Uterine Fibroids Increase the Risk of Thyroid Cancer
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Sampled Participants
2.3. Statistical Analysis
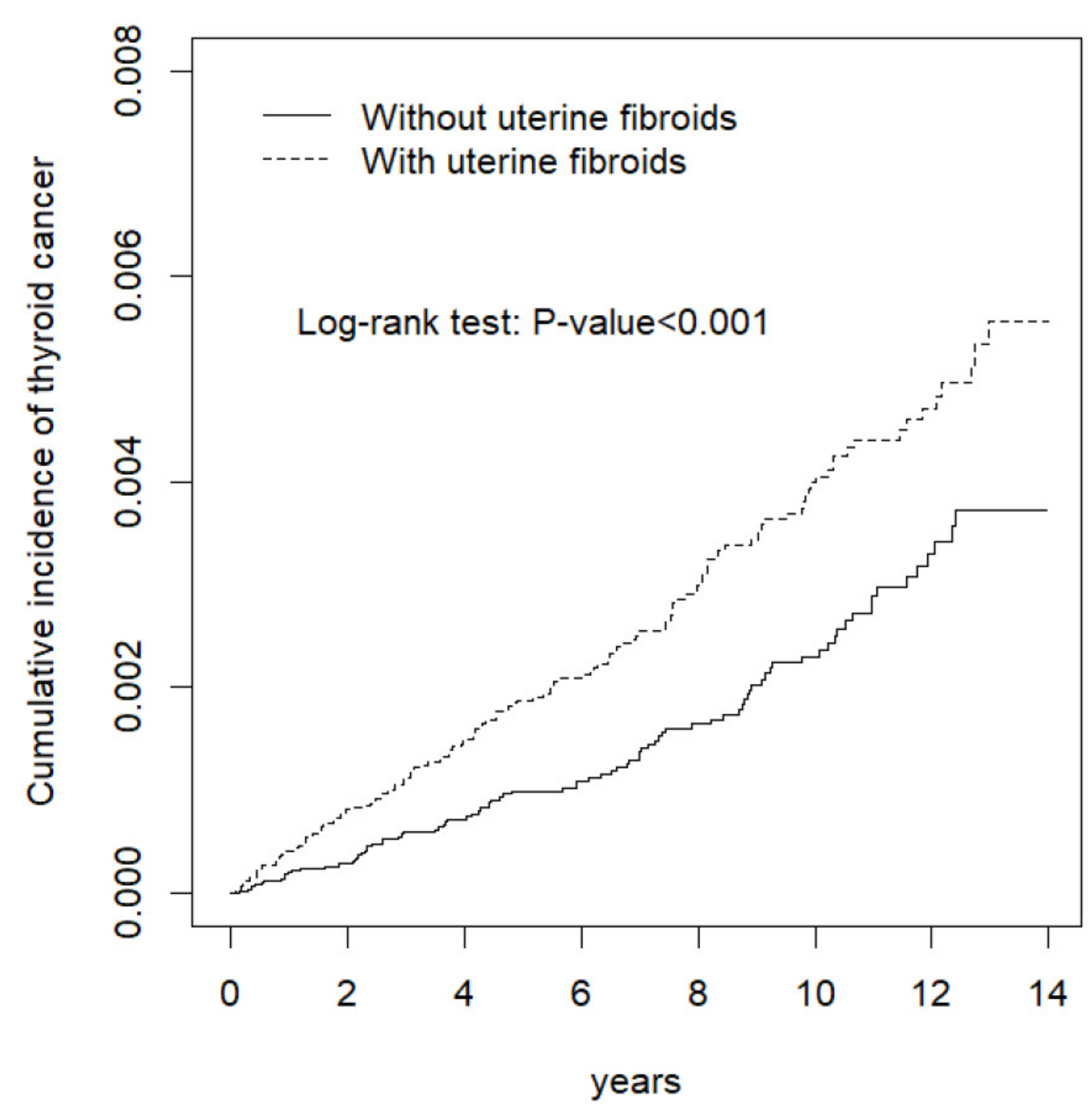
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
References
- Cheng, M.H.; Chao, H.T.; Wang, P.H. Unusual clinical presentation of uterine myomas. Taiwan J. Obstet. Gynecol. 2007, 46, 323–324. [Google Scholar] [CrossRef][Green Version]
- Laughlin, S.K.; Schroeder, J.C.; Baird, D.D. New directions in the epidemiology of uterus fibroids. Semin. Reprod. Med. 2010, 28, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.F.; Patel, A. The frequency of uterine leiomyomas. Am. J. Clin. Pathol. 1990, 94, 435–438. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, M.M.; Chennathukuzhi, V.M. Recent advances in uterine fibroid etiology. Semin. Reprod. Med. 2017, 35, 181–189. [Google Scholar] [CrossRef]
- Sparic, R.; Mirkovic, L.; Malvasi, A.; Tinelli, A. Epidemiology of uterine myomas: A review. Int. J. Fertil. Steril. 2016, 9, 424–435. [Google Scholar]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013, 2013, 96521. [Google Scholar] [CrossRef]
- Cancer Statistics Annual Report: Taiwan Cancer Registry. Available online: http://tcr.cph.ntu.edu.tw/main.php?Page=N2 (accessed on 22 May 2020).
- Braganza, M.Z.; de González, A.B.; Schonfeld, S.J.; Wentzensen, N.; Brenner, A.V.; Kitahara, C.M. Benign breast and gynecologic conditions, reproductive and hormonal factors, and risk of thyroid cancer. Cancer Prev. Res. (Phila.) 2014, 7, 418–425. [Google Scholar] [CrossRef]
- Shah, J.P. Thyroid carcinoma: Epidemiology, histology, and diagnosis. Clin. Adv. Hematol. Oncol. 2015, 13, 3–6. [Google Scholar]
- Liu, F.-C.; Lin, H.-T.; Lin, S.-F.; Kuo, C.-F.; Chung, T.-T.; Yu, H.-P. Nationwide cohort study on the epidemiology and survival outcomes of thyroid cancer. Oncotarget 2017, 22, 78429–78451. [Google Scholar] [CrossRef][Green Version]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and clinical perspectives on thyroid cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Thyroid Carcinoma. Version 2. 2014. National Comprehensive Cancer Network. Updated 12 August 2014. Available online: http://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (accessed on 22 May 2020).
