Gender Differences in Medication Use: A Drug Utilization Study Based on Real World Data
Abstract
1. Introduction
2. Methods
- All 84 ATC second-level groups available on the Campania market were identified.
- Twenty ATC second-level groups with a prevalence rate >3% were selected for the study.
- From these 20 ATC second-level groups, all 57 pharmacological/therapeutic subgroups (ATC IV) accounting for >90% of the total volume expressed in defined daily dose (DDD) were selected.
2.1. Drug Utilization Indicators
2.2. Statistical Analysis
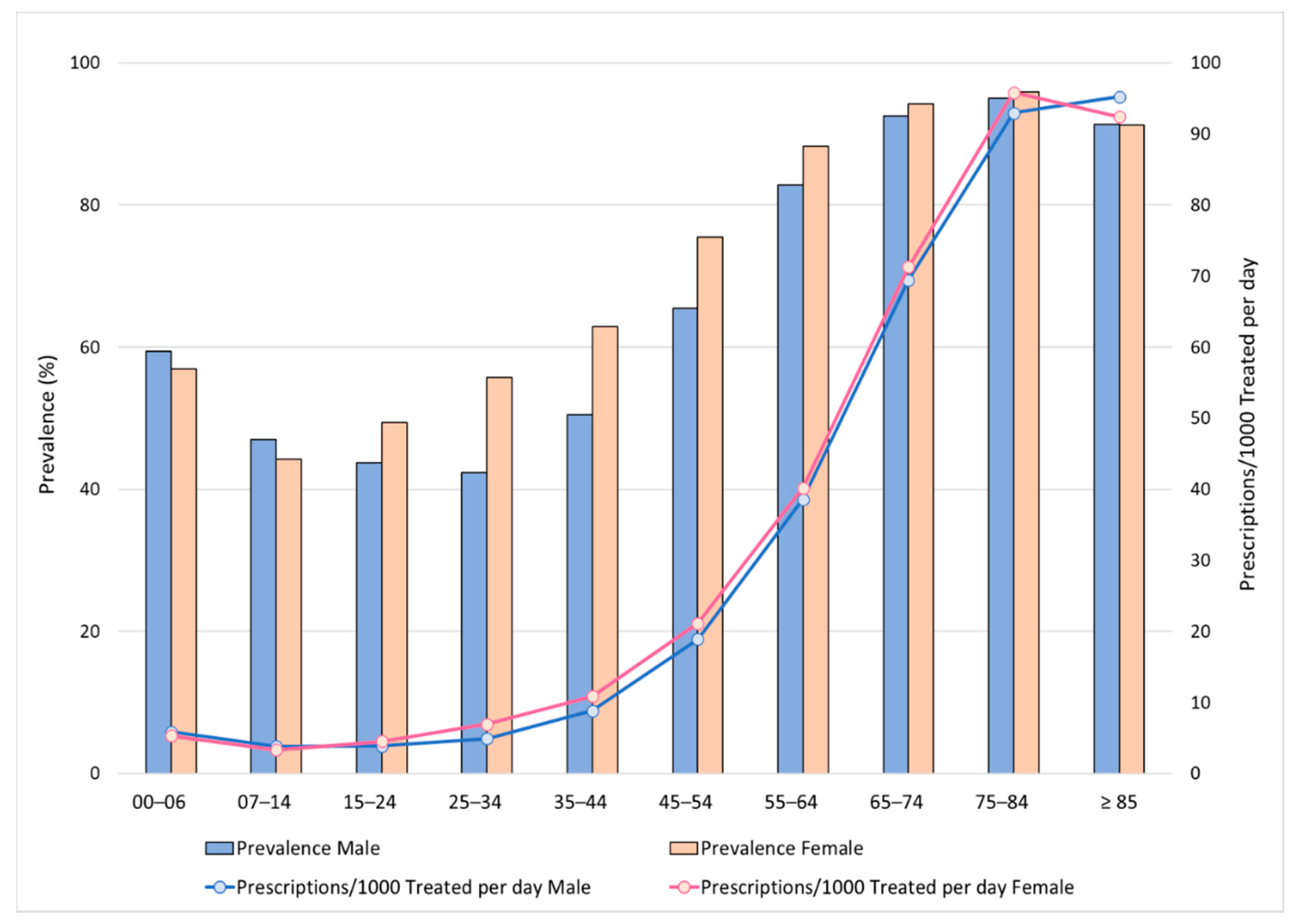
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. The rational use of drugs. In Proceedings of the WHO Report of the Conferences of Experts, Nairobi, Kenya, 25–29 November 1985. [Google Scholar]
- Soldin, O.P.; Chung, S.H.; Mattison, D.R. Sex differences in drug disposition. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V. Sex and gender differences in health. Science & Society Series on Sex and Science. EMBO Rep. 2012, 13, 596–603. [Google Scholar] [PubMed]
- Gulbins, H.; Vogel, B.; Reichenspurner, H. Gender effects on health care costs in cardiovascular medicine-a black box? Thorac. Cardiovasc. Surg. 2013, 61, 74–78. [Google Scholar] [PubMed]
- Owens, G.M. Gender differences in health care expenditures, resource utilization, and quality of care. J. Manag. Care Pharm. 2008, 14, 2–6. [Google Scholar] [CrossRef]
- Putignano, D.; Bruzzese, D.; Orlando, V.; Fiorentino, D.; Tettamanti, A.; Menditto, E. Differences in drug use between men and women: An Italian cross sectional study. BMC Women’s Health 2017, 17, 73. [Google Scholar] [CrossRef]
- World Health Organization. What Do We Mean by “Sex” and “Gender”. Available online: http://www.who.int/gender/whatisgender/en (accessed on 1 February 2020).
