaction with Soil Carbon Dynamics Following a Wetland Drying Process in Mu Us Sandy Land" International Journal of Environmental Research and Public Health 17, no. 12: 4199. https://doi.org/10.3390/ijerph17124199
APA StyleHe, H., Liu, Y., Hu, Y., Zhang, M., Wang, G., & Shen, W. (2020). Soil Microbial Community and Its Interaction with Soil Carbon Dynamics Following a Wetland Drying Process in Mu Us Sandy Land. International Journal of Environmental Research and Public Health, 17(12), 4199. https://doi.org/10.3390/ijerph17124199



