Nontuberculous Mycobacterial Disease and Molybdenum in Colorado Watersheds
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.1.1. NTM Species
2.1.2. Socio-Demographic Data
2.1.3. Environmental Exposure Data
2.1.4. Water-Quality Data Compilation
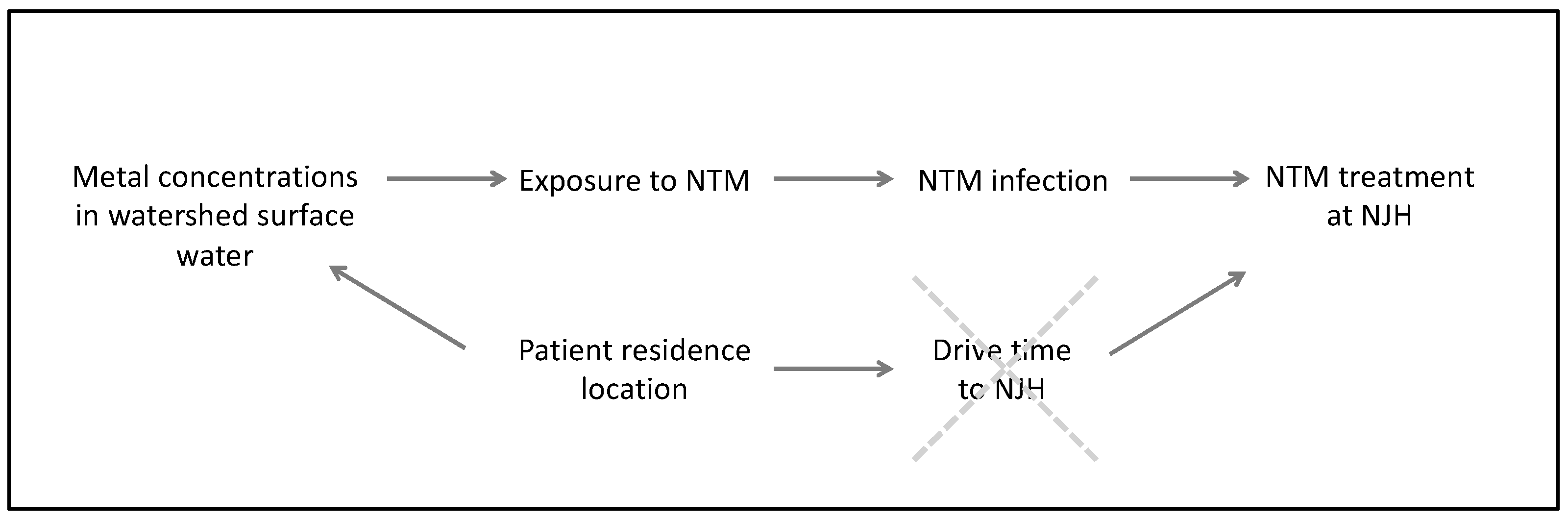
2.2. Statistical Analysis
2.2.1. Principal Component Analysis (PCA)
2.2.2. Poisson Regression Models
Principal Component Regression Model
Poisson Regression Models with Individual Metals
Sensitivity Analyses
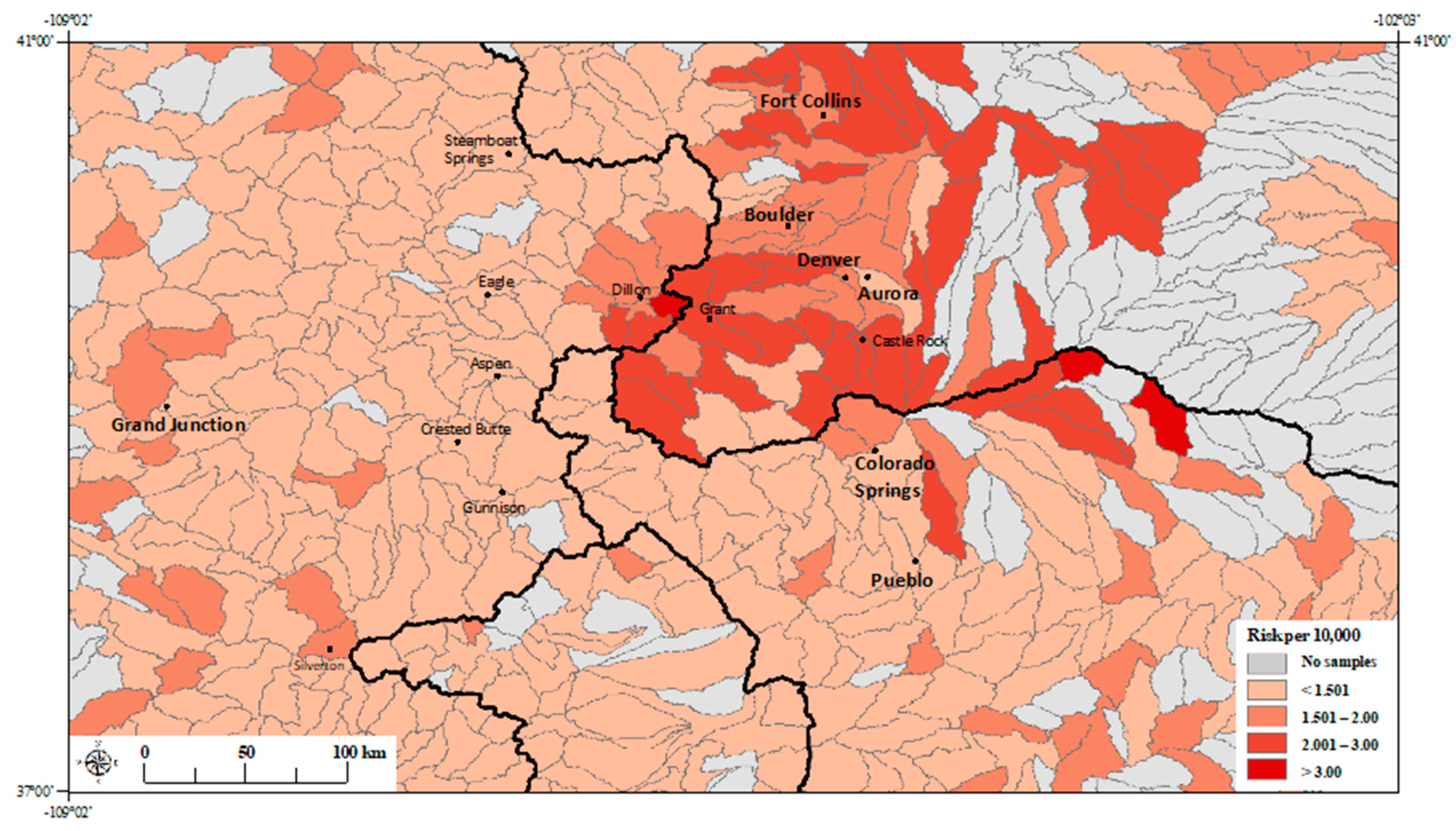
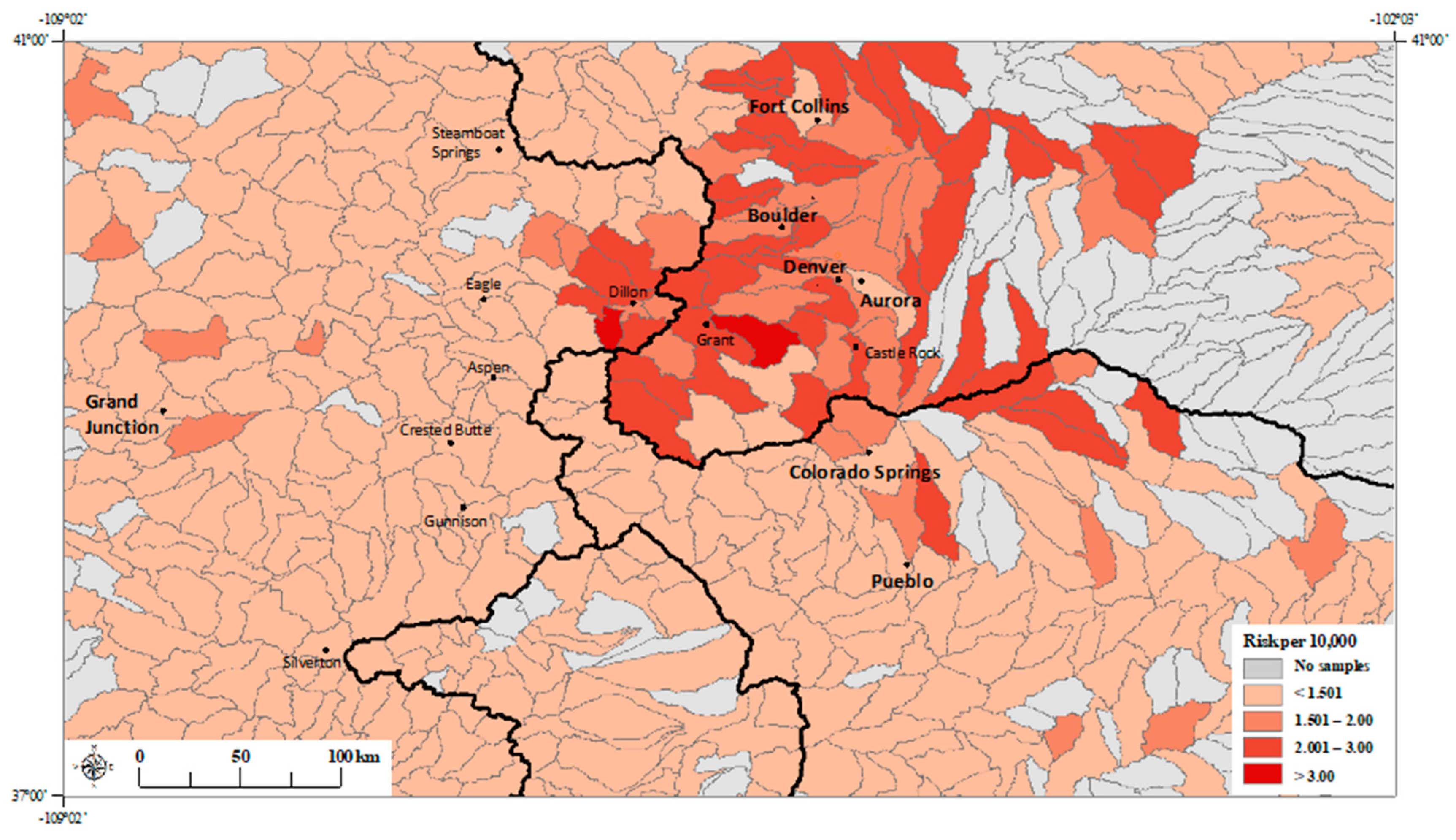
3. Results
3.1. Principal Component Regression Model
3.2. Poisson Regression Models with Individual Metals
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef]
- Winthrop, K.L.; McNelley, E.; Kendall, B.; Marshall-Olson, A.; Morris, C.; Cassidy, M.; Saulson, A.; Hedberg, K. Pulmonary Nontuberculous Mycobacterial Disease Prevalence and Clinical Features. Am. J. Respir. Crit. Care Med. 2010, 182, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Bottai, D.; Stinear, T.P.; Supply, P.J.; Brosch, R. Mycobacterial Pathogenomics and Evolution. Microbiol. Spectr. 