- Loikas, D.; Wettermark, B.; von Euler, M.; Bergman, U.; Schenck-Gustafsson, K. Differences in drug utilisation between men and women: A cross-sectional analysis of all dispensed drugs in Sweden. BMJ Open 2013, 3, e002378. [Google Scholar] [CrossRef]
- Campbell, C.I.; Weisner, C.; Leresche, L.; Ray, G.T.; Saunders, K.; Sullivan, M.D.; BantaGreen, C.J.; Merrill, J.O.; Silverberg, M.J.; Boudreau, D.; et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am. J. Public Health 2010, 100, 2541–2547. [Google Scholar] [CrossRef]
- Johnell, K.; Fastbom, J. Gender and use of hypnotics or sedatives in old age: A nationwide register-based study. Int. J. Clin. Pharm. 2011, 33, 788–793. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J. Sex and gender differences in therapy of type 2 diabetes. Diabetes Res. Clin. Pract. 2017, 131, 230–241. [Google Scholar] [CrossRef]
- Fernández-Liz, E.; Modamio, P.; Catalán, A.; Lastra, C.F.; Rodríguez, T.; Mariño, E.L. Identifying how age and gender influence prescription drug use in a primary health care environment in Catalonia, Spain. Br. J. Clin. Pharmacol. 2008, 65, 407–417. [Google Scholar] [CrossRef]
- Roe, C.M.; McNamara, A.M.; Motheral, B.R. Gender- and age-related prescription drug use patterns. Ann. Pharmacother. 2002, 36, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Glaeske, G.; Gerdau-Heitmann, C.; Hofel, F.; Schicktanz, C. “Gender-specific drug prescription in Germany” results from prescriptions analyses. Handb. Exp. Pharmacol. 2012, 214, 149–167. [Google Scholar]
- Sondik, E.J.M.; Jennifer, H.; Gentleman, J.F. Summary Health Statistics for the U.S. Population: National Health Interview Survey, 2009; National Health Survey: Hyattsville, MD, USA, 2010; Volume 248.
