The Effects of Social, Personal, and Behavioral Risk Factors and PM2.5 on Cardio-Metabolic Disparities in a Cohort of Community Health Center Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Aim
2.2. Design
2.3. Sample
2.4. Procedures
2.5. Statistical Analysis
3. Results
4. Discussion
- (1)
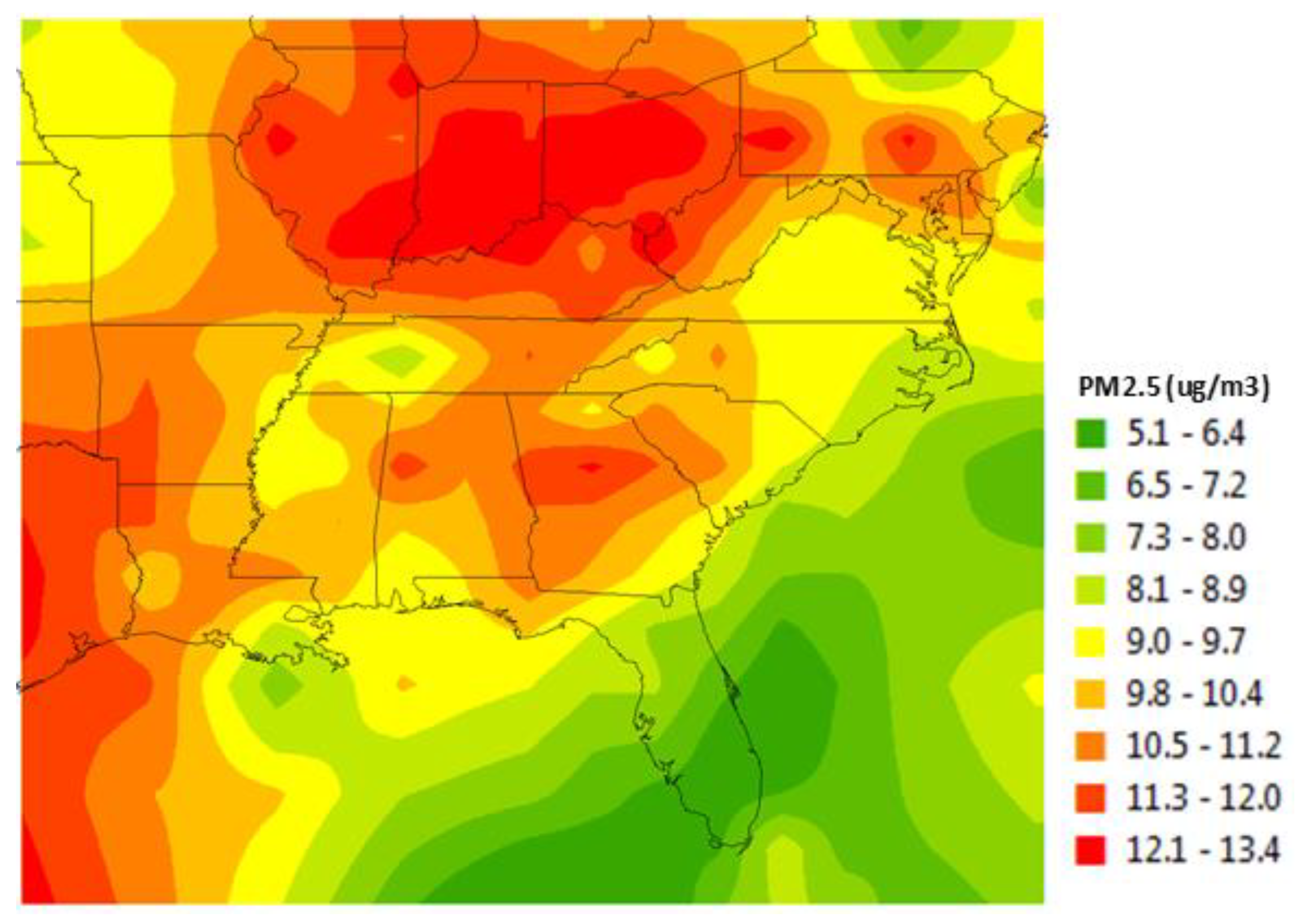
- Residents of communities with exposure to higher levels of PM2.5 annual concentrations are more likely to have reported a CMD.
- (2)
- Race, a social risk factor for disparities in health, is not predictive of CMD when behavioral, clinical, and environmental risk factors are accounted for in the model. Similarly, residence in an urban or rural setting is not associated with CMD after PM2.5 and other risk factor information are taken into consideration.
- (3)
- A significant residual variation in the presence of CMD among participants across states was found, perhaps reflecting differences in environmental exposures, social policies, and other place-based factors. These differences will be explored further in future analyses.
- (4)
- Multiple individual and environmental risk factors are associated with self-reported CMD, consistent with a multifactorial etiology of these conditions. Our results are generally consistent with previously published literature. We found statistically significant positive associations between CMD and marital status, BMI, education, gender, age, employment, and higher concentrations of PM2.5.
