Clinical Reasoning Behind Non-Pharmacological Interventions for the Management of Headaches: A Narrative Literature Review
Abstract
:1. Introduction
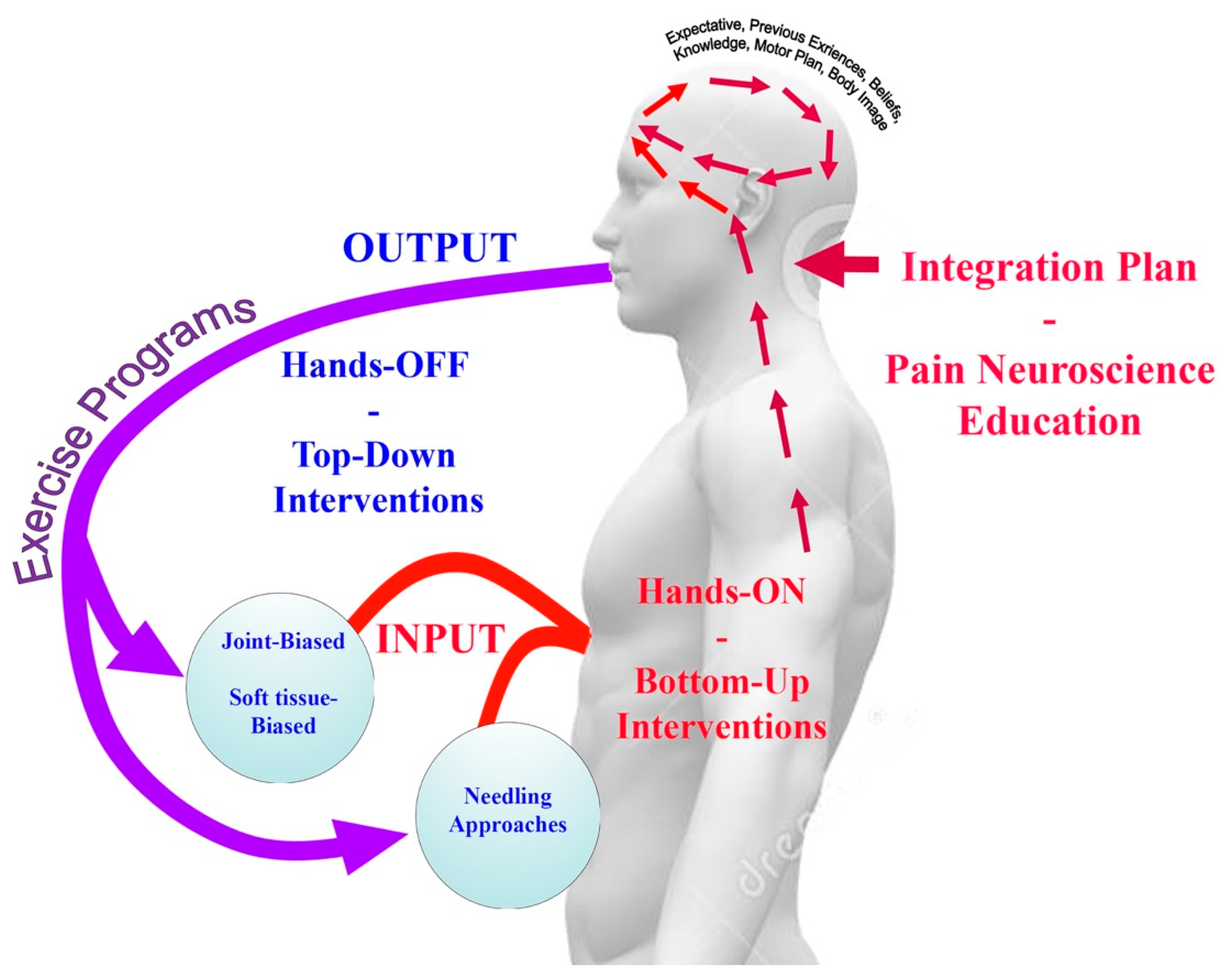
2. Bottom Up (Hands-ON) or Top Down (Hands-OFF) Interventions
3. Literature Search
3.1. Search Strategy
3.2. Inclusion Criteria and Data Extraction
3.3. Data Mapping
4. Scientific Evidence of Joint-biased Interventions for Headaches
5. Scientific Evidence of Soft Tissue-biased Interventions for Headaches
6. Scientific Evidence of Needling Therapies Interventions for Headaches
7. Scientific Evidence of Exercise Interventions for Headaches
8. Scientific Evidence of Cognitive Interventions for Headaches
9. Summarizing the Literature: which Headache Patients Can Benefit from Non-Pharmacological Interventions?
10. Clinical Reasoning for the Management of Headaches
11. Future Research Questions
12. Conclusions
Key Findings
- Treatment strategies used for the management of headache should include beyond tissue- based impairment therapies (bottom-up interventions) and central nervous system interventions (top down interventions).
- Bottom-up (hand-on) strategies can include joint-biased, soft-tissue biased, and needling therapies whereas top-down (hand-off) strategies include exercise and cognitive interventions.
- Evidence shows that the effectiveness of non-pharmacological interventions for headaches depends on a clinical reasoning since not all strategies are equally effective for all headaches.
- Evidence of non-pharmacological interventions is more controversial for migraine than for TTH or CeH, since migraine pathogenesis mainly involves activation of sub-cortical structures and the trigemino-vascular system whereas pathogenesis of TTH or CeH is associated to musculoskeletal impairments of the cervical spine.
Author Contributions
Funding
Conflicts of Interest
References
- Steiner, T.J.; Stovner, L.J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lantéri-Minet, M.; Rastenyte, D.; Ruiz de la Torre, E.; Tassorelli, C.; Barré, J.; et al. The impact of headache in Europe: Principal results of the Eurolight project. J. Headache Pain 2014, 15, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Headache Society, ICHD-III Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 3 editions. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, T.; Manzoni, G.C.; Russo, M.; Camarda, C.; Taga, A.; Veronesi, L.; Pasquarella, C.; Sansebastiano, G.; Torelli, P. Prevalence of tension-type headache in adult general population: The PACE study and review of the literature. Neurol. Sci. 2013, 34, S137–S138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burch, R.; Rizzoli, P.; Loder, E. The prevalence and impact of migraine and severe headache in the United States: Figures and trends from government health studies. Headache 2018, 58, 496–505. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.S.; Sibbritt, D.W.; Adams, J. A critical review of manual therapy use for headache disorders: Prevalence, profiles, motivations, communication and self-reported effectiveness. BMC Neurol 2017, 17, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendtsen, L.; Evers, S.; Linde, M.; Mitsikostas, D.D.; Sandrini, G.; Schoenen, J.; EFNS. EFNS guideline on the treatment of tension-type headache—Report of an EFNS task force. Eur. J. Neurol. 