- Sleator, D.J.D. Towards accurate prescribing analysis in general practice: Accounting for the effects of practice demography. Br. J. Gen. Pract. 1993, 43, 102–106. [Google Scholar] [PubMed]
- Lloyd, D.C.E.F.; Harris, C.M.; Roberts, D.J. Specific therapeutic age-sex related prescribing units (STAR-PUs): Weightings for analysing general practices’ prescribing in England. BMJ 1995, 311, 991–994. [Google Scholar] [CrossRef]
- Roberts, S.J.; Harris, C.M. Age, sex and temporary resident originated prescribing units (ASTRO-PUs): New weightings for analysing prescribing of general practices in England. BMJ 1993, 307, 485–488. [Google Scholar] [CrossRef][Green Version]
- Melzer, D.; Tavakoly, B.; Winder, R.E.; Masoli, J.A.; Henley, W.E.; Ble, A.; Richards, S.H. Much more medicine for the oldest old: Trends in UK electronic clinical records. Age Ageing 2015, 44, 46–53. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Selection of Essential Drugs; Technical Report Series No. 615; World Health Organization: Geneva, Switzerland, 1977. [Google Scholar]
- Iolascon, G.; Gimigliano, F.; Moretti, A.; Riccio, I.; Di Gennaro, M.; Illario, M.; Monetti, V.M.; Orlando, V.; Menditto, E. Rates and reasons for lack of persistence with anti-osteoporotic drugs: Analysis of the Campania region database. Clin. Cases Miner. Bone Metab. 2016, 13, 126–129. [Google Scholar] [CrossRef]
- Menditto, E.; Cahir, C.; Aza-Pascual-Salcedo, M.; Bruzzese, D.; Poblador-Plou, B.; Malo, S.; Costa, E.; González-Rubio, F.; Gimeno-Miguel, A.; Orlando, V.; et al. Adherence to chronic medication in older populations: Application of a common protocol among three European cohorts. Patient Prefer Adherence 2018, 12, 1975–1987. [Google Scholar] [CrossRef]
- Menditto, E.; Guerriero, F.; Orlando, V.; Crola, C.; Di Somma, C.; Illario, M.; Morisky, D.E.; Colao, A. Self-assessment of adherence to medication: A case study in Campania region community-dwelling population. J. Aging Res. 2015, 2015. [Google Scholar] [CrossRef]
- Casula, M.; Catapano, A.L.; Piccinelli, R.; Menditto, E.; Manzoli, L.; De Fendi, L.; Orlando, V.; Flacco, M.E.; Gambera, M.; Filippi, A.; et al. Assessment and potential determinants of compliance and persistence to antiosteoporosis therapy in Italy. Am. J. Manag. Care 2014, 20, e138–e145. [Google Scholar]
- Guerriero, F.; Orlando, V.; Monetti, V.M.; Russo, V.; Menditto, E. Biological therapy utilization, switching, and cost among patients with psoriasis: Retrospective analysis of administrative databases in Southern Italy. Clin. Outcomes Res. 2017, 9, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Juste, A.; Menditto, E.; Orlando, V.; Monetti, V.M.; Gimeno-Miguel, A.; González-Rubio, F.; Aza-Pascual-Salcedo, M.M.; Cahir, C.; Prados-Torres, A.; Riccard, G. Treatment patterns of diabetes in Italy: A population-based study. Front. Pharmacol. 2019, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- WHOCC-ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 7 May 2018).
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment. 2019. Available online: https://www.whocc.no/news/guidelines_for_atc_classification_and_ddd_assignment (accessed on 9 May 2019).
- Demo-Geodemo. Mappe, Popolazione, Statistiche Demografiche dell’ISTAT. Available online: http://demo.istat.it/. (accessed on 7 February 2019).
