Risk of Herpes Zoster in Patients with Adhesive Capsulitis of the Shoulder
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Main Outcome and Comorbidities
2.4. Statistical Analysis
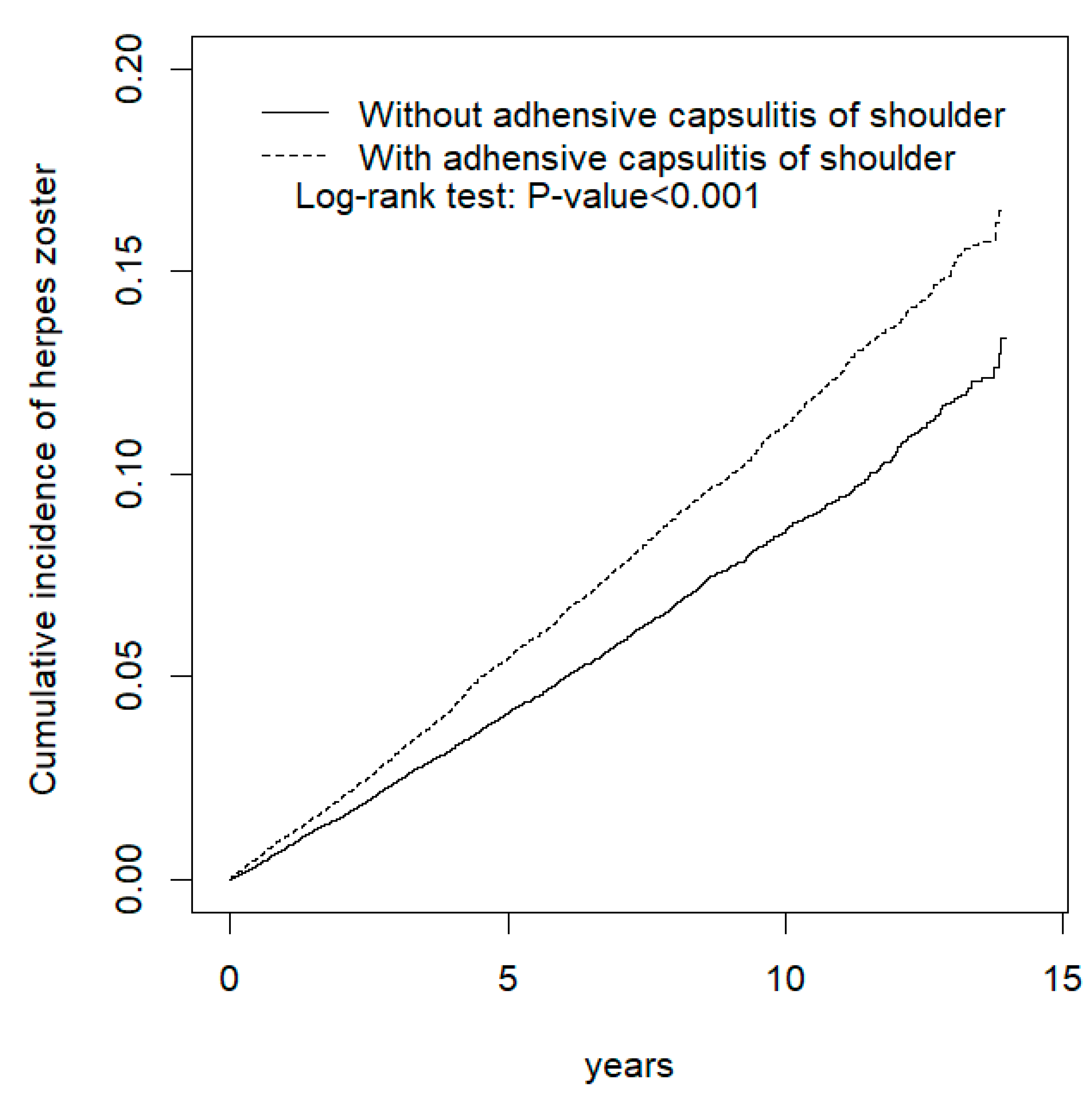
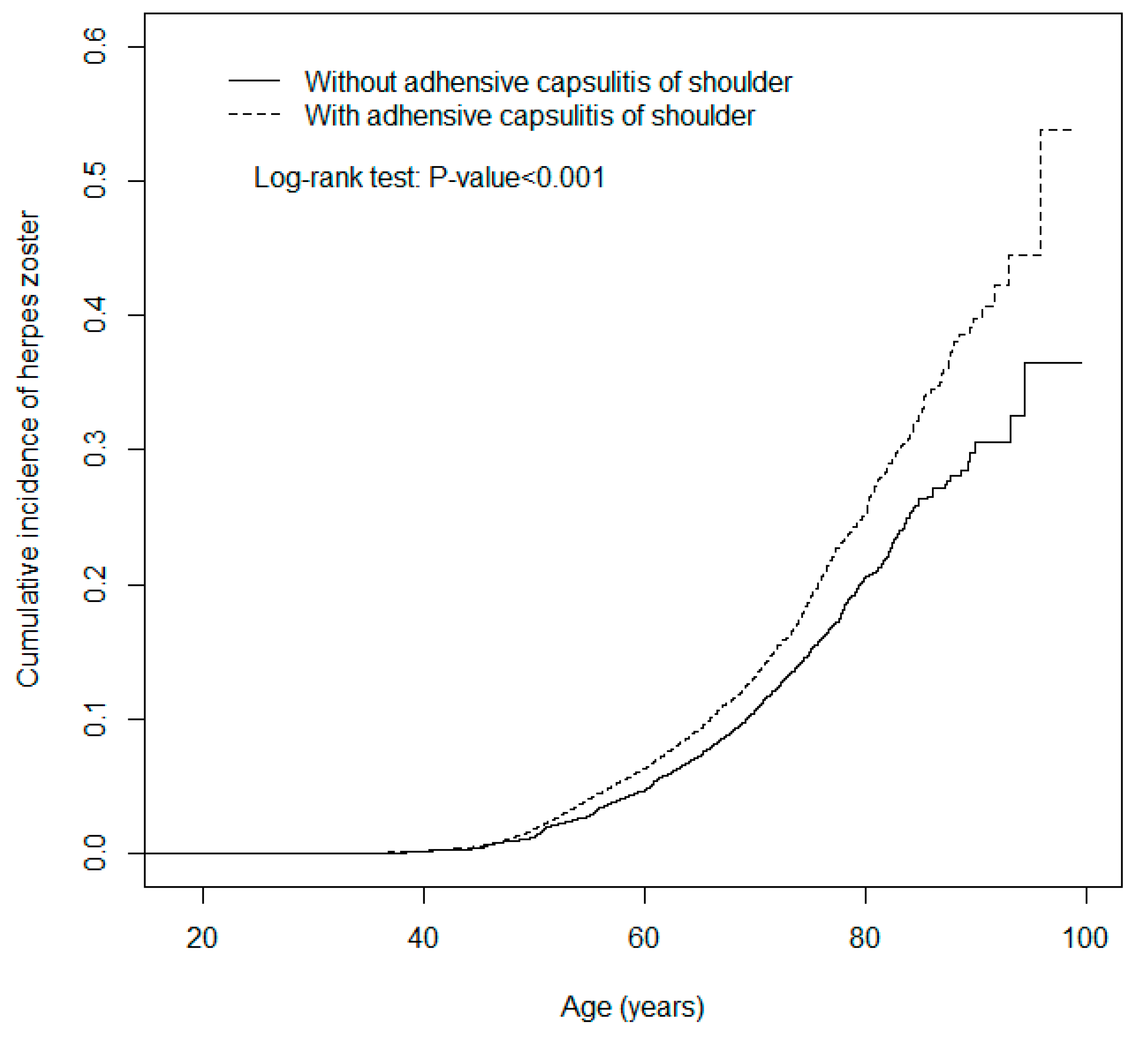
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Compliance with Ethical Standards
References
- Le, H.V.; Lee, S.J.; Nazarian, A.; Rodriguez, E.K. Adhesive capsulitis of the shoulder: Review of pathophysiology and current clinical treatments. Shoulder Elb. 2017, 9, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Bae, K.C.; Kim, D.H. Treatment strategy for frozen shoulder. Clin. Orthop. Surg. 2019, 11, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J. Adhesive Capsulitis: Diagnosis and management. Am. Fam. Physician 2019, 99, 297–300. [Google Scholar] [PubMed]
- Feli, K.H.; Ediale, C.E.; McMichael, A.J. Update in herpes zoster prevention and the role of dermatologists. J. Drugs Dermatol. 2019, 18, 18–22. [Google Scholar] [PubMed]
- Wolfson, L.J.; Daniels, V.J.; Altland, A.; Black, W.; Huang, W.; Ou, W. The impact of varicella vaccination on the incidence of varicella and herpes zoster in the United States: Updated evidence from observational databases, 1991–2016. Clin. Infect. Dis. 2020, 70, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Lin, C.L.; Kao, C.H. Balanitis is a risk factor for herpes zoster. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Wang, Y.C.; Kao, C.H. Dyshidrosis is a risk factor for herpes zoster. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Lin, C.L.; Kao, C.H. Association between chronic interstitial cystitis and herpes zoster. Int. J. Environ. Res. Public Health 2020, 17, 2228. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Chang, C.S.; Muo, C.H.; Kao, C.H. High prevalence of herpes zoster in patients with depression. J. Clin. Psychiatry 2015, 76, e1099–e1104. [Google Scholar] [CrossRef] [PubMed]