2010, 17, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Granella, F.; Prudenzano, M.; Pini, L.A.; Guidetti, V.; Bono, G.; Pinessi, L.; Alessandri, M.; Antonaci, F.; Fanciullacci, M.; et al. Italian guidelines for primary headaches: 2012 revised version. J. Headache Pain 2012, 13, S31–S70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Probyn, K.; Bowers, H.; Mistry, D.; Caldwell, F.; Underwood, M.; Patel, S.; Sandhu, H.K.; Matharu, M.; Pincus, T.; CHESS team. Non-pharmacological self-management for people living with migraine or tension-type headache: A systematic review including analysis of intervention components. BMJ Open 2017, 7, e01667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaibi, A.; Russell, M. Manual therapies for primary chronic headaches: A systematic review of randomized controlled trials. J. Headache Pain 2014, 15, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaibi, A.; Tuchin, P.J.; Russell, M.B. Manual therapies for migraine: A systematic review. J. Headache Pain 2011, 12, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaibi, A.; Russell, M.B. Manual therapies for cervicogenic headache: A systematic review. J. Headache Pain 2012, 13, 351–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa-Jiménez, J.A.; Lozano-López, C.; Angulo-Díaz-Parreño, S.; Rodríguez-Fernández, Á.L.; De-la-Hoz-Aizpurua, J.L.; Fernández-de-las-Peñas, C. Multimodal manual therapy vs. pharmacological care for management of tension type headache: A meta-analysis of randomized trials. Cephalalgia 2015, 35, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, K.; Allers, A.; Schulte, L.; May, A. Efficacy of interventions used by physiotherapists for patients with headache and migraine-systematic review and meta-analysis. Cephalalgia 2016, 36, 474–492. [Google Scholar] [CrossRef] [PubMed]
- Falsiroli Maistrello, L.; Rafanelli, M.; Turolla, A. Manual therapy and quality of life in people with headache: Systematic review and meta-analysis of randomized controlled trials. Curr. Pain Headache Rep. 2019, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Lozano-López, C.; Mesa-Jiménez, J.; de-la-Hoz-Aizpurúa, J.L.; Pareja-Grande, J.; Fernández- de-las-Peñas, C. Efficacy of manual therapy in the treatment of tension-type headache: A systematic review from 2000–2013. Neurologia 2016, 31, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.A.; Fernández-de-las-Peñas, C.; Bond, S. Mechanisms of chronic pain—Key considerations for appropriate physical therapy management. J. Man. Manip. Ther. 2017, 25, 118–127. [Google Scholar] [CrossRef] [PubMed]
- De Tommaso, M.; Fernández-de-las-Peñas, C. Tension type headache. Curr. Rheumatol. Rev. 2016, 12, 127–139. [Google Scholar] [CrossRef] [PubMed]
- De Tommaso, M.; Sciruicchio, V. Migraine and central sensitization: Clinical features, main comorbidities and therapeutic perspectives. Curr. Rheumatol. Rev. 2016, 12, 11326. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Van Houdenhova, B.; Oostendorp, R.A.B. Recognition of central sensitization in patients with musculoskeletal pain: Application of pain neurophysiology in manual therapy practice. Man. Ther. 2010, 15, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bialosky, J.E.; Beneciuk, J.M.; Bishop, M.D.; Coronado, R.A.; Penza, C.W.; Simon, C.; George, S.Z. Unraveling the mechanisms of manual therapy: Modelling an approach. J. Orthop. Sports Phys. Ther. 2018, 48, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Lluch Girbés, E.; Meeus, M.; Baert, I.; Nijs, J. Balancing “hands-on” with “hands-off” physical therapy interventions for the treatment of central sensitization pain in osteoarthritis. Man. Ther. 2015, 20, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Gallace, A.; Spence, C. The science of interpersonal touch: An overview. Neurosci. Biobehav. Rev. 2010, 34, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Autret, A.; Valade, D.; Debiais, S. Placebo