- Newman, S.C. Biostatistical Methods in Epidemiology; John Wiley and Sons: New York, NY, USA, 2001. [Google Scholar]
- Manteuffel, M.; Williams, S.; Chen, W.; Verbrugge, R.R.; Pittman, D.G.; Steinkellner, A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J. Womens Health 2014, 23, 112–119. [Google Scholar] [CrossRef] [PubMed]
- The Medicines Utilisation Monitoring Centre. National Report on Medicines Use in Italy. Year 2018; Italian Medicines Agency: Rome, Italy, 2019. Available online: https://www.aifa.gov.it/web/guest/-/national-report-on-medicines-use-in-italy-year-2018-english-edition- (accessed on 8 May 2019).
- Stock, S.A.; Stollenwerk, B.; Redaelli, M.; Civello, D.; Lauterbach, K.W. Sex differences in treatment patterns of six chronic diseases: An analysis from the German statutory health insurance. J. Womens Health 2008, 17, 343–354. [Google Scholar] [CrossRef]
- Van Boxel, O.S.; Hagenaars, M.P.; Smout, A.J.P.M.; Siersema, P.D. Socio-demographic factors influence chronic proton pump inhibitor use by a large population in the Netherlands. Aliment. Pharmacol. Ther. 2009, 29, 571–579. [Google Scholar] [CrossRef]
- Davis, J.S.; Lee, H.Y.; Kim, J.; Advani, S.M.; Peng, H.L.; Banfield, E.; Frazier-Wood, A.C. Use of non-steroidal anti-inflammatory drugs in US adults: Changes over time and by demographic. Open Heart 2017, 4, e000550. [Google Scholar] [CrossRef]
- Santalucia, P.; Franchi, C.; Djade, C.D.; Tettamanti, M.; Pasina, L.; Corrao, S.; Mannucci, P.M. Gender difference in drug use in hospitalized elderly patients. Eur. J. Intern. Med. 2015, 26, 483–490. [Google Scholar] [CrossRef]
- Iolascon, G.; Gimigliano, F.; Orlando, V.; Capaldo, A.; Di Somma, C.; Menditto, E. Osteoporosis drugs in real-world clinical practice: An analysis of persistence. Aging Clin. Exp. Res. 2013, 25, S137–S141. [Google Scholar] [CrossRef]
- Cawthon, P.M. Gender differences in osteoporosis and fractures. Clin. Orthop. Relat. Res. 2011, 469, 1900–1905. [Google Scholar] [CrossRef]
- Pinto-Meza, A.; Usall, J.; Serrano-Blanco, A.; Suárez, D.; Haro, J.M. Gender differences in response to antidepressant treatment prescribed in primary care. Does menopause make a difference? J. Affect. Disord. 2006, 93, 53–60. [Google Scholar] [CrossRef]
- Richardson, J.; Holdcroft, A. Gender differences and pain medication. Womens Health 2009, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Bassols, A.; Bosch, F.; Baños, J.E. How does the general population treat their pain? A survey in Catalonia, Spain. J. Pain Symptom Manag. 2002, 23, 318–328. [Google Scholar] [CrossRef]
- Bauer, M.; Glenn, T.; Pilhatsch, M.; Pfennig, A.; Whybrow, P.C. Gender differences in thyroid system function: Relevance to bipolar disorder and its treatment. Bipolar Disord. 2014, 16, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, C.; Kahan, T.; Schiöler, L.; Hjerpe, P.; Hasselström, J.; Wettermark, B.; Manhem, K. Gender differences in antihypertensive drug treatment: Results from the Swedish Primary Care Cardiovascular Database (SPCCD). J. Am. Soc. Hypertens. 2014, 8, 882–890. [Google Scholar] [CrossRef]