2014, 2, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O., III. Surrounded by mycobacteria: Nontuberculous mycobacteria in the human environment. J. Appl. Microbiol. 2009, 107, 356–367. [Google Scholar]
- Strollo, S.E.; Adjemian, J.; Adjemian, M.K.; Prevots, D.R. The Burden of Pulmonary Nontuberculous Mycobacterial Disease in the United States. Ann. Am. Thorac. Soc. 2015, 12, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Gebert, M.J.; Delgado-Baquerizo, M.; Oliverio, A.M.; Webster, T.M.; Nichols, L.M.; Honda, J.R.; Chan, E.D.; Adjemian, J.; Dunn, R.R.; Fierer, N. Ecological Analyses of Mycobacteria in Showerhead Biofilms and Their Relevance to Human Health. MBio 2018, 9, e01614-18. [Google Scholar] [CrossRef]
- Falkinham, J.O., III; Parker, B.C.; Gruft, H. Epidemiology of infection by nontuberculous mycobacteria. I. Geographic distribution in the eastern United States. Am. Rev. Respir. Dis. 1980, 121, 931–937. [Google Scholar] [PubMed]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Falkinham, J.O., III; Holland, S.M.; Prevots, D.R. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am. J. Respir. Crit. Care Med. 2012, 186, 553–558. [Google Scholar] [CrossRef]