and other psychological interactions in headache treatment. J. Headache Pain 2012, 13, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luedtke, K.; Boissonnault, W.; Caspersen, N.; Castien, R.; Chaibi, A.; Falla, D.; Fernández-de Las-Peñas, C.; Hall, T.; Hirsvang, J.R.; Horre, T.; et al. International consensus on the most useful physical examination tests used by physiotherapists for patients with headache: A Delphi study. Man. Ther. 2016, 23, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Meer, H.A.; Visscher, C.M.; Vredeveld, T.; Nijhuis van der Sanden, M.W.; Engelbert, R.; Speksnijder, C.M. The diagnostic accuracy of headache measurement instruments: A systematic review and meta-analysis focusing on headaches associated with musculoskeletal symptoms. Cephalalgia 2019, 39, 1313–1332. [Google Scholar] [CrossRef] [PubMed]
- Ashina, S.; Bendtsen, L.; Lyngberg, A.C.; Lipton, R.B.; Hajiyeva, N.; Jensen, R. Prevalence of neck pain in migraine and tension-type headache: A population study. Cephalalgia 2015, 35, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Mintken, P.E.; DeRosa, C.; Little, T.; Smith, B.; American Academy of Orthopaedic Manual Physical Therapists. AAOMPT clinical guidelines: A model for standardizing manipulation terminology in physical therapy practice. J. Orthop. Sports Phys. Ther. 2008, 38, A1–A6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenssinck, M.; Damen, L.; Verhagen, A.; Berger, M.; Passchier, J.; Koes, B. The effectiveness of physiotherapy and manipulation in patients with tension-type headache: A systematic review. Pain 2004, 112, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Bronfort, G.; Nilsson, N.; Haas, M.; Evans, R.; Goldsmith, C.H.; Assendelft, W.J.; Bouter, L.M. Non-invasive physical treatments for chronic/recurrent headache. Cochrane Database Syst. Rev. 2004, 3, CD001878. [Google Scholar]
- Posadzki, P.; Ernst, E. Spinal manipulations for the treatment of migraine: A systematic review of randomized clinical trials. Cephalalgia 2011, 31, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Ernst, E. Systematic reviews of spinal manipulations for headaches: An attempt to clear up the confusion. Headache 2011, 51, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Ernst, E. Spinal manipulations for cervicogenic headaches: A systematic review of randomized clinical trials. Headache 2011, 51, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Racicki, S.; Gerwin, S.; Diclaudio, S.; Reinmann, S.; Donaldson, M. Conservative physical therapy management for the treatment of cervicogenic headache: A systematic review. J. Man. Manip. Ther. 2013, 21, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rist, P.M.; Hernandez, A.; Bernstein, C.; Kowalski, M.; Osypiuk, K.; Vining, R.; Long, C.R.; Goertz, C.; Song, R.; Wayne, P.M. The impact of spinal manipulation on migraine pain and disability: A systematic review and meta-analysis. Headache 2019, 59, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.D.; Arnold, S.; Tetley, K.; Voight, K.; Frank, R. Mobilization and manipulation of the cervical spine in patients with cervicogenic headache: Any Scientific Evidence? Front. Neurol. 2016, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, E. Manipulation of the cervical spine: A systematic review of case reports of serious adverse events, 1995–2001. Med. J. Aust. 2002, 176, 376–380. [Google Scholar] [CrossRef]