- Safran, O.; El-Haj, M.; Leibowitz, G.; Beyth, S.; Furman, Z.; Milgrom, C.; Kandel, L. Should patients with frozen shoulder be screened for diabetes mellitus? Orthop. J. Sports Med. 2017, 5, 2325967117716450. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Kashid, M.; Chakrabarty, B.; Upreti, V.; Shaki, O. Is it necessary to screen patient with adhesive capsulitis of shoulder for diabetes mellitus? J. Fam. Med. Prim. Care 2019, 8, 2927–2932. [Google Scholar]
- Alhashimi, R.A.H. Analytical observational study of frozen shoulder among patients with diabetes mellitus. Joints 2018, 6, 141–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.P.; Fann, C.Y.; Chiu, Y.H.; Yen, M.F.; Chen, L.S.; Chen, H.H.; Pan, S.L. Association of diabetes mellitus with the risk of developing adhesive capsulitis of the shoulder: A longitudinal population-based followup study. Arthritis Care Res. (Hoboken) 2013, 65, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Zreik, N.H.; Malik, R.A.; Charalambous, C.P. Adhesive capsulitis of the shoulder and diabetes: A meta-analysis of prevalence. Muscles Ligaments Tendons J. 2016, 6, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, N.; Xu, H.; Wang, H.; Huang, J. Case control study of risk factors for frozen shoulder in China. Int. J. Rheum. Dis. 2015, 18, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Kingston, K.; Curry, E.J.; Galvin, J.W.; Li, X. Shoulder adhesive capsulitis: Epidemiology and predictors of surgery. J. Shoulder Elb. Surg. 2018, 27, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Tang, Y.; Xue, Y.; Yang, Z.; Li, Z.; He, D.; Zhao, Y.; Zong, Y. A report on the prevalence of depression and anxiety in patients with frozen shoulder and their relations to disease status. Psychol. Health Med. 2014, 19, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Toprak, M.; Erden, M. Sleep quality, pain, anxiety, depression and quality of life in patients with frozen shoulder1. J. Back Musculoskelet. Rehabil. 2019, 32, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.H.; Moradi, A.; Bidgoli, H.F.; Zarei, B. The relationship between depression or anxiety symptoms and objective and subjective symptoms of patients with frozen shoulder. Int. J. Prev. Med. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed]