- Guenego, A.; Mesrine, S.; Dartois, L.; Leenhardt, L.; Clavel-Chapelon, F.; Kvaskoff, M.; Boutron-Ruault, M.C.; Bonnet, F. Relation between hysterectomy, oophorectomy and the risk of incident differentiated thyroid cancer: The E3N cohort. Clin. Endocrinol. 2019, 90, 360–368. [Google Scholar] [CrossRef]
- Luo, J.; Hendryx, M.; Manson, J.E.; Liang, X.; Margolis, K.L. Hysterectomy, oophorectomy, and risk of thyroid cancer. J. Clin. Endocrinol. Metab. 2016, 101, 3812–3819. [Google Scholar] [CrossRef]
- Mack, W.J.; Preston-Martin, S.; Bernstein, L.; Qian, D.; Xiang, M. Reproductive and hormonal risk factors for thyroid cancer in Los Angeles county females. Cancer Epidemiol. Biomarkers Prev. 1999, 8, 991–997. [Google Scholar]
- Manta, L.; Suciu, N.; Toader, O.; Purcărea, R.M.; Constantin, A.; Popa, F. The etiopathogenesis of uterine fibromatosis. J. Med. Life 2016, 9, 39–45. [Google Scholar]
- Parker, W.H. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007, 87, 725–736. [Google Scholar] [CrossRef]
- Ron, E.; Schneider, A.B. Thyroid Cancer. In Cancer Epidemiology and Prevention; Schottenfeld, D., Fraumeni, J.F., Jr., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 975–994. [Google Scholar]
- Magri, F.; Capelli, V.; Rotondi, M.; Leporati, P.; La Manna, L.; Ruggiero, R.; Malovini, A.; Bellazzi, R.; Villani, L.; Chiovato, L. Expression of estrogen and androgen receptors in differentiated thyroid cancer: An additional criterion to assess the patient’s risk. Endocr. Relat. Cancer 2012, 19, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Moleti, M.; Sturniolo, G.; Di Mauro, M.; Russo, M.; Vermiglio, F. Female reproductive factors and differentiated thyroid cancer. Front. Endocrinol. (Lausanne) 2017, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Yane, K.; Kitahori, Y.; Konishi, N.; Okaichi, K.; Ohnishi, T.; Miyahara, H.; Matsunaga, T.; Lin, J.C.; Hiasa, Y. Expression of the estrogen receptor in human thyroid neoplasms. Cancer Lett. 1994, 84, 59–66. [Google Scholar] [CrossRef]
- Faria, C.C.; Peixoto, M.S.; Carvalho, D.P.; Fortunato, R.S. The emerging role of estrogens in thyroid Redox homeostasis and carcinogenesis. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Klinge, C.M.; Goldstein, R.E. Estradiol induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors α and β. Int. J. Oncol. 2010, 36, 1067–1080. [Google Scholar]