5. Conclusions
Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CMD | Cardio-metabolic disease |
| SCCS | Southern Community Cohort Study |
| PM2.5 | Particulate matter smaller than 2.5 micrometers in diameter |
| BMI | Body mass index |
| GLMM | Generalized linear mixed model |
References
- Su, J.B. Vascular endothelial dysfunction and pharmacological treatment. World J. Cardiol. 2015, 7, 719–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, X.; Shen, P.; Si, Y.; Liu, X.; Xu, Z.; Wu, J.; Zhang, J.; Lu, P.; Lin, H.; et al. Multimorbidity of cardiometabolic diseases: Prevalence and risk for mortality from one million chinese adults in a longitudinal cohort study. BMJ Open 2019, 9, e024476. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration; Di Angelantonio, E.; Kaptoge, S.; Wormser, D.; Willeit, P.; Butterworth, A.S.; Bansal, N.; O’Keeffe, L.M.; Gao, P.; Wood, A.M.; et al. Association of cardiometabolic multimorbidity with mortality. JAMA 2015, 314, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Murphy, S.L.; Kochanek, K.D.; Bastian, B.; Arias, E. Final Data for 2016. National Vital Statistics Reports; National Center for Health Statistics: Hyattsville, MD, USA, 2018. [Google Scholar]
- Juarez, P.D.; Matthews-Juarez, P.; Hood, D.B.; Im, W.; Levine, R.S.; Kilbourne, B.J.; Langston, M.A.; Al-Hamdan, M.Z.; Crosson, W.L.; Estes, M.G.; et al. The public health exposome: A population-based, exposure science approach to health disparities research. Int. J. Environ. Res. Public Health 2014, 11, 12866–12895. [Google Scholar] [CrossRef]
- Shahraki, M.; Shahraki, T.; Shidfar, F.; Ansari, H. Which modifiable, non-modifiable, and socioeconomic factors have more effect on cardiovascular risk factors in overweight and obese women? J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2012, 17, 676–680. [Google Scholar]
- Balfour, P.C.; Rodriguez, C.J.; Ferdinand, K.C. The role of hypertension in race-ethnic disparities in cardiovascular disease. Curr. Cardiovasc. Risk Rep. 2015, 9, 18. [Google Scholar] [CrossRef]
- Logan, J.; Barksdale, D.J. Allostasis and allostatic load: Expanding the discourse on stress and cardiovascular disease. J. Clin. Nurs. 2008, 17, 201–208. [Google Scholar] [CrossRef]
- Mattei, J.; Demissie, S.; Falcon, L.M.; Ordovas, J.M.; Tucker, K.L. Allostatic load is associated with chronic conditions in the boston puerto rican health study. Soc. Sci. Med. 2010, 70, 1988–1996. [Google Scholar] [CrossRef]
- McCaffery, J.M.; Marsland, A.L.; Strohacker, K.; Muldoon, M.F.; Manuck, S.B. Factor structure underlying components of allostatic load. PLoS ONE 2012, 7, e47246. [Google Scholar] [CrossRef]
- Musunuru, K. Atherogenic dyslipidemia: Cardiovascular risk and dietary intervention. Lipids 2010, 45, 907–914. [Google Scholar] [CrossRef]
- Holven, K.B.; Retterstøl, K.; Ueland, T.; Ulven, S.M.; Nenseter, M.S.; Sandvik, M.; Narverud, I.; Berge, K.E.; Ose, L.; Aukrust, P.; et al. Subjects with low plasma hdl cholesterol levels are characterized by an inflammatory and oxidative phenotype. PLoS ONE 2013, 8, e78241. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.; Predazzi, I.M.; Williams, S.M.; Bush, W.S.; Kim, Y.; Havas, S.; Toth, P.P.; Fazio, S.; Miller, M. Is isolated low high-density lipoprotein cholesterol a cardiovascular disease risk factor? New insights from the framingham offspring study. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Hulsegge, G.; Spijkerman, A.M.W.; van der Schouw, Y.T.; Bakker, S.J.L.; Gansevoort, R.T.; Smit, H.A.; Verschuren, W.M.M. Trajectories of metabolic risk factors and biochemical markers prior to the onset of cardiovascular disease - the doetinchem cohort study. PLoS ONE 2016, 11, e0155978. [Google Scholar] [CrossRef]
- Stout, R. The impact of insulin upon atherosclerosis. Horm. Metab. Res. 1994, 26, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Alderman, M.H. Serum uric acid and cardiovascular mortality: The nhanes i epidemiologic follow-up study, 1971–1992. JAMA 2000, 283, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.; Sund, M.; Fröhlich, M.; Fischer, H.G.; Löwel, H.; Döring, A.; Hutchinson, W.L.; Pepys, M.B. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men - results from the monica (monitoring trends and determinants in cardiovascular disease) augsburg cohort study, 1984 to 1992. Circulation 1999, 99, 237–242. [Google Scholar]
- Peng, J.; Luo, F.; Ruan, G.; Peng, R.; Li, X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017, 16, 233. [Google Scholar] [CrossRef]
- Tracy, R.P. Thrombin, inflammation, and cardiovascular disease: An epidemiologic perspective. Chest 2003, 124, 49S–57S. [Google Scholar] [CrossRef]
- Hoek, G.; Brunekreef, B.; Fischer, P.; van Wijnen, J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology 2001, 12, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Katzke, V.A.; Sookthai, D.; Johnson, T.; Kühn, T.; Kaaks, R. Blood lipids and lipoproteins in relation to incidence and mortality risks for cvd and cancer in the prospective epic-heidelberg cohort. BMC Med. 2017, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, P.; Vaag, A.; Kyvik, K.; Beck-Nielsen, H. Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia 2001, 44, 537–543. [Google Scholar] [CrossRef]
- Elder, S.J.; Lichtenstein, A.H.; Pittas, A.G.; Roberts, S.B.; Fuss, P.J.; Greenberg, A.S.; McCrory, M.A.; Bouchard, T.J.; Saltzman, E.; Neale, M.C. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J. Lipid Res. 2009, 50, 1917–1926. [Google Scholar] [CrossRef]
- Danesh, J.; Collins, R.; Appleby, P.; Peto, R. Association of fibrinogen, c-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. JAMA 1998, 279, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Graham, I.M.; Daly, L.E.; Refsum, H.M.; Robinson, K.; Brattström, L.E.; Ueland, P.M.; Palma-Reis, R.J.; Boers, G.H.; Sheahan, R.G.; Israelsson, B. Plasma homocysteine as a risk factor for vascular disease: The european concerted action project. JAMA 1997, 277, 1775–1781. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Diabetes Report Card 2017; US DHHS: Atlanta, GA, USA, 2018.