- Cammarota, S.; Bruzzese, D.; Catapano, A.L.; Citarella, A.; De Luca, L.; Manzoli, L.; Masulli, M.; Menditto, E.; Mezzetti, A.; Riegler, S.; et al. Lower incidence of macrovascular complications in patients on insulin glargine versus those on basal human insulins: A population-based cohort study in Italy. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 10–17. [Google Scholar] [CrossRef]
- Illario, M.; Vollenbroek-Hutten, M.M.R.; Molloy, D.W.; Menditto, E.; Iaccarino, G.; Eklund, P. Active and healthy ageing and independent living. J. Aging Res. 2015, 2015. [Google Scholar] [CrossRef]
- Illario, M.; Vollenbroek-Hutten, M.M.R.; Molloy, D.W.; Menditto, E.; Iaccarino, G.; Eklund, P. Active and healthy ageing and independent living. J. Aging Res. 2016, 2016. [Google Scholar] [CrossRef]
- Coretti, S.; Romano, F.; Orlando, V.; Codella, P.; Prete, S.; Di Brino, E.; Ruggeri, M. Economic evaluation of screening programs for hepatitis C virus infection: Evidence from literature. Risk Manag. Healthc. Policy 2015, 8, 45. [Google Scholar]
- Menditto, E.; Orlando, V.; Coretti, S.; Putignano, D.; Fiorentino, D.; Ruggeri, M. Doctors commitment and long-term effectiveness for cost containment policies: Lesson learned from biosimilar drugs. Clin. Outcomes Res. 2015, 7, 575. [Google Scholar]
- Scala, D.; Menditto, E.; Caruso, G.; Monetti, V.M.; Orlando, V.; Guerriero, F.; Buonomo, G.; Caruso, D.; D’Avino, M. Are you more concerned about or relieved by medicines? An explorative randomized study of the impact of telephone counseling by pharmacists on patients’ beliefs regarding medicines and blood pressure control. Patient Educ. Couns. 2018, 101, 679–686. [Google Scholar] [CrossRef]
- Ruggeri, M.; Manca, A.; Coretti, S.; Codella, P.; Iacopino, V.; Romano, F.; Mascia, D.; Orlando, V.; Cicchetti, A. Investigating the generalizability of economic evaluations conducted in Italy: A critical review. Value Health 2015, 18, 709–720. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Male | Female | Overall |
|---|---|---|---|
| Number of treated patients | 1,785,518 (45.8%) | 2,113,842 (54.2%) | 3,899,360 |
| Prevalence of drug use/100 inhabitants (95% CI) | 62.84 (62.75–62.94) | 70.79 (70.70–71.89) | 66.92 (66.85–67.00) |
| Number of prescriptions | 25,454,363 (44.4%) | 31,903,087 (55.6%) | 57,357,450 |
| DDD per treated patient | 495.6 | 481.5 | 488.0 |
| Cost per treated patient (€) | 227.2 | 225.6 | 226.3 |
| ATC II | Description | Adjusted Prevalence % (CI) | Adjusted RR (95% CI) Male/Female | ||
|---|---|---|---|---|---|
| Male | Female | Overall | |||
| J01 | Antibacterials for systemic use | 43.33 (43.26−43.41) | 50.55 (50.48−50.64) | 47.50 (47.45−47.56) | 0.857 (0.856−0.858) |