- Adjemian, J.; Daniel-Wayman, S.; Ricotta, E.; Prevots, D.R. Epidemiology of Nontuberculous Mycobacteriosis. Semin. Respir. Crit. Care Med. 2018, 39, 325–335. [Google Scholar] [PubMed]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am. J. Respir. Crit. Care Med. 2012, 185, 881–886. [Google Scholar]
- Adjemian, J.; Olivier, K.N.; Prevots, D.R. Epidemiology of Pulmonary Nontuberculous Mycobacterial Sputum Positivity in Patients with Cystic Fibrosis in the United States, 2010–2014. Ann. Am. Thorac. Soc. 2018, 15, 817–826. [Google Scholar] [CrossRef]
- Adjemian, J.; Olivier, K.N.; Prevots, D.R. Nontuberculous Mycobacteria among Patients with Cystic Fibrosis in the United States. Screening Practices and Environmental Risk. Am. J. Respir. Crit. Care Med. 2014, 190, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Frankland, T.B.; Daida, Y.G.; Honda, J.R.; Olivier, K.N.; Zelazny, A.; Honda, S.; Prevots, D.R. Epidemiology of Nontuberculous Mycobacterial Lung Disease and Tuberculosis, Hawaii, USA. Emerg. Infect. Dis. 2017, 23, 439–447. [Google Scholar] [CrossRef]
- Lipner, E.; Knox, D.; French, J.; Rudman, J.; Strong, M.; Crooks, J.L. A Geospatial Epidemiologic Analysis of Nontuberculous Mycobacterial Infection: An Ecological Study in Colorado. Ann. Am. Thorac. Soc. 2017, 14, 1523–1532. [Google Scholar] [CrossRef]
- Chou, M.P.; Clements, A.C.; Thomson, R.M. A spatial epidemiological analysis of nontuberculous mycobacterial infections in Queensland, Australia. BMC Infect. Dis. 2014, 14, 279. [Google Scholar] [CrossRef] [PubMed]
- Center for International Earth Science Information Network (CIESIN) Columbia University. U.S. Census Grids (Summary File 1) 2010; Center NSDaA, Ed.; SEDAC: Washington, DC, USA, 2017.
- U.S. Department of Agriculture, Natural Resources Conservation Service (USDA-NRCS) and the Environmental Protection Agency (EPA). The Watershed Boundary Dataset (WBD). Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/main/national/water/watersheds/ (accessed on 12 June 2015).
- US Geological Survey UDoA, National Water Quality Monitoring Council: Water Quality Portal. 2012. Available online: https://www.waterqualitydata.us/portal/ (accessed on 8 August 2019).