- Geiss, L.S.; Wang, J.; Cheng, Y.J.; Thompson, T.J.; Barker, L.; Li, Y.; Albright, A.L.; Gregg, E.W. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, united states, 1980-2012prevalence and incidence trends for diabetes among adultsprevalence and incidence trends for diabetes among adults. JAMA 2014, 312, 1218–1226. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Hartiala, J.; Breton, C.V.; Tang, W.H.W.; Lurmann, F.; Hazen, S.L.; Gilliland, F.D.; Allayee, H. Ambient air pollution is associated with the severity of coronary atherosclerosis and incident myocardial infarction in patients undergoing elective cardiac evaluation. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2016, 5, e003947. [Google Scholar] [CrossRef]
- Mustafić, H.; Jabre, P.; Caussin, C.; Murad, M.H.; Escolano, S.; Tafflet, M.; Périer, M.C.; Marijon, E.; Vernerey, D.; Empana, J.P.; et al. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA 2012, 307, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Pope Iii, C.A.; Renlund, D.G.; Kfoury, A.G.; May, H.T.; Horne, B.D. Relation of heart failure hospitalization to exposure to fine particulate air pollution. Am. J. Cardiol. 2008, 102, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Link, M.S.; Luttmann-Gibson, H.; Schwartz, J.; Mittleman, M.A.; Wessler, B.; Gold, D.R.; Dockery, D.W.; Laden, F. Acute exposure to air pollution triggers atrial fibrillation. J. Am. Coll. Cardiol. 2013, 62, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Kampfrath, T.; Maiseyeu, A.; Ying, Z.; Shah, Z.; Deiuliis, J.A.; Xu, X.; Kherada, N.; Brook, R.D.; Reddy, K.M.; Padture, N.P.; et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via nadph oxidase and tlr4 pathways. Circ. Res. 2011, 108, 716–726. [Google Scholar] [CrossRef]
- Nelin, T.D.; Joseph, A.M.; Gorr, M.W.; Wold, L.E. Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol. Lett. 2012, 208, 293–299. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S. Particulate matter, air pollution, and blood pressure. J. Am. Soc. Hypertens. 2009, 3, 332–350. [Google Scholar] [CrossRef]
- Yang, B.-Y.; Qian, Z.; Howard, S.W.; Vaughn, M.G.; Fan, S.-J.; Liu, K.-K.; Dong, G.-H. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ. Pollut. 2018, 235, 576–588. [Google Scholar] [CrossRef]
- Coogan, P.F.; White, L.F.; Yu, J.; Burnett, R.T.; Seto, E.; Brook, R.D.; Palmer, J.R.; Rosenberg, L.; Jerrett, M. Pm(2.5) and diabetes and hypertension incidence in the black women’s health study. Epidemiology 2016, 27, 202–210. [Google Scholar]
- Dzhambov, A.M.; Dimitrova, D.D. Exposures to road traffic, noise, and air pollution as risk factors for type 2 diabetes: A feasibility study in bulgaria. Noise Health 2016, 18, 133–142. [Google Scholar] [CrossRef]
- Qiu, H.; Sun, S.; Tsang, H.; Wong, C.-M.; Lee, R.S.-y.; Schooling, C.M.; Tian, L. Fine particulate matter exposure and incidence of stroke. Neurology 2017, 88, 1709. [Google Scholar] [CrossRef]
- Wang, Y.; Eliot, M.N.; Wellenius, G.A. Short-term changes in ambient particulate matter and risk of stroke: A systematic review and meta-analysis. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2014, 3, e000983. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual. Atmos. Health 2011, 4, 79–93. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, Q. Fine particulate matter air pollution and atherosclerosis: Mechanistic insights. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 2863–2868. [Google Scholar] [CrossRef]
- Seo, S.; Choi, S.; Kim, K.; Kim, S.M.; Park, S.M. Association between urban green space and the risk of cardiovascular disease: A longitudinal study in seven korean metropolitan areas. Environ. Int. 2019, 125, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental risk factors for developing type 2 diabetes mellitus: A systematic review. Int. J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Giskes, K.; van Lenthe, F.; Avendano-Pabon, M.; Brug, J. A systematic review of environmental factors and obesogenic dietary intakes among adults: Are we getting closer to understanding obesogenic environments? Obes. Rev. 2011, 12, e95–e106. [Google Scholar] [CrossRef] [PubMed]
- Pool, L.R.; Ning, H.; Lloyd-Jones, D.M.; Allen, N.B. Trends in racial/ethnic disparities in cardiovascular health among us adults from 1999–2012. J. Am. Heart Assoc. 2017, 6, e006027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Gong, W. Type 2 diabetes mellitus and neighborhood deprivation index: A spatial analysis in zhejiang, china. J. Diabetes Investig. 2019, 10, 272–282. [Google Scholar] [CrossRef]
- Sundquist, K.; Theobald, H.; Yang, M.; Li, X.; Johansson, S.-E.; Sundquist, J. Neighborhood violent crime and unemployment increase the risk of coronary heart disease: A multilevel study in an urban setting. Soc. Sci. Med. 2006, 62, 2061–2071. [Google Scholar] [CrossRef]
- Sundquist, J.; Johansson, S.E.; Yang, M.; Sundquist, K. Low linking social capital as a predictor of coronary heart disease in sweden: A cohort study of 2.8 million people. Soc. Sci. Med. 2006, 62, 954–963. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Rural Americans at Higher Risk of Death from Five Leading Causes. Available online: https://www.cdc.gov/media/releases/2017/p0112-rural-death-risk.html (accessed on 28 July 2019).