- Puentedura, E.J.; March, J.; Anders, J.; Perez, A.; Landers, M.R.; Wallmann, H.W.; Cleland, J.A. Safety of cervical spine manipulation: Are adverse events preventable and are manipulations being performed appropriately?: A review of 134 case reports. J. Man. Manip. Ther. 2012, 20, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.M.; Tarp, S.; Christensen, R.; Bliddal, H.; Klokker, L.; Henriksen, M. The risk associated with spinal manipulation: An overview of reviews. Syst. Rev. 2017, 6, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, T.P.; Heldarskard, G.F.; Kolding, L.T.; Hvedstrup, J.; Schytz, H.W. Myofascial trigger points in migraine and tension-type headache. J. Headache Pain 2018, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Abboud, J.; Marchand, A.A.; Sorra, K.; Descarreaux, M. Musculoskeletal physical outcome measures in individuals with tension-type headache: A scoping review. Cephalalgia 2013, 33, 1319–1336. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Cuadrado, M.L.; Arendt-Nielsen, L.; Simons, D.G.; Pareja, J.A. Myofascial trigger points and sensitisation: An updated pain model for tension type headache. Cephalalgia 2007, 27, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Salom-Moreno, J.; Ge, H.Y.; Dommerholt, J. Manual treatment of myofascial trigger points. In Manual Therapy for Musculoskeletal Pain Syndromes of the Upper and Lower Quadrants: An Evidence and Clinical-Informed Approach; Fernández-de-las-Peñas, C., Cleland, J.A., Dommerholt, J., Eds.; Elsevier: London, UK, 2015; pp. 678–689. [Google Scholar]
- Falsiroli Maistrello, L.; Geri, T.; Gianola, S.; Zaninetti, M.; Testa, M. Effectiveness of trigger point manual treatment on the frequency, intensity, and duration of attacks in primary headaches: A systematic review and meta-analysis of randomized controlled trials. Front. Neurol. 2018, 9, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castien, R.F.; van der Windt, D.A.; Grooten, A.; Dekker, J. Effectiveness of manual therapy for chronic tension-type headache: A pragmatic, randomised, clinical trial. Cephalalgia 2011, 31, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, Z.; Wei, N.; Chang, W.; Chen, W.; Sui, H.J. Effectiveness of physical therapy on the suboccipital area of patients with tension-type headache: A meta-analysis of randomized controlled trials. Medicine 2019, 98, e15487. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Cuadrado, M.L. Therapeutic options for cervicogenic headache. Expert Rev. Neurother. 2014, 14, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.S.; Kuruvilla, D.; Blumenfeld, A.; Charleston L 4th Sorrell, M.; Robertson, C.E.; Grosberg, B.M.; Bender, S.D.; Napchan, U.; Ashkenazi, A.; Peripheral Nerve Blocks and Other Interventional Procedures Special Interest Section of the American Headache Society. Trigger point injections for headache disorders: Expert consensus methodology and narrative review. Headache 2014, 54, 1441–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunning, J.; Butts, R.; Mourad, F.; Young, I.; Flannagan, S.; Perreault, T. Dry needling: Literature review with implications for clinical practice guidelines. Phys. Ther. Rev. 2014, 19, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Linde, K.; Allais, G.; Brinkhaus, B.; Fei, Y.; Mehring, M.; Shin, B.C.; Vickers, A.; White, A.R. Acupuncture for the prevention of tension-type headache. Cochrane Database Syst Rev. 2016, 4, CD007587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linde, K.; Allais, G.; Brinkhaus, B.; Fei, Y.; Mehring, M.; Vertosick, E.A.; Vickers, A.; White, A.R. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016, 6, CD001218. [Google Scholar] [CrossRef] [PubMed]