| Variable | Adhesive Capsulitis of the Shoulder | p-Value | |
|---|---|---|---|
| No | Yes | ||
| N = 30,239 | N = 30,239 | ||
| Age, year | 0.99 | ||
| ≤49 | 8342 (27.6) | 8342 (27.6) | |
| 50–64 | 13,352 (44.2) | 13,352 (44.2) | |
| 65+ | 8545 (28.3) | 8545 (28.3) | |
| Median (IQR) † | 55.6 (47.9–66.3) | 56.3 (49.2–66.6) | <0.001 |
| Sex | 0.99 | ||
| Female | 18,433 (61.0) | 18,433 (61.0) | |
| Male | 11,806 (39.0) | 11,806 (39.0) | |
| Comorbidity | |||
| Diabetes | 2553 (8.44) | 4021 (13.3) | <0.001 |
| CAD | 5385 (17.8) | 7570 (25.0) | <0.001 |
| Depression | 1591 (5.26) | 2429 (8.03) | <0.001 |
| Chronic kidney disease | 647 (2.14) | 690 (2.28) | 0.23 |
| Obesity | 431 (1.43) | 608 (2.01) | <0.001 |
| Cancer | 961 (3.18) | 1062 (3.51) | 0.02 |
| Variable | Event | PY | Rate # | Crude HR (95% CI) | Adjusted HR & (95% CI) |
|---|---|---|---|---|---|
| Adhesive capsulitis of the shoulder | |||||
| No | 1817 | 204,507 | 8.88 | 1.00 | 1.00 |
| Yes | 2421 | 205,670 | 11.8 | 1.33 (1.25, 1.41) *** | 1.28 (1.20, 1.36) *** |
| Age, year | |||||
| ≤49 | 763 | 125,368 | 6.09 | 1.00 | 1.00 |
| 50–64 | 1956 | 179,862 | 10.9 | 1.81 (1.66, 1.97) *** | 1.74 (1.60, 1.90) *** |
| 65+ | 1519 | 104,976 | 14.5 | 2.43 (2.22, 2.65) *** | 2.22 (2.03, 2.44) *** |
| Sex | |||||
| Female | 2718 | 255,139 | 10.7 | 1.08 (1.02, 1.15) * | 1.09 (1.02, 1.16) * |
| Male | 1520 | 155,068 | 9.80 | 1.00 | 1.00 |
| Comorbidity | |||||
| Diabetes | |||||
| No | 3688 | 370,547 | 9.95 | 1.00 | 1.00 |
| Yes | 550 | 39,659 | 13.9 | 1.42 (1.29, 1.55) *** | 1.11 (1.01, 1.21) * |
| CAD | |||||
| No | 3069 | 327,661 | 9.37 | 1.00 | 1.00 |
| Yes | 1169 | 82,545 | 14.2 | 1.53 (1.43, 1.63) *** | 1.16 (1.07, 1.24) *** |
| Depression | |||||
| No | 3923 | 386,997 | 10.1 | 1.00 | 1.00 |
| Yes | 315 | 23,209 | 13.6 | 1.37 (1.22, 1.53) *** | 1.20 (1.07, 1.34) ** |
| Chronic kidney disease | |||||
| No | 4126 | 403,717 | 10.2 | 1.00 | 1.00 |
| Yes | 112 | 6490 | 17.3 | 1.74 (1.44, 2.10) *** | 1.35 (1.12, 1.63) ** |
| Obesity | |||||
| No | 4185 | 404,328 | 10.4 | 1.00 | 1.00 |
| Yes | 53 | 5878 | 9.02 | 0.89 (0.68, 1.17) | |
| Cancer | |||||