- Hicken, M.T.; Adar, S.D.; Hajat, A.; Kershaw, K.N.; Do, D.P.; Barr, R.G.; Kaufman, J.D.; Diez Roux, A.V. Air pollution, cardiovascular outcomes, and social disadvantage: The multi-ethnic study of atherosclerosis. Epidemiology 2016, 27, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Becerra, M.; Jagai, J.S.; Ard, K.; Sargis, R.M. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care 2018, 41, 193. [Google Scholar] [CrossRef]
- Erqou, S.; Clougherty Jane, E.; Olafiranye, O.; Magnani Jared, W.; Aiyer, A.; Tripathy, S.; Kinnee, E.; Kip Kevin, E.; Reis Steven, E. Particulate matter air pollution and racial differences in cardiovascular disease risk. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Signorello, L.B.; Hargreaves, M.K.; Blot, W.J. The southern community cohort study: Investigating health disparities. J. Health Care Poor Underserved 2010, 21, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Cosselman, K.E.; Navas-Acien, A.; Kaufman, J.D. Environmental factors in cardiovascular disease. Nat. Rev. Cardio.l 2015, 12, 627–642. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead exposure and cardiovascular disease—A systematic review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef]
- Orioli, R.; Cremona, G.; Ciancarella, L.; Solimini, A.G. Association between pm10, pm2.5, no2, o3 and self-reported diabetes in italy: A cross-sectional, ecological study. PLoS ONE 2018, 13, e0191112. [Google Scholar] [CrossRef]
- Smith, A.H.; O Lingas, E.; Rahman, M. Contamination of Drinking Water by Arsenic in Bangladesh: A Public Health Emergency. Bull. World Health Organ. 2000, 78, 1093–1103. [Google Scholar]
- Peters, J.L.; Perlstein, T.S.; Perry, M.J.; McNeely, E.; Weuve, J. Cadmium exposure in association with history of stroke and heart failure. Environ. Res. 2010, 110, 199–206. [Google Scholar] [CrossRef]
- Edwards, J.R.; Prozialeck, W.C. Cadmium, diabetes and chronic kidney disease. Toxicol. Appl. Pharmacol. 2009, 238, 289–293. [Google Scholar] [CrossRef]
- Bulka, C.M.; Daviglus, M.L.; Persky, V.W.; Durazo-Arvizu, R.A.; Lash, J.P.; Elfassy, T.; Lee, D.J.; Ramos, A.R.; Tarraf, W.; Argos, M. Association of occupational exposures with cardiovascular disease among us hispanics/latinos. Heart 2019, 105, 439. [Google Scholar] [CrossRef] [PubMed]
- Rinsky, J.L.; Hoppin, J.A.; Blair, A.; He, K.; Beane Freeman, L.E.; Chen, H. Agricultural exposures and stroke mortality in the agricultural health study. J. Toxicol. Environ. Health. Part A 2013, 76. [Google Scholar] [CrossRef] [PubMed]
- Sandler, D.P.; Kamel, F.; Montgomery, M.P.; Saldana, T.M.; Alavanja, M.C.R. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural health study, 1993–2003. Am. J. Epidemiol. 2008, 167, 1235–1246. [Google Scholar]
- Wilcosky, T.C.; Simonsen, N.R. Solvent exposure and cardiovascular disease. Am. J. Ind. Med. 1991, 19, 569–586. [Google Scholar] [CrossRef]
- Mohammadi, A.; Azhdarpoor, A.; Shahsavani, A.; Tabatabaee, H. Health impacts of exposure to pm10 on inhabitants of shiraz, iran. Health Scope 2015, 4. [Google Scholar] [CrossRef]
- Kowalska, M.; Kocot, K. Short-term exposure to ambient fine particulate matter (pm2,5 and pm10) and the risk of heart rhythm abnormalities and stroke. Postepy Higieny i Medycyny Doswiadczalnej 2016, 70, 1017–1025. [Google Scholar] [CrossRef]
- Lee, F.-Y.; Chen, W.-K.; Lin, C.-L.; Kao, C.-H. Carbon monoxide poisoning and subsequent cardiovascular disease risk: A nationwide population-based cohort study. Medicine 2015, 94, e624. [Google Scholar] [CrossRef]
- Hampson, N.B.; Weaver, L.K. Carbon monoxide poisoning and risk for ischemic stroke. Eur. J. Intern. Med. 2016, 31, e7. [Google Scholar] [CrossRef]
- Huang, C.-C.; Ho, C.-H.; Chen, Y.-C.; Lin, H.-J.; Hsu, C.-C.; Wang, J.-J.; Su, S.-B.; Guo, H.-R. Increased risk for diabetes mellitus in patients with carbon monoxide poisoning. Oncotarget 2017, 8, 63680–63690. [Google Scholar] [CrossRef]
- Goodman, J.E.; Prueitt, R.L.; Sax, S.N.; Pizzurro, D.M.; Lynch, H.N.; Zu, K.; Venditti, F.J. Ozone exposure and systemic biomarkers: Evaluation of evidence for adverse cardiovascular health impacts. Crit. Rev. Toxicol. 2015, 45, 412–452. [Google Scholar] [CrossRef]