| A02 | Drugs for acid related disorders | 24.96 (24.90−25.02) | 30.88 (30.81−30.94) | 28.45 (28.41−28.50) | 0.808 (0.807−0.810) |
| M01 | Anti-inflammatory and antirheumatic products | 18.67 (18.61−18.71) | 25.66 (25.60−25.72) | 22.55 (22.51−22.59) | 0.727 (0.726−0.728) |
| C09 | Agents acting on the renin–angiotensin system | 22.16 (22.10−22.22) | 23.48 (23.42−23.54) | 23.25 (23.21−23.29) | 0.943 (0.942−0.946) |
| R03 | Drugs for obstructive airway diseases | 15.37 (15.31−15.42) | 17.02 (16.98−17.07) | 16,40 (16.36−16.43) | 0.902 (0.901−0.905) |
| H02 | Corticosteroids for systemic use | 13.59 (13.55−13.64) | 16.81 (16.77−16.86) | 15.41 (15.38−15.44) | 0.808 (0.807−0.810) |
| C10 | Lipid modifying agents | 14.00 (13.95−14.05) | 14.31 (14.27−14.36) | 14.41 (14.38−14.45) | 0.977 (0.976−0.980) |
| C07 | Beta blocking agents | 11.57 (11.53−11.61) | 14.53 (14.49−14.58) | 13.34 (13.31−13.37) | 0.796 (0.794−0.798) |
| A11 | Vitamins | 4.43 (4.41−4.46) | 20.54 (20.49−20.59) | 12.94 (12.92−12.98) | 0.215 (0.215−0.216) |
| B01 | Antithrombotic agents | 10.73 (10.69−10.77) | 12.00 (11.96−12.04) | 11.65 (11.62−11.68) | 0.894 (0.892−0.896) |
| R06 | Antihistamines for systemic use | 7.55 (7.52−7.59) | 9.73 (9.70−9.77) | 8.74 (8.72−8.77) | 0.776 (0.774−0.779) |
| A07 | Antidiarrheals. Intestinal anti-inflammatory/anti-infective agents | 6.44 (6.41−6.47) | 7.37 (7.34−7.40) | 7.02 (6.99−7.04) | 0.873 (0.871−0.877) |
| A10 | Drugs used in diabetes | 6.80 (6.77−6.83) | 6.25 (6.22−6.28) | 6.65 (6.62−6.67) | 1.088 (1.085−1.092) |
| C08 | Calcium channel blockers | 6.38 (6.35−6.42) | 6.48 (6.45−6.51) | 6.57 (6.55−6.59) | 0.985 (0.982−0.989) |
| N06 | Psychoanaleptics | 3.66 (3.64−3.68) | 7.07 (7.04−7.10) | 5.51 (5.49−5.53) | 0.517 (0.515−0.519) |
| C03 | Diuretics | 4.27 (4.24−4.29) | 6.31 (6.28−6.34) | 5.47 (5.45−5.49) | 0.676 (0.673−0.679) |
| B03 | Antianemic preparations | 2.77 (2.75−2.79) | 6.72 (6.69−6.75) | 4.89 (4.87−4.91) | 0.412 (0.410−0.414) |
| H03 | Thyroid therapy | 1.52 (1.51−1.54) | 6.47 (6.44−6.50) | 4.13 (4.11−4.14) | 0.235 (0.234−0.236) |
| N02 | Analgesics | 2.80 (2.77−2.81) | 5.50 (5.46−5.52) | 4.25 (4.24−4.28) | 0.509 (0.507−0.511) |
| N03 | Antiepileptics | 2.97 (2.95−2.99) | 3.80 (3.77−3.82) | 3.44 (3.43−3.46) | 0.781 (0.777−0.785) |
| ATC II | Description | DDD/Treated | Sporadic Users (%) | Cost/Treated (€) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Overall | Male | Female | Overall | Male | Female | Overall | ||
| J01 | Antibacterials for systemic use | 18.6 | 18.8 | 18.7 | 47.6 | 43.9 | 45.6 | 29.1 | 28.8 | 28.9 |
| A02 | Drugs for acid related disorders | 110.1 | 110.1 | 110.1 | 26.5 | 26.1 | 26.3 | 64.1 | 63.1 | 63.5 |
| M01 | Anti-inflammatory and antirheumatic products | 37.5 | 44.6 | 41.7 | 54.4 | 47.1 | 50.1 | 10.1 | 12.3 | 11.4 |