- France, S.; Bown, J.; Nowosilskyj, M.; Mott, M.; Rand, S.; Walters, J. Evidence for the use of dry needling and physiotherapy in the management of cervicogenic or tension type headache: A systematic review. Cephalalgia 2014, 34, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, A.; Nakhostin Ansari, N.; Naghdi, S. Comparison of acute effects of superficial and deep dry needling into trigger points of suboccipital and upper trapezius muscles in patients with cervicogenic headache. J. Bodyw. Mov. Ther. 2017, 21, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Gildir, S.; Tüzün, E.H.; Eroğlu, G.; Eker, L. A randomized trial of trigger point dry needling versus sham needling for chronic tension-type headache. Medicine 2019, 98, e14520. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, H.B.; Handberg, G.; Graven-Nielsen, T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain 2014, 155, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Daenen, L.; Varkey, E.; Kellmann, M.; Nijs, J. Exercise, not to exercise, or how to exercise in patients with chronic pain? Applying science to practice. Clin. J. Pain 2015, 31, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Courtney, C.A. Clinical reasoning for manual therapy management of tension type and cervicogenic headache. J. Man. Manip. Ther. 2014, 22, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Fernández-de-las-Peñas, C. Physical therapy and exercise in headache. Cephalalgia 2008, 28, S36–S38. [Google Scholar] [CrossRef] [PubMed]
- Hindiyeh, N.A.; Krusz, J.C.; Cowan, R.P. Does exercise make migraines worse and tension type headaches better? Curr. Pain Headache Rep. 2013, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Madsen, B.K.; Søgaard, K.; Andersen, L.L.; Skotte, J.; Jensen, R. Neck and shoulder muscle strength in patients with tension-type headache: A case-control study. Cephalalgia 2016, 36, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-de-las-Peñas, C.; Falla, D.; Arendt-Nielsen, L.; Farina, D. Cervical muscle co activation in isometric contractions is enhanced in chronic tension-type headache patients. Cephalalgia 2008, 28, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Madsen, B.K.; Søgaard, K.; Andersen, L.L.; Skotte, J.; Tornøe, B.; Jensen, R. Neck/shoulder function in tension-type headache patients and the effect of strength training. J. Pain Res. 2018, 11, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, B.K.; Søgaard, K.; Andersen, L.L.; Tornøe, B.; Jensen, R.H. Efficacy of strength training on tension-type headache: A randomised controlled study. Cephalalgia 2018, 38, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Van Ettekoven, H.; Lucas, C. Efficacy of physiotherapy including a cranio-cervical training programme for tension-type headache: A randomized clinical trial. Cephalalgia 2006, 26, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Castien, R.; Blankenstein, A.; van der Windt, D.; Heymans, M.W.; Dekker, J. The working mechanism of manual therapy in participants with chronic tension-type headache. J. Orthop. Sports Phys. Ther. 2013, 43, 693–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florencio, L.L.; de Oliveira, I.V.; Lodovichi, S.S.; Bragatto, M.M.; Benatto, M.T.; Dach, F.; Fernández-de-las-Peñas, C.; Bevilaqua-Grossi, D. Cervical muscular endurance performance in women with and without migraine. J. Orthop. Sports Phys. Ther. 2019, 49, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Florencio, L.L.; Oliveira, A.S.; Lemos, T.W.; Carvalho, G.F.; Dach, F.; Bigal, M.E.; Falla, D.; Fernández-de-Las-Peñas, C.; Bevilaqua-Grossi, D. Patients with chronic, but not episodic, migraine display altered activity of their neck extensor muscles. J. Electromyogr. Kinesiol. 