- Srebot, V.; Gianicolo, E.A.L.; Rainaldi, G.; Trivella, M.G.; Sicari, R. Ozone and cardiovascular injury. Cardiovasc. Ultrasound 2009, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Jerrett, M.; Brook, R.; White, L.F.; Burnett, R.T.; Yu, J.; Su, J.; Seto, E.; Marshall, J.; Palmer, J.R.; Rosenberg, L.; et al. Ambient ozone and incident diabetes: A prospective analysis in a large cohort of african american women. Environ. Int. 2017, 102, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kopp, A.; Lu, H.; Bai, L.; Kwong, J.C.; Chen, H.; Van Ryswyk, K.; Weichenthal, S.; Burnett, R.T.; Hatzopoulou, M.; Jerrett, M.; et al. Associations of long-term exposure to ultrafine particles and nitrogen dioxide with increased incidence of congestive heart failure and acute myocardial infarction. Am. J. Epidemiol. 2018, 188, 151–159. [Google Scholar]
- Amancio, C.T.; Nascimento, L.F.C. Association of sulfur dioxide exposure with circulatory system deaths in a medium-sized city in brazil. Braz. J. Med. Biol. Res. 2012, 45, 1080–1085. [Google Scholar] [CrossRef]
- Gaglioti, A.H.; Xu, J.; Rollins, L.; Baltrus, P.; O’Connell, L.K.; Cooper, D.L.; Hopkins, J.; Botchwey, N.D.; Akintobi, T.H. Neighborhood environmental health and premature death from cardiovascular disease. Prev. Chronic Dis. 2018, 15, E17. [Google Scholar] [CrossRef]
- Kwon, I.; Choi, S.; Mittman, B.; Bharmal, N.; Liu, H.; Vickrey, B.; Song, S.; Araiza, D.; McCreath, H.; Seeman, T.; et al. Study protocol of “worth the walk”: A randomized controlled trial of a stroke risk reduction walking intervention among racial/ethnic minority older adults with hypertension in community senior centers. BMC Neurol. 2015, 15, 91. [Google Scholar] [CrossRef]
- Sundquist, K.; Eriksson, U.; Mezuk, B.; Ohlsson, H. Neighborhood walkability, deprivation and incidence of type 2 diabetes: A population-based study on 512,061 swedish adults. Health Place 2015, 31, 24–30. [Google Scholar] [CrossRef]
- Pham, D.Q.; Ommerborn, M.J.; Hickson, D.A.; Taylor, H.A.; Clark, C.R. Neighborhood safety and adipose tissue distribution in african americans: The jackson heart study. PLoS ONE 2014, 9, e105251. [Google Scholar] [CrossRef]
- Evenson, K.R.; Block, R.; Diez Roux, A.V.; McGinn, A.P.; Wen, F.; Rodríguez, D.A. Associations of adult physical activity with perceived safety and police-recorded crime: The multi-ethnic study of atherosclerosis. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 146. [Google Scholar] [CrossRef]
- Lindberg, R.; Sidebottom, A.C.; McCool, B.; Pereira, R.F.; Sillah, A.; Boucher, J.L. Changing the restaurant food environment to improve cardiovascular health in a rural community: Implementation and evaluation of the heart of new ulm restaurant programme. Public Health Nutr. 2018, 21, 992–1001. [Google Scholar] [CrossRef]
- Christine, P.J.; Auchincloss, A.H.; Bertoni, A.G.; Carnethon, M.R.; Sánchez, B.N.; Moore, K.; Adar, S.D.; Horwich, T.B.; Watson, K.E.; Diez Roux, A.V. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: The multi-ethnic study of atherosclerosis (mesa). JAMA Intern. Med. 2015, 175, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Poelman, M.; Strak, M.; Schmitz, O.; Hoek, G.; Karssenberg, D.; Helbich, M.; Ntarladima, A.-M.; Bots, M.; Brunekreef, B.; Grobbee, R.; et al. Relations between the residential fast-food environment and the individual risk of cardiovascular diseases in the netherlands: A nationwide follow-up study. Eur. J. Prev. Cardiol. 2018, 25, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Hu, J.; Kershaw, K.; Hastings, K.G.; López, L.; Cullen, M.R.; Harrington, R.A.; Palaniappan, L.P. County-level hispanic ethnic density and cardiovascular disease mortality. J. Am. Heart Assoc. 2018, 7, e009107. [Google Scholar] [CrossRef] [PubMed]
- Gebreab, S.Y.; Diez Roux, A.V.; Brenner, A.B.; Hickson, D.A.; Sims, M.; Subramanyam, M.; Griswold, M.E.; Wyatt, S.B.; James, S.A. The impact of lifecourse socioeconomic position on cardiovascular disease events in african americans: The jackson heart study. J. Am. Heart Assoc. 2015, 4, e001553. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.F.; Liang, L.-J.; Vassar, S.D.; Stein-Merkin, S.; Longstreth, W.T., Jr.; Ovbiagele, B.; Yan, T.; Escarce, J.J. Neighborhood disadvantage and ischemic stroke: The cardiovascular health study (chs). Stroke 2011, 42, 3363–3368. [Google Scholar] [CrossRef]