- Greve, A.I.; Van Metre, P.C.; Wilson, J.T. Identification of Water-Quality Trends Using Sediment Cores from Dillon Reservoir, Summit County, Colorado; United States of the Interior USGS: Denver, CO, USA, 2001. [Google Scholar]
- Bivand, R.; Keitt, T.; Rowlingson, B. Rdal: Bindings for the ‘Geospatial’ Data Abstraction Library. R Package Version 1.4-8. 2019. Available online: https://CRAN.R-project.org/package=rgdal (accessed on 25 April 2020).
- Roger, S.; Bivand, E.P.; Gomez-Rubio, C. Applied Spatial Data Analysis with R, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Gelman, A.; Su, Y.-S. Data Analysis Using Regression and Multilevel/Hierarchical Models: R package version 1.10-1. 2018. Available online: https://CRAN.R-project.org/package=arm (accessed on 25 April 2020).
- Wickham, H.; Francois, R.; Henry, L.; Muller, K. Dplyr: A Grammar of Data Manipulation. R package version 0.8.3. 2019. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 25 April 2020).
- Josse, J.; Husson, F. missMDA: A Package for Handling Missing Values in Multivariate Data Analysis. J. Stat. Softw. 2016, 70. [Google Scholar] [CrossRef]
- Bivand, R.; Rundel, C.; Pebesma, E.; Stuetz, R.; Hufthammer, K.O.; Giraaudoux, P.; Davis, M.; Santilli, S. Interface to Geometry Engine-Open Source (‘GEOS’); Rgeos: Washington, DC, USA, 2019. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25. [Google Scholar] [CrossRef]
- Colorado Foundation for Water Education: Citizen’s Guide to Where Your Water Comes From. Denver, CO. 2005. Available online: https://www.colorado.gov/pacific/sites/default/files/Citizen%27s%20Guide%20to%20Where%20Your%20Water%20Comes%20From.pdf (accessed on 25 April 2020).
- Molybdenum. Available online: http://coloradogeologicalsurvey.org/mineral-resources/metallic-minerals/moylbdenum/ (accessed on 25 April 2020).
- Levillain, F.; Poquet, Y.; Mallet, L.; Mazeres, S.; Marceau, M.; Brosch, R.; Bange, F.-C.; Supply, P.J.; Magalon, A.; Neyrolles, O. Horizontal acquisition of a hypoxia-responsive molybdenum cofactor biosynthesis pathway contributed to Mycobacterium tuberculosis pathoadaptation. PLOS Pathog. 2017, 13, e1006752. [Google Scholar] [CrossRef]
- Williams, M.J.; Kana, B.D.; Mizrahi, V. Functional Analysis of Molybdopterin Biosynthesis in Mycobacteria Identifies a Fused Molybdopterin Synthase in Mycobacterium tuberculosis. J. Bacteriol. 2010, 193, 98–106. [Google Scholar] [CrossRef]
- McGuire, A.M.; Weiner, B.; Park, S.T.; Wapinski, I.; Raman, S.; Dolganov, G.; Peterson, M.; Riley, R.; Zucker, J.; Abeel, T.; et al. Comparative analysis of Mycobacterium and related Actinomycetes yields insight into the evolution of Mycobacterium tuberculosis pathogenesis. BMC Genom. 2012, 13, 120. [Google Scholar] [CrossRef]
- Oh, J.; Shin, S.H.; Choi, R.; Kim, S.; Park, H.-D.; Kim, S.-Y.; Han, S.A.; Koh, W.-J.; Lee, S.-Y. Assessment of 7 trace elements in serum of patients with nontuberculous mycobacterial lung disease. J. Trace Elem. Med. Boil. 2019, 53, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. Molybdenum in natural waters: A review of occurrence, distributionsand controls. Appl. Geochem. 2017, 84, 387–432. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water, 3rd ed.; Survey USG, Ed.; US Geological Survey: Alexandria, VA, USA, 1985.