2016, 30, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Florencio, L.L.; de Oliveira, A.S.; Carvalho, G.F.; Tolentino Gde, A.; Dach, F.; Bigal, M.E.; Fernández-de-las-Peñas, C.; Bevilaqua Grossi, D. Cervical muscle strength and muscle coactivation during isometric contractions in patients with migraine: A cross-sectional study. Headache 2015, 55, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Kay, T.M.; Paquin, J.P.; Blanchette, S.; Lalonde, P.; Christie, T.; Dupont, G.; Graham, N.; Burnie, S.J.; Gelley, G.; et al. Exercises for mechanical neck disorders. Cochrane Database Syst Rev. 2015, 1, CD004250. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, A.; Kindelan-Calvo, P.; Agudo-Carmona, D.; Muñoz-Plata, R.; López-de Uralde-Villanueva, I.; La Touche, R. Therapeutic exercise as treatment for migraine and tension-type headaches: A systematic review of randomised clinical trials. Rev. Neurol. 2013, 57, 433–443. [Google Scholar] [PubMed]
- Lemmens, J.; De Pauw, J.; Van Soom, T.; Michiels, S.; Versijpt, J.; van Breda, E.; Castien, R.; De Hertogh, W. The effect of aerobic exercise on the number of migraine days, duration and pain intensity in migraine: A systematic literature review and meta-analysis. J. Headache Pain 2019, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Varkey, E.; Cider, A.; Carlsson, J.; Linde, M. Exercise as migraine prophylaxis: A randomized study using relaxation and topiramate as controls. Cephalalgia 2011, 31, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Machado-Oliveira, L.; da Silva Gauto, Y.O.; de Santana Neto, F.J.; da Silva, M.G.; Germano-Soares, A.H.; Diniz, P.R.B. Effects of Different Exercise Intensities on Headache: A Systematic Review. Am. J. Phys Med. Rehabil. 2020, 99, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Gaul, C.; van Doorn, C.; Webering, N.; Dlugaj, M.; Katsarava, Z.; Diener, H.C.; Fritsche, G. Clinical outcome of a headache-specific multidisciplinary treatment program and adherence to treatment recommendations in a tertiary headache center: An observational study. J. Headache Pain 2011, 12, 475–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benatto, M.T.; Bevilaqua-Grossi, D.; Carvalho, G.F.; Bragatto, M.M.; Pinheiro, C.F.; Straceri Lodovichi, S.; Dach, F.; Fernández-de-las-Peñas, C.; Florencio, L.L. Kinesiophobia is associated with migraine. Pain Med. 2019, 20, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Ceña, D.; Neira-Martín, B.; Silva-Hernández, L.; Mayo-Canalejo, D.; Florencio, L.L.; Fernández-de-las-Peñas, C.; García-Moreno, H.; García-Azorín, D.; Cuadrado, M.L. Living with chronic migraine: A qualitative study on female patients’ perspectives from a specialised headache clinic in Spain. BMJ Open 2017, 7, e017851. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, A.B.W.; Davis-Martin, R.E.; Houle, T.T.; Turner, D.P.; Smitherman, T.A. Perceived triggers of primary headache disorders: A meta-analysis. Cephalalgia 2018, 38, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Bussone, G.; D’Amico, D.; Cortelli, P.; Cevoli, S.; Manzoni, G.C.; Torelli, P.; Tonini, M.C.; Allais, G.; De Simone, R.; et al. Headache, anxiety and depressive disorders: The HADAS study. J. Headache Pain 2010, 11, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri, S.; Behnezhad, S.; Azad, E. Migraine headache and depression in adults: A systematic review and meta-analysis. Neuropsychiatrie 2019, 33, 131–140. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Fernández-Muñoz, J.J.; Palacios-Ceña, M.; Parás-Bravo, P.; Cigarán-Méndez, M.; Navarro-Pardo, E. Sleep disturbances in tension-type headache and migraine. Ther. Adv. Neurol. Disord. 2017, 11, 1756285617745444. [Google Scholar] [CrossRef] [PubMed]