- Li, S.; Bruen, B.K.; Lantz, P.M.; Mendez, D. Impact of health insurance expansions on nonelderly adults with hypertension. Prev. Chronic Dis. 2015, 12, E105. [Google Scholar] [CrossRef]
- Medford-Davis, L.N.; Fonarow, G.C.; Bhatt, D.L.; Xu, H.; Smith, E.E.; Suter, R.; Peterson, E.D.; Xian, Y.; Matsouaka, R.A.; Schwamm, L.H. Impact of insurance status on outcomes and use of rehabilitation services in acute ischemic stroke: Findings from get with the guidelines-stroke. J. Am. Heart Assoc. 2016, 5, e004282. [Google Scholar] [CrossRef]
- Stark Casagrande, S.; Cowie, C.C. Health insurance coverage among people with and without diabetes in the u.S. Adult population. Diabetes Care 2012, 35, 2243–2249. [Google Scholar] [CrossRef]
- Booth, J.; Connelly, L.; Lawrence, M.; Chalmers, C.; Joice, S.; Becker, C.; Dougall, N. Evidence of perceived psychosocial stress as a risk factor for stroke in adults: A meta-analysis. BMC Neurol. 2015, 15, 233. [Google Scholar] [CrossRef]
- Grigsby-Toussaint, D.S.; Jones, A.; Kubo, J.; Bradford, N. Residential segregation and diabetes risk among latinos. Ethn. Dis. 2015, 25, 451–458. [Google Scholar] [CrossRef]
- Kershaw, K.N.; Osypuk, T.L.; Do, D.P.; Chavez, P.J.; Diez Roux, A.V. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: The multi-ethnic study of atherosclerosis. Circulation 2015, 131, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.C.; Baek, J.; Smith, M.A.; Morgenstern, L.B.; Lisabeth, L.D. Residential ethnic segregation and stroke risk in mexican americans: The brain attack surveillance in corpus christi project. Ethn. Dis. 2015, 25, 11–18. [Google Scholar] [PubMed]
- Schoenthaler, A.; Montague, E.; Baier Manwell, L.; Brown, R.; Schwartz, M.D.; Linzer, M. Patient-physician racial/ethnic concordance and blood pressure control: The role of trust and medication adherence. Ethn. Health 2014, 19, 565–578. [Google Scholar] [CrossRef]
- Heisler, M.; Bouknight, R.R.; Hayward, R.A.; Smith, D.M.; Kerr, E.A. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J. Gen. Intern. Med. 2002, 17, 243–252. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Bandosz, P.; Rehm, C.D.; Penalvo, J.; Whitsel, L.; Gaziano, T.; Conrad, Z.; Wilde, P.; Micha, R.; Lloyd-Williams, F.; et al. Reducing us cardiovascular disease burden and disparities through national and targeted dietary policies: A modelling study. PLoS Med. 2017, 14, e1002311. [Google Scholar] [CrossRef] [PubMed]
- Jilcott Pitts, S.B.; Smith, T.W.; Thayer, L.M.; Drobka, S.; Miller, C.; Keyserling, T.C.; Ammerman, A.S. Addressing rural health disparities through policy change in the stroke belt. J. Public Health Manag. Pract. JPHMP 2013, 19, 503–510. [Google Scholar] [CrossRef]
- Shaikh, S.; Jagai, J.S.; Ashley, C.; Zhou, S.; Sargis, R.M. Underutilized and under threat: Environmental policy as a tool to address diabetes risk. Curr. Diabetes Rep. 2018, 18, 25. [Google Scholar] [CrossRef]
- Ackermann, R.T.; Kenrik Duru, O.; Albu, J.B.; Schmittdiel, J.A.; Soumerai, S.B.; Wharam, J.F.; Ali, M.K.; Mangione, C.M.; Gregg, E.W.; Group, N.-D.S. Evaluating diabetes health policies using natural experiments: The natural experiments for translation in diabetes study. Am. J. Prev. Med. 2015, 48, 747–754. [Google Scholar] [CrossRef]
- Al-Hamdan, M.Z.; Crosson, W.L.; Economou, S.A.; Estes, M.G.; Estes, S.M.; Hemmings, S.N.; Kent, S.T.; Puckett, M.; Quattrochi, D.A.; Rickman, D.L.; et al. Environmental public health applications using remotely sensed data. Geocarto Int. 2014, 29, 85–98. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Snijders, T.A.; Bosker, R.J. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling, 2nd ed.; Sage Publications: London, UK, 2012. [Google Scholar]