| C09 | Agents acting on the renin–angiotensin system | 368.2 | 350.8 | 359.1 | 6.8 | 6.8 | 6.8 | 80.3 | 79.4 | 79.9 |
| R03 | Drugs for obstructive airway diseases | 83.8 | 70.9 | 76.9 | 51.8 | 54.9 | 53.5 | 118.8 | 95.0 | 106 |
| H02 | Corticosteroids for systemic use | 29.0 | 30.8 | 30 | 68.4 | 65.4 | 66.7 | 7.8 | 8.6 | 8.2 |
| C10 | Lipid modifying agents | 273.6 | 221.2 | 246.6 | 10.6 | 11.2 | 10.9 | 114.3 | 92.5 | 103.0 |
| C07 | Beta blocking agents | 155.0 | 168.4 | 162.6 | 11.0 | 10.6 | 10.8 | 38.6 | 39.4 | 39.0 |
| A11 | Vitamins | 86.2 | 97.8 | 95.8 | 37.6 | 24.2 | 26.5 | 40.4 | 51.1 | 49.3 |
| B01 | Antithrombotic agents | 189.9 | 174.2 | 181.5 | 21.7 | 24.0 | 22.9 | 38.6 | 44.7 | 41.9 |
| R06 | Antihistamines for systemic use | 57.3 | 59.3 | 58.4 | 63.9 | 63.1 | 63.4 | 13.4 | 13.5 | 13.5 |
| A07 | Antidiarrheals. Intestinal anti-inflammatory | 41.5 | 34.1 | 37.5 | 60.6 | 58.1 | 59.2 | 58.1 | 50.7 | 54.1 |
| A10 | drugs used in diabetes | 271.2 | 267.4 | 269.3 | 8.4 | 8.5 | 8.5 | 123.2 | 124.3 | 123.7 |
| C08 | Calcium channel blockers | 297.3 | 267.8 | 282.2 | 14.4 | 16.9 | 15.7 | 61.5 | 59.6 | 60.5 |
| N06 | Psychoanaleptics | 182.4 | 192.2 | 189 | 26.5 | 22.7 | 24.0 | 81.8 | 82.0 | 81.9 |
| C03 | Diuretics | 175.7 | 149.2 | 159.7 | 32.1 | 34.3 | 33.4 | 25.8 | 22.2 | 23.6 |
| B03 | Antianemic preparations | 116.7 | 100.7 | 105 | 48.9 | 53.2 | 52.1 | 24.6 | 20.2 | 21.4 |
| H03 | Thyroid therapy | 160.3 | 147.0 | 149.4 | 24.0 | 19.8 | 20.6 | 17.9 | 17.7 | 17.7 |
| N02 | Analgesics | 21.5 | 23.2 | 22.6 | 61.1 | 53.9 | 56.2 | 74.5 | 71.3 | 72.3 |
| N03 | Antiepileptics | 142.8 | 111.3 | 124.9 | 24.7 | 27.0 | 26.0 | 179.0 | 151.4 | 163.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, V.; Mucherino, S.; Guarino, I.; Guerriero, F.; Trama, U.; Menditto, E. Gender Differences in Medication Use: A Drug Utilization Study Based on Real World Data. Int. J. Environ. Res. Public Health 2020, 17, 3926. https://doi.org/10.3390/ijerph17113926
Orlando V, Mucherino S, Guarino I, Guerriero F, Trama U, Menditto E. Gender Differences in Medication Use: A Drug Utilization Study Based on Real World Data. International Journal of Environmental Research and Public Health. 2020; 17(11):3926. https://doi.org/10.3390/ijerph17113926
Chicago/Turabian StyleOrlando, Valentina, Sara Mucherino, Ilaria Guarino, Francesca Guerriero, Ugo Trama, and Enrica Menditto. 2020. "Gender Differences in Medication Use: A Drug Utilization Study Based on Real World Data" International Journal of Environmental Research and Public Health 17, no. 11: 3926. https://doi.org/10.3390/ijerph17113926
APA StyleOrlando, V., Mucherino, S., Guarino, I., Guerriero, F., Trama, U., & Menditto, E. (2020). Gender Differences in Medication Use: A Drug Utilization Study Based on Real World Data. International Journal of Environmental Research and Public Health, 17(11), 3926. https://doi.org/10.3390/ijerph17113926