- Chen, G.G.; Vlantis, C.A.; Zeng, Q.; van Hasselt, C.A. Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer. Curr. Cancer Drug Targets 2008, 8, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, L.; Xiao, H. Review of factors related to thet hyroid cancer epidemic. Int. J. Endocrinol. 2017, 2017, 5308635. [Google Scholar] [CrossRef]
- Lumbiganon, P.; Rugpao, S.; Phandhu-fung, S.; Laopaiboon, M.; Vudhikamraksa, N.; Werawatakul, Y. Protective effect of depot-medroxyprogesterone acetate on surgically treated uterine leiomyomas: A multicentre case—Control study. Br. J. Obstet. Gynaecol. 1996, 103, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.K.; Pike, M.C.; Vessey, M.P.; Bull, D.; Yeates, D.; Casagrande, J.T. Risk factors for uterine fibroids: Reduced risk associated with oral contraceptives. Br. Med. J. (Clin. Res. Ed.) 1986, 293, 359–362. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Platz, E.A.; Beane Freeman, L.E.; Hsing, A.W.; Linet, M.S.; Park, Y.; Schairer, C.; Schatzkin, A.; Shikany, J.M.; Berrington de González, A. Obesity and thyroid cancer risk among U.S. men and women: A pooled analysis of five prospective studies. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 464–472. [Google Scholar] [CrossRef]
- Rossing, M.A.; Voigt, L.F.; Wicklund, K.G.; Daling, J.R. Reproductive factors and risk of papillary thyroid cancer in women. Am. J. Epidemiol. 2000, 151, 765–772. [Google Scholar] [CrossRef]
- Lun, Y.; Wu, X.; Xia, Q.; Han, Y.; Zhang, X.; Liu, Z.; Wang, F.; Duan, Z.; Xin, S.; Zhang, J. Hashimoto’s thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngol. Head Neck Surg. 2013, 148, 396–402. [Google Scholar] [CrossRef]
- Kim, M.-H.; Park, Y.R.; Lim, D.-J.; Yoon, K.-H.; Kang, M.-I.; Cha, B.-Y.; Lee, K.-W.; Son, H.-Y. The relationship between thyroid nodules and uterine fibroids. Endocr. J. 2010, 57, 615–621. [Google Scholar] [CrossRef]
- Spinos, N.; Terzis, G.; Crysanthopoulou, A.; Adonakis, G.; Markou, K.B.; Vervita, V.; Koukouras, D.; Tsapanos, V.; Decavalas, G.; Kourounis, G.; et al. Increased frequency of thyroid nodules and breast fibroadenomas in women with uterine fibroids. Thyroid 2007, 17, 1257–1259. [Google Scholar] [CrossRef]
| Uterine Fibroids | p-Value | ||
|---|---|---|---|
| No | Yes | ||
| (N = 57,066) | (N = 57,066) | ||
| Age stratified | 0.34 | ||