- Garramone, F.; Baiano, C.; Russo, A.; D’Iorio, A.; Tedeschi, G.; Trojano, L.; Santangelo, G. Personality profile and depression in migraine: A meta-analysis. Neurol. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Benito-González, E.; Palacios-Ceña, M.; Fernández-Muñoz, J.J.; Castaldo, M.; Wang, K.; Catena, A.; Arendt-Nielsen, L.; Fernández-de-las-Peñas, C. Variables associated with sleep quality in chronic tension-type headache: A cross-sectional and longitudinal design. PLoS ONE 2018, 13, e0197381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huguet, A.; McGrath, P.J.; Stinson, J.; Tougas, M.E.; Doucette, S. Efficacy of psychological treatment for headaches: An overview of systematic reviews and analysis of potential modifiers of treatment efficacy. Clin. J. Pain 2014, 30, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.H.; Cho, E.Y.; Kim, S.M.; Yoon, S. Efficacy of psychological treatment for headache disorder: A systematic review and meta-analysis. J. Headache Pain 2019, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Law, E.; Dudeney, J.; Palermo, T.M.; Stewart, G.; Eccleston, C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst. Rev. 2018, 9, CD003968. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.P.; Martin, P.R.; Boschen, M.J. Psychological sleep interventions for migraine and tension-type headache: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 6411. [Google Scholar] [CrossRef] [PubMed]
- Nestoriuc, Y.; Rief, W.; Martin, A. Meta-analysis of biofeedback for tension-type headache: Efficacy, specificity, and treatment moderators. J. Consult. Clin. Psychol. 2008, 76, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Nestoriuc, Y.; Martin, A. Efficacy of biofeedback for migraine: A meta-analysis. Pain 2007, 128, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Physicians (UK) National Clinical Guideline Centre. National Clinical Guideline C. National Institute for Health and Clinical Excellence: Guidance. Headaches: Diagnosis and Management of Headaches in Young People and Adults; Royal College of Physicians (UK) National Clinical Guideline Centre: London, UK, 2012. [Google Scholar]
- Kindelan-Calvo, P.; Gil-Martínez, A.; Paris-Alemany, A.; Pardo-Montero, J.; Muñoz-García, D.; Angulo-Díaz-Parreño, S.; La Touche, R. Effectiveness of therapeutic patient education for adults with migraine. A systematic review and meta-analysis of randomized controlled trials. Pain Med. 2014, 15, 1619–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-de-las-Peñas, C.; Cuadrado, M.L. Physical therapy for headaches. Cephalalgia 2016, 36, 1134–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jull, G.; Stanton, W. Predictors of responsiveness to physiotherapy management of cervicogenic headache. Cephalalgia 2005, 25, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Cleland, J.A.; Palomeque-del-Cerro, L.; Caminero, A.B.; Guillem-Mesado, A.; Jiménez-García, R. Development of a clinical prediction rule for identifying women with tension-type headache who are likely to achieve short-term success with joint mobilization and muscle trigger point therapy. Headache 2011, 51, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Castien, R.F.; van der Windt, D.A.; Blankenstein, A.H.; Heymans, M.W.; Dekker, J. Clinical variables associated with recovery in patients with chronic tension-type headache after treatment with manual therapy. Pain 2012, 153, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.J. Cervicogenic headache: Evidence that the neck is a pain generator. Headache 2010, 50, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.A.; Buse, D.C.; Andrasik, F.; Lipton, R.B. Non-pharmacologic treatments for migraine and tension-type headache: How to choose and when to use. Curr. Treat. Options Neurol. 