- Kaback, D.S. Transport of molybdenum in mountainous streams, Colorado. Geochim. Cosmochim. Acta 1976, 40, 581–582. [Google Scholar] [CrossRef]
- Kubota, J.; Lemon, E.R.; Allaway, W.H. The Effect of Soil Moisture Content Upon the Uptake of Molybdenum, Copper, and Cobalt by Alsike Clover. Soil Sci. Soc. Am. J. 1963, 27, 679–683. [Google Scholar] [CrossRef]
- Kirschner, R.A.; Parker, B.C.; Falkinham, J.O. Epidemiology of Infection by Nontuberculous Mycobacteria: Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum in Acid, Brown-Water Swamps of the Southeastern United States and Their Association with Environmental Variables. Am. Rev. Respir. Dis. 1992, 145, 271–275. [Google Scholar] [CrossRef]
- Thorel, M.F.; Falkinham, J.O., III. Moreau RG: Environmental mycobacteria from alpine and subalpine habitats. FEMS Microbiol. Ecol. 2004, 49, 343–347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jenne, E.A. Trace element sorption by sediments and soils—sites and processes. In Molybdenum in the Environment; Marcel Dekker: New York, NY, USA, 1977. [Google Scholar]
- Falkinham, J.O., III. Ecology of nontuberculous mycobacteria--where do human infections come from? Semin. Respir. Crit. Care Med. 2013, 34, 95–102. [Google Scholar] [CrossRef]
- Lande, L.; Alexander, D.C.; Wallace, R.J.; Kwait, R.; Iakhiaeva, E.; Williams, M.; Cameron, A.D.S.; Olshefsky, S.; Devon, R.; Vasireddy, R.; et al. Mycobacterium avium in Community and Household Water, Suburban Philadelphia, Pennsylvania, USA, 2010–2012. Emerg. Infect. Dis. 2019, 25, 473–481. [Google Scholar] [CrossRef]
- Falkinham, J.O., III. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg. Infect. Dis. 2011, 17, 419–424. [Google Scholar] [CrossRef]
- Honda, J.R.; Hasan, N.A.; Davidson, R.M.; Williams, M.D.; Epperson, L.E.; Reynolds, P.R.; Smith, T.; Iakhiaeva, E.; Bankowski, M.J.; Wallace, R.J., Jr.; et al. Environmental Nontuberculous Mycobacteria in the Hawaiian Islands. PLoS Negl. Trop. Dis. 2016, 10, e0005068. [Google Scholar] [CrossRef]
- Falkinham, J.O.; Norton, C.D.; Lechevallier, M.W. Factors Influencing Numbers of Mycobacterium avium, Mycobacterium intracellulare, and Other Mycobacteria in Drinking Water Distribution Systems. Appl. Environ. Microbiol. 2001, 67, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- King, D.N.; Donohue, M.J.; Vesper, S.J.; Villegas, E.N.; Ware, M.W.; Vogel, M.; Furlong, E.F.; Kolpin, D.W.; Glassmeyer, S.T.; Pfaller, S.L. Microbial pathogens in source and treated waters from drinking water treatment plants in the United States and implications for human health. Sci. Total. Environ. 2016, 562, 987–995. [Google Scholar] [PubMed]
- Falkinham, J.O., III. Environmental sources of nontuberculous mycobacteria. Clin. Chest Med. 2015, 36, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bull Run Water Treatment. 2019. Water Quality Report. West Slope Water District. Available online: http://www.wswd.org/document_center/Water%20Quality/2019%20Water%20Quality%20Report.pdf (accessed on 25 April 2020).