- Taylor, A.L.; Ziesche, S.; Yancy, C.; Carson, P.; D’Agostino, R.; Ferdinand, K.; Taylor, M.; Adams, K.; Sabolinski, M.; Worcel, M.; et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N. Engl. J. Med. 2004, 351, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Braveman, P.A.; Egerter, S.A.; Cubbin, C.; Marchi, K.S. An approach to studying social disparities in health and health care. Am. J. Public Health 2004, 94, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- LaVeist, T.; Pollack, K.; Thorpe, R.; Fesahazion, R.; Gaskin, D. Place, not race: Disparities dissipate in southwest baltimore when blacks and whites live under similar conditions. Health Aff. 2011, 30, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Jermendy, G.; Horváth, T.; Littvay, L.; Steinbach, R.; Jermendy, Á.L.; Tárnoki, Á.D.; Tárnoki, D.L.; Métneki, J.; Osztovits, J. Effect of genetic and environmental influences on cardiometabolic risk factors: A twin study. Cardiovasc. Diabetol. 2011, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Braveman, P.A.; Cubbin, C.; Egerter, S.; Williams, D.R.; Pamuk, E. Socioeconomic disparities in health in the united states: What the patterns tell us. Am. J. Public Health 2010, 100 (Suppl. 1), S186–S196. [Google Scholar] [CrossRef]
- Barouki, R.; Gluckman, P.D.; Grandjean, P.; Hanson, M.; Heindel, J.J. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health 2012, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Geronimus, A.T.; Bound, J.; Colen, C.G. Geronimus et al. Respond. Am. J. Public Health 2011, 101, 1541. [Google Scholar] [CrossRef]
- Wild, C.P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Tisnado, D.M.; Adams, J.L.; Liu, H.; Damberg, C.L.; Hu, F.A.; Chen, W.-P.; Carlisle, D.M.; Mangione, C.M.; Kahn, K.L. Does the concordance between medical records and patient self-report vary with patient characteristics? Health Serv. Outcomes Res. Methodol. 2006, 6, 157–175. [Google Scholar] [CrossRef]
| Domain | Subdomain | Environmental Stressor | Cardiovascular Disease (CVD) | Stroke | Diabetes |
|---|---|---|---|---|---|
| Natural | Metals | Lead | Cosselman [58] | Navas [59] | Orioli [60] |
| Arsenic | Smith [61] | Smith [61] | Smith [61] | ||
| Cosselman [58] | |||||
| Cadmium | Cosselman [58] | Peters [62] | Edwards [63] | ||
| Solvents and pesticides | Solvents | Bulka [64] | Rinsky [65] | Montgomery [66] | |
| Pesticides | Wilcosky [67] | ||||
| Air pollution | PM10, PM2.5, ultrafine PM | Mohammadi, [68] | Kowalska [69] | ||
| Gases | Carbon monoxide | Lee [70] | Hampson [71] | Huang [72] | |
| Ozone | Goodman [73] | Srebot [74] | Jerrett [75] | ||
| Nitrogen dioxide Sulfur dioxide | Kopp [76] | Amancio [77] | Coogan [40] | ||
| Built | Neighborhood conditions | Walkability | Gaglioti [78] | Kwon [79] | Sundquist [80] |
| Perceived/actual safety | Pham [81] | ||||
| Evonson [82] | |||||
| Access to healthy foods | Availability of healthy or unhealthy stores/restaurants | Lindberg [83] | Christine [84] | ||
| Gaglioti [78] | |||||
| Peolman [85] | |||||
| Social | Demographic | Population density | Rodriguez [86] | ||
| Socioeconomic status (SES) | Gebreab [87] | ||||
| Social supports | Brown [88] | Zhang [50] | |||
| Access to health care | Access to insurance and health care services | Li [89] | Medford-Davis [90] | Stark [91] | |
| Social stressors | Community stressors Residential segregation | Ford [91] | Booth [92] | Grigsby-Toussaint [93] | |
| Kershaw [94] | Patel [95] | ||||
| Cultural influences | Lack of trust in health care providers | Schoenthaler [96] | Heisler [97] | ||
| Socio-cultural beliefs and norms | |||||
| Policy | Dietary policy | Pearson [98] | Jilcott Pitts [99] | ||
| Physical activity policy | Jilcott Pitts [99] | ||||
| Endocrine-disrupting chemicals policies | Shaikh [100] | ||||
| diabetes care and prevention policy | Ackermann [101] |
| Characteristics | % of Sample | % with CMD | Sig. |
|---|---|---|---|
| All Participants | 100.0 | 29.2 | |
| Gender | <0.001 | ||
| Male | 39.6 | 27.6 | |
| Female | 60.4 | 30.3 | |
| Race | 0.072 | ||
| Black | 66.1 | 28.9 | |
| Male (n = 13,292) | 27.2 | 25.9 | |
| Female (n = 18,986) | 38.9 | 31.0 | |
| White | 33.9 | 29.7 | |
| Male (n = 6046) | 12.4 | 31.1 | |
| Female (n = 10,475) | 21.5 | 28.9 | |
| Education (years completed) | 0.001 | ||
| Less than 9 years | 7.5 | 41.7 | |
| 9–11 years | 21.0 | 32.4 | |
| 12 years (or GED) | 34.3 | 27.8 | |
| Vocational/technical | 5.0 | 29.9 | |
| Some college | 20.1 | 27.0 | |