| ≤34 | 10,577 (20.8) | 10,613 (20.9) | |
| 35–49 | 32,102 (63.2) | 32,237 (69.5) | |
| 50+ | 8087 (15.9) | 7916 (15.6) | |
| Age, mean ± SD a | 41.9 ± 9.40 | 42.2 ± 8.76 | <0.001 |
| Monthly insured income (NTD) † | 0.99 | ||
| <15,000 | 16,230 (32.0) | 16,223 (32.0) | |
| 15,000–30,000 | 17,549 (34.6) | 17,538 (34.6) | |
| >30,000 | 16,987 (33.5) | 17,005 (33.5) | |
| Urbanization level ‡ | |||
| 1 (highest) | 18,249 (36.0) | 18,215 (35.9) | |
| 2 | 15,210 (30.0) | 15,202 (30.0) | |
| 3 | 8434 (16.6) | 8448 (16.6) | |
| 4 (lowest) | 8873 (17.5) | 8901 (17.5) | |
| Occupation category | 0.92 | ||
| Office worker | 30,225 (59.6) | 30,196 (59.5) | |
| Laborer | 17,096 (33.7) | 17,132 (33.8) | |
| Other & | 3415 (6.73) | 3438 (6.77) | |
| Comorbidity | |||
| Hashimoto’s thyroditis | 95 (0.19) | 94 (0.19) | 0.94 |
| Goiter | 3202 (6.31) | 3369 (6.64) | 0.03 |
| Hypertension | 6889 (13.6) | 7039 (13.9) | 0.17 |
| Diabetes | 797 (1.57) | 873 (1.72) | 0.06 |
| Hyperlipidemia | 7006 (13.8) | 7153 (14.1) | 0.18 |
| COPD | 2971 (5.85) | 3140 (6.19) | 0.03 |
| Alcohol-related illness | 679 (1.34) | 703 (1.38) | 0.52 |
| Uterine Fibroids | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Variable | Event | PY | Rate # | Event | PY | Rate # | Crude HR (95% CI) | Adjusted HR $ (95% CI) |
| All | 89 | 377,567 | 2.36 | 148 | 379,170 | 3.90 | 1.65 (1.27, 2.15) *** | 1.64 (1.26, 2.13) *** |
| Age group, years | ||||||||
| ≤34 | 16 | 75,278 | 2.13 | 19 | 76,688 | 2.48 | 1.16 (0.60, 2.26) | 1.12 (0.58, 2.19) |
| 35–49 | 60 | 244,733 | 2.45 | 102 | 246,238 | 4.14 | 1.69 (1.23, 2.32) ** | 1.67 (1.22, 2.30) ** |
| 50+ | 13 | 57,555 | 2.26 | 27 | 56,245 | 4.80 | 2.12 (1.10, 4.12) * | 1.93 (0.99, 3.76) |
| Insured monthly income (NTD) † | ||||||||
| <15,000 | 25 | 107,172 | 2.33 | 30 | 107,592 | 2.79 | 1.19 (0.70, 2.03) | 1.18 (0.69, 2.01) |
| 15,000–30,000 | 28 | 135,197 | 2.07 | 52 | 136,471 | 3.81 | 1.84 (1.16, 2.91) ** | 1.81 (1.15, 2.87) * |
| >30,000 | 36 | 135,197 | 2.66 | 66 | 135,108 | 4.88 | 1.83 (1.22, 2.75) ** | 1.82 (1.21, 2.73) ** |
| Urbanization level ‡ | ||||||||
| 1 (highest) | 32 | 137,981 | 2.32 | 51 | 138,141 | 3.69 | 1.59 (1.02, 2.48) * | 1.59 (1.02, 2.47) * |
| 2 | 30 | 113,164 | 2.65 | 49 | 113,672 | 4.31 | 1.62 (1.03, 2.56) * | 1.60 (1.01, 2.51) * |
| 3 | 16 | 61,871 | 2.59 | 15 | 62,703 | 2.39 | 0.92 (0.46, 1.87) | 0.91 (0.45, 1.84) |