- Nishiuchi, Y.; Maekura, R.; Kitada, S.; Tamaru, A.; Taguri, T.; Kira, Y.; Hiraga, T.; Hirotani, A.; Yoshimura, K.; Miki, M.; et al. The Recovery of Mycobacterium avium-intracellulare Complex (MAC) from the Residential Bathrooms of Patients with Pulmonary MAC. Clin. Infect. Dis. 2007, 45, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.; Tolson, C.; Carter, R.; Coulter, C.; Huygens, F.; Hargreaves, M. Isolation of Nontuberculous Mycobacteria (NTM) from Household Water and Shower Aerosols in Patients with Pulmonary Disease Caused by NTM. J. Clin. Microbiol. 2013, 51, 3006–3011. [Google Scholar] [PubMed]
- Ivahnenko, T.; Ortiz, R.F.; Stogner, R. Characterization of streamflow, water quality, and instantaneous dissolved solids, selenium, and uranium loads in selected reaches of the Arkansas River, southeastern Colorado, 2009–2010. Sci. Investig. Rep. 2013, 5234. [Google Scholar]

| Exposure Characteristics | Median ± SD (µg/L) |
|---|---|
| Aluminum | 18 ± 4371.6 |
| Arsenic | <0.5 ± 49.9 |
| Cadmium | 0.1 ± 50.6 |
| Calcium | 32,110 ± 70,745.7 |
| Copper | 1.6 ± 440.8 |
| Iron | 38 ± 26,245.6 |
| Lead | <0.5 ± 326.4 |
| Magnesium | 6691 ± 40,822.9 |
| Manganese | 22.6 ± 7406.7 |
| Molybdenum | 4.3 ± 18.8 |
| Nickel | 1.2 ± 37.2 |
| pH | 7.93± 0.77 |
| Potassium | 1347 ± 6884.6 |
| Selenium | 0.06 ± 48.0 |
| Sodium | 6100 ± 123,203.3 |
| Zinc | 17 ± 5951.9 |
| Characteristics | Relative Risk (95% CI) p-Value |
|---|---|
| Age: ≥65 years (%) | 0.969 (0.059, 15.319) 0.813 |
| Race: Non-White a | 0.118 (0.046, 0.298) 1.57 × 10−6 |
| Drive Time (>2.0 h to NJH) | 0.634 (0.485, 0.821) 1.14 × 10−5 |
| Principal Component 1 | 1.054 (1.0007, 1.111) 0.049 |
| Principal Component 2 | 1.035 (0.979, 1.094) 0.223 |
| Principal Component 3 | 1.083 (1.009, 1.161) 0.026 |
| Characteristics | Relative Risk (95% CI) p-Value |
|---|---|
| Age: ≥65 years (%) | 2.16 (0.119, 37.6) 0.599 |
| Race: Non-White a | 0.076 (0.027, 0.211) 8.3 × 10−7 |
| Drive Time (>2.0 h to NJH) | 0.513 (0.393, 0.663) 5.1 × 10−7 |
| pH | 0.810 (0.543, 1.22) 0.306 |
| Arsenic (1-log unit) | 0.946 (0.869, 1.03) 0.082 |
| Calcium (1-log unit) | 1.95 (1.36, 2.77) 0.0003 |
| Magnesium (1-log unit) | 0.625 (0.475, 0.820) 0.0007 |
| Molybdenum (1-log unit) | 1.22 (1.07, 1.39) 0.0030 |
| Potassium (1-log unit) | 0.988 (0.785, 1.24) 0.918 |
| Selenium (1-log unit) | 0.966 (0.906, 1.03) 0.297 |
| Sodium (1-log unit) | 1.04 (0.882, 1.23) 0.637 |
| Characteristics | Relative Risk (95% CI) p-Value | Characteristics | Relative Risk (95% CI) p-Value | Characteristics | Relative Risk (95% CI) p-Value |
|---|---|---|---|---|---|
| Age: ≥65 years (%) | 1.32 (0.08, 20.5) 0.845 | Age: ≥65 years (%) | 1.42 (0.092, 21.0) 0.801 | Age: ≥65 years (%) | 0.86 (0.05, 13.7) 0.916 |
| Race: Non-Whitea | 0.14 (0.06, 0.32) 2.4 × 10−6 | Race: Non-White a | 0.20 (0.09, 0.44) 5.9 × 10−6 | Race: Non-White m a | 0.16 (0.07, 0.34) 3.7 × 10−6 |