2011, 13, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Galea, O.; Thomas, L.; Jull, G.; Treleaven, J. Cervical musculoskeletal impairments in migraine and tension type headache: A systematic review and meta-analysis. Musculoskelet. Sci. Pract. 2019, 42, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Szikszay, T.M.; Hoenick, S.; von Korn, K.; Meise, R.; Schwarz, A.; Starke, W.; Luedtke, K. Which examination tests detect differences in cervical musculoskeletal impairments in people with migraine? A systematic review and meta-analysis. Phys Ther. 2019, 99, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Côté, P.; Yu, H.; Shearer, H.M.; Randhawa, K.; Wong, J.J.; Mior, S.; Ameis, A.; Carroll, L.J.; Nordin, M.; Varatharajan, S.; et al. Non-pharmacological management of persistent headaches associated with neck pain: A clinical practice guideline from the Ontario protocol for traffic injury management (OPTIMa) collaboration. Eur. J. Pain 2019, 23, 1051–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, A.J.; Rees, R.W.; Zollman, C.E.; McCarney, R.; Smith, C.M.; Ellis, N.; Fisher, P.; van Haselen, R.; Wonderling, D.; Grieve, R. Acupuncture of chronic headache disorders in primary care: Randomised controlled trial and economic analysis. Health Technol. Assess. 2004, 8, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, C.M.; Reinhold, T.; Jena, S.; Brinkhaus, B.; Willich, S.N. Cost-effectiveness of acupuncture treatment in patients with headache. Cephalalgia 2008, 28, 334–345. [Google Scholar] [CrossRef] [PubMed]
| Intervention | Tension Type Headache | Migraine | Cervicogenic Headache |
|---|---|---|---|
| Joint-biased interventions | − | +/− | + |
| Soft-tissues interventions | + | +/− | ? |
| Needling interventions | |||
| Acupuncture | + | + | ? |
| Dry Needling | ? | ? | ? |
| Exercises | |||
| Localized | + | ? | + |
| Aerobic | + | + | ? |
| Cognitive Interventions | + | + | ? |
| Pain Neuroscience Education | ? | + | ? |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-de-las-Peñas, C.; Florencio, L.L.; Plaza-Manzano, G.; Arias-Buría, J.L. Clinical Reasoning Behind Non-Pharmacological Interventions for the Management of Headaches: A Narrative Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 4126. https://doi.org/10.3390/ijerph17114126
Fernández-de-las-Peñas C, Florencio LL, Plaza-Manzano G, Arias-Buría JL. Clinical Reasoning Behind Non-Pharmacological Interventions for the Management of Headaches: A Narrative Literature Review. International Journal of Environmental Research and Public Health. 2020; 17(11):4126. https://doi.org/10.3390/ijerph17114126
Chicago/Turabian StyleFernández-de-las-Peñas, César, Lidiane L. Florencio, Gustavo Plaza-Manzano, and José L. Arias-Buría. 2020. "Clinical Reasoning Behind Non-Pharmacological Interventions for the Management of Headaches: A Narrative Literature Review" International Journal of Environmental Research and Public Health 17, no. 11: 4126. https://doi.org/10.3390/ijerph17114126
APA StyleFernández-de-las-Peñas, C., Florencio, L. L., Plaza-Manzano, G., & Arias-Buría, J. L. (2020). Clinical Reasoning Behind Non-Pharmacological Interventions for the Management of Headaches: A Narrative Literature Review. International Journal of Environmental Research and Public Health, 17(11), 4126. https://doi.org/10.3390/ijerph17114126