| No | 4103 | 399,593 | 10.3 | 1.00 | 1.00 |
| Yes | 135 | 10,613 | 12.7 | 1.27 (1.07, 1.50) ** | 1.09 (0.92, 1.29) |
| Adhesive Capsulitis of the Shoulder | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Variables | Event | PY | Rate # | Event | PY | Rate # | Crude HR (95% CI) | Adjusted HR & (95% CI) |
| Age, years | ||||||||
| ≤49 | 300 | 62,903 | 4.77 | 463 | 62,465 | 7.41 | 1.56 (1.35, 1.80) *** | 1.52 (1.31, 1.75) *** |
| 50–64 | 855 | 89,760 | 9.53 | 1101 | 90,101 | 12.2 | 1.28 (1.17, 1.40) *** | 1.26 (1.15, 1.38) *** |
| 65+ | 662 | 51,843 | 12.8 | 857 | 53,133 | 16.1 | 1.26 (1.14, 1.40) *** | 1.22 (1.10, 1.35) *** |
| Sex | ||||||||
| Female | 1196 | 127,118 | 9.41 | 1522 | 128,021 | 11.9 | 1.26 (1.17, 1.36) *** | 1.22 (1.13, 1.32) *** |
| Male | 621 | 77,389 | 8.02 | 899 | 77,679 | 11.6 | 1.44 (1.30, 1.60) *** | 1.40 (1.26, 1.55) *** |
| Comorbidity § | ||||||||
| No | 1165 | 151,759 | 7.68 | 1350 | 130,055 | 10.4 | 1.35 (1.25, 1.46) *** | 1.36 (1.26, 1.47) *** |
| Yes | 652 | 52,747 | 12.4 | 1071 | 75,644 | 14.2 | 1.14 (1.04, 1.26) ** | 1.16 (1.06, 1.28) ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-Y.; Ke, D.-S.; Lin, C.-L.; Kao, C.-H. Risk of Herpes Zoster in Patients with Adhesive Capsulitis of the Shoulder. Int. J. Environ. Res. Public Health 2020, 17, 3592. https://doi.org/10.3390/ijerph17103592
Hsu C-Y, Ke D-S, Lin C-L, Kao C-H. Risk of Herpes Zoster in Patients with Adhesive Capsulitis of the Shoulder. International Journal of Environmental Research and Public Health. 2020; 17(10):3592. https://doi.org/10.3390/ijerph17103592
Chicago/Turabian StyleHsu, Chao-Yu, Der-Shin Ke, Cheng-Li Lin, and Chia-Hung Kao. 2020. "Risk of Herpes Zoster in Patients with Adhesive Capsulitis of the Shoulder" International Journal of Environmental Research and Public Health 17, no. 10: 3592. https://doi.org/10.3390/ijerph17103592
APA StyleHsu, C.-Y., Ke, D.-S., Lin, C.-L., & Kao, C.-H. (2020). Risk of Herpes Zoster in Patients with Adhesive Capsulitis of the Shoulder. International Journal of Environmental Research and Public Health, 17(10), 3592. https://doi.org/10.3390/ijerph17103592