| Drive Time (>2.0 h to NJH) | 0.52 (0.40, 0.66) 1.7 × 10−7 | Drive Time (>2.0 h to NJH) | 0.55 (0.43, 0.69) 9.2 × 10−7 | Drive Time (>2.0 h to NJH) | 0.55 (0.43, 0.69) 7.54 × 10−7 |
| Calcium (1-log unit) | 1.19 (1.05, 1.35) 0.0055 | Magnesium (1-log unit) | 1.06 (0.96, 1.17) 0.232 | Molybdenum (1-log unit) | 1.17 (1.05, 1.29) 0.0041 |
| Characteristics | Relative Risk (95% CI) p-Value |
|---|---|
| Age: ≥65 years (%) | 1.17 (0.065, 20.1) 0.915 |
| Race: Non-White a | 0.09 (0.03, 0.26) 1.1 × 10−5 |
| Drive Time (>2.0 h to NJH) | 0.57 (0.44, 0.73) 1.3 × 10−5 |
| pH | 0.99 (0.68, 1.45) 0.964 |
| Aluminum (1-log unit) | 1.01 (0.96, 1.07) 0.615 |
| Arsenic (1-log unit) | 0.90 (0.82, 0.99) 0.022 |
| Cadmium (1-log unit) | 1.00 (0.96, 1.04) 0.917 |
| Manganese (1-log unit) | 1.06 (0.96, 1.18) 0.255 |
| Molybdenum (1-log unit) | 1.23 (1.06, 1.41) 0.0049 |
| Selenium (1-log unit) | 1.00 (0.94, 1.06) 0.899 |
| Zinc (1-log unit) | 1.02 (0.93, 1.13) 0.653 |
| Characteristics | Relative Risk (95% CI) p-Value | Characteristics | Relative Risk (95% CI) p-Value |
|---|---|---|---|
| Age: ≥65 years (%) | 2.12 (0.14, 30.4) 0.583 | Age: ≥65 years (%) | 0.86 (0.051, 13.7) 0.916 |
| Race: Non-White a | 0.23 (0.11, 0.48) 8.3 × 10−5 | Race: Non-White a | 0.16 (0.07, 0.34) 3.7 × 10−6 |
| Drive Time (>2.0 h to NJH) | 0.56 (0.44, 0.70) 1.4 × 10−6 | Drive Time (>2.0 h to NJH) | 0.55 (0.43, 0.69) 7.54 × 10−7 |
| Arsenic (1-log unit) | 0.96 (0.89, 1.01) 0.113 | Molybdenum (1-log unit) | 1.17 (1.05, 1.29) 0.0041 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/1.0/).
Share and Cite
Lipner, E.M.; French, J.; Bern, C.R.; Walton-Day, K.; Knox, D.; Strong, M.; Prevots, D.R.; Crooks, J.L. Nontuberculous Mycobacterial Disease and Molybdenum in Colorado Watersheds. Int. J. Environ. Res. Public Health 2020, 17, 3854. https://doi.org/10.3390/ijerph17113854
Lipner EM, French J, Bern CR, Walton-Day K, Knox D, Strong M, Prevots DR, Crooks JL. Nontuberculous Mycobacterial Disease and Molybdenum in Colorado Watersheds. International Journal of Environmental Research and Public Health. 2020; 17(11):3854. https://doi.org/10.3390/ijerph17113854
Chicago/Turabian StyleLipner, Ettie M., Joshua French, Carleton R. Bern, Katherine Walton-Day, David Knox, Michael Strong, D. Rebecca Prevots, and James L. Crooks. 2020. "Nontuberculous Mycobacterial Disease and Molybdenum in Colorado Watersheds" International Journal of Environmental Research and Public Health 17, no. 11: 3854. https://doi.org/10.3390/ijerph17113854
APA StyleLipner, E. M., French, J., Bern, C. R., Walton-Day, K., Knox, D., Strong, M., Prevots, D. R., & Crooks, J. L. (2020). Nontuberculous Mycobacterial Disease and Molybdenum in Colorado Watersheds. International Journal of Environmental Research and Public Health, 17(11), 3854. https://doi.org/10.3390/ijerph17113854