| 4 (lowest) | 11 | 64,551 | 2.59 | 33 | 64,653 | 5.10 | 3.00 (1.52, 5.93) ** | 2.96 (1.49, 5.85) ** |
| Occupation category | ||||||||
| Office worker | 54 | 223,429 | 2.42 | 80 | 224,066 | 3.57 | 1.48 (1.05, 2.08) * | 1.46 (1.04, 2.06) * |
| Laborer | 30 | 130,080 | 2.31 | 58 | 130,767 | 4.44 | 1.92 (1.24, 2.99) ** | 1.90 (1.22, 2.95) ** |
| Other & | 5 | 24,058 | 2.08 | 10 | 24,337 | 4.11 | 1.98 (0.68, 5.78) | 1.94 (0.66, 5.68) |
| Comorbidity | ||||||||
| No | 52 | 266,441 | 1.95 | 84 | 266,508 | 3.15 | 1.61 (1.14, 2.28) ** | 1.61 (1.14, 2.27) ** |
| Yes | 37 | 111,126 | 3.33 | 64 | 112,662 | 5.68 | 1.71 (1.14, 2.56) ** | 1.70 (1.14, 2.55) * |
| Follow-up period | ||||||||
| <1 year | 10 | 50,599 | 1.98 | 21 | 50,618 | 4.15 | 2.10 (0.99, 4.46) | 2.04 (0.96, 4.33) |
| 1–5 years | 33 | 169,545 | 1.95 | 62 | 169,907 | 3.65 | 1.87 (1.23, 2.86) ** | 1.84 (1.21, 2.81) ** |
| ≧5 years | 46 | 157,423 | 2.92 | 65 | 158,645 | 4.10 | 1.40 (0.96, 2.04) | 1.40 (0.96, 2.04) |
| Variable | Event | PY | Rate # | Adjusted HR (95% CI) | Adjusted HR † (95% CI) |
|---|---|---|---|---|---|
| Control patients | 89 | 377,567 | 2.36 | 1.00 | |
| Uterine fibroids patients | |||||
| Myomectomy | |||||
| No | 106 | 275,774 | 3.84 | 1.61 (1.21, 2.13) ** | 1.00 |
| Yes | 42 | 103,397 | 4.06 | 1.71 (1.19, 2.47) ** | 1.06 (0.74, 1.52) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.-M.; Chung, L.-M.; Lin, C.-L.; Kao, C.-H. Uterine Fibroids Increase the Risk of Thyroid Cancer. Int. J. Environ. Res. Public Health 2020, 17, 3821. https://doi.org/10.3390/ijerph17113821
Sun L-M, Chung L-M, Lin C-L, Kao C-H. Uterine Fibroids Increase the Risk of Thyroid Cancer. International Journal of Environmental Research and Public Health. 2020; 17(11):3821. https://doi.org/10.3390/ijerph17113821
Chicago/Turabian StyleSun, Li-Min, Li-Min Chung, Cheng-Li Lin, and Chia-Hung Kao. 2020. "Uterine Fibroids Increase the Risk of Thyroid Cancer" International Journal of Environmental Research and Public Health 17, no. 11: 3821. https://doi.org/10.3390/ijerph17113821
APA StyleSun, L.-M., Chung, L.-M., Lin, C.-L., & Kao, C.-H. (2020). Uterine Fibroids Increase the Risk of Thyroid Cancer. International Journal of Environmental Research and Public Health, 17(11), 3821. https://doi.org/10.3390/ijerph17113821