| College graduate | 7.7 | 24.7 | |
| Graduate school | 3.2 | 21.4 | |
| Doctorate | 1.3 | 19.5 | |
| Marital Status | 0.001 | ||
| Married or with partner | 34.3 | 29.6 | |
| Divorced | 34.4 | 29.1 | |
| Widowed | 9.4 | 41.6 | |
| Single | 21.9 | 23.5 | |
| Household Income | 0.001 | ||
| <$15,000 | 56.4 | 32.1 | |
| $15,000–$24,999 | 21.1 | 27.6 | |
| $25,000–$49,999 | 13.9 | 25.7 | |
| $50,000–$99,999 | 6.6 | 21.1 | |
| >$100,000 | 2.0 | 14.5 | |
| Residence | 0.001 | ||
| Urban | 54.2 | 26.4 | |
| Rural | 45.8 | 32.5 | |
| Air Quality Inside | 0.105 | ||
| Poor | 5.8 | 28.3 | |
| Fair | 28.5 | 28.7 | |
| Good | 52.7 | 29.4 | |
| Excellent | 12.9 | 30.1 | |
| Air Quality Outside | 0.001 | ||
| Poor | 7.2 | 31.5 | |
| Fair | 34.4 | 28.3 | |
| Good | 46.3 | 29.6 | |
| Excellent | 12.1 | 28.8 | |
| Body Mass Index (BMI) | 0.001 | ||
| Less than or equal 18.5 | 1.3 | 16.8 | |
| 18.5–25 | 24.0 | 17.2 | |
| 25–30 | 29.5 | 25.3 | |
| 30–35 | 21.9 | 34.0 | |
| 35–40 | 12.0 | 40.5 | |
| 40 or higher | 11.2 | 45.3 | |
| Employment Status | 0.001 | ||
| Employed | 38.5 | 19.9 | |
| Not employed | 61.5 | 35.0 | |
| Age | 0.001 | ||
| Senior 65 years and older | 10.3 | 45.2 | |
| 40–64 years old | 89.7 | 27.3 | |
| Smoking Status | 0.001 | ||
| Current | 42.0 | 23.9 | |
| Former | 22.6 | 38.0 | |
| Never | 35.4 | 29.8 | |
| Hypercholesterolemia | 0.001 | ||
| No | 65.3 | 19.4 | |
| Yes | 34.7 | 47.6 | |
| Hypertension | 0.001 | ||
| No | 44.4 | 14.3 | |
| Yes | 55.6 | 41.1 |
| Model Term | Coefficient | Std. Error | T-Value | p-Value | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval for Exp (Coefficient) | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| Intercept | −3.511 | 0.1482 | −23.692 | 0 | −3.802 | −3.221 | 0.030 | 0.022 | 0.04 |
| Enrollment Age | 0.025 | 0.001 | 24.005 | 0 | 0.023 | 0.027 | 1.025 | 1.023 | 1.027 |
| Education | |||||||||
| Doctorate | −0.2 | 0.1407 | −1.419 | 0.156 | −0.475 | 0.076 | 0.819 | 0.622 | 1.079 |
| Masters | −0.282 | 0.0579 | −4.867 | 0 | −0.395 | −0.168 | 0.754 | 0.673 | 0.845 |
| College | −0.142 | 0.0638 | −2.232 | 0.026 | −0.267 | −0.017 | 0.867 | 0.765 | 0.983 |
| Some College | −0.128 | 0.0355 | −3.607 | 0 | −0.198 | −0.059 | 0.880 | 0.820 | 0.943 |
| Vocational | −0.034 | 0.0353 | −0.953 | 0.341 | −0.103 | 0.036 | 0.967 | 0.902 | 1.036 |
| High School | −0.162 | 0.0286 | −5.673 | 0 | −0.218 | −0.106 | 0.850 | 0.804 | 0.899 |
| Some High School | −0.064 | 0.0488 | −1.311 | 0.19 | −0.16 | 0.032 | 0.938 | 0.852 | 1.032 |
| Less 9 years education | REF | - | - | - | - | - | - | - | - |
| Marital Status | |||||||||
| Single | −0.109 | 0.038 | −2.878 | 0.004 | −0.184 | −0.035 | 0.896 | 0.832 | 0.966 |
| Widowed | 0.068 | 0.037 | 1.835 | 0.067 | −0.005 | 0.14 | 1.070 | 0.995 | 1.151 |
| Divorced | −0.021 | 0.0287 | −0.727 | 0.467 | −0.077 | 0.035 | 0.979 | 0.926 | 1.036 |
| Married | REF | - | - | - | - | - | - | - | - |
| Income | |||||||||
| $100,000 plus | −0.713 | 0.0677 | −10.521 | 0 | −0.845 | −0.58 | 0.490 | 0.429 | 0.56 |
| $50,000–$99,000 | −0.381 | 0.06 | −6.356 | 0 | −0.499 | −0.264 | 0.683 | 0.607 | 0.768 |
| $25,000–$49,000 | −0.199 | 0.0429 | −4.632 | 0 | −0.283 | −0.115 | 0.820 | 0.754 | 0.892 |
| $15,000–$24,000 | −0.124 | 0.0233 | −5.337 | 0 | −0.170 | −0.079 | 0.883 | 0.844 | 0.924 |
| Less than $15,000 | REF | - | - | - | - | - | - | - | - |
| Rural or Farm | |||||||||
| Rural or Farm | 0.036 | 0.025 | 1.435 | 0.151 | −0.013 | 0.085 | 1.037 | 0.987 | 1.089 |
| Urban | REF | - | - | - | - | - | - | - | - |
| Air Quality Outside | |||||||||
| Excellent | −0.113 | 0.0384 | −2.951 | 0.003 | −0.189 | −0.038 | 0.893 | 0.828 | 0.963 |
| Good | −0.033 | 0.036 | −0.921 | 0.357 | −0.104 | 0.037 | 0.967 | 0.902 | 1.038 |
| Fair | −0.061 | 0.0342 | −1.777 | 0.076 | −0.128 | 0.006 | 0.941 | 0.880 | 1.006 |
| Poor | REF | - | - | - | - | - | - | - | - |
| Air Quality Inside | |||||||||
| Excellent | 0.15 | 0.0515 | 2.913 | 0.004 | 0.049 | 0.251 | 1.162 | 1.050 | 1.285 |
| Good | 0.01 | 0.0458 | 0.218 | 0.827 | −0.080 | 0.1 | 1.010 | 0.923 | 1.105 |
| Fair | 0.038 | 0.0424 | 0.888 | 0.374 | −0.045 | 0.121 | 1.038 | 0.956 | 1.128 |
| Poor | REF | - | - | - | - | - | - | - | - |
| BMI | |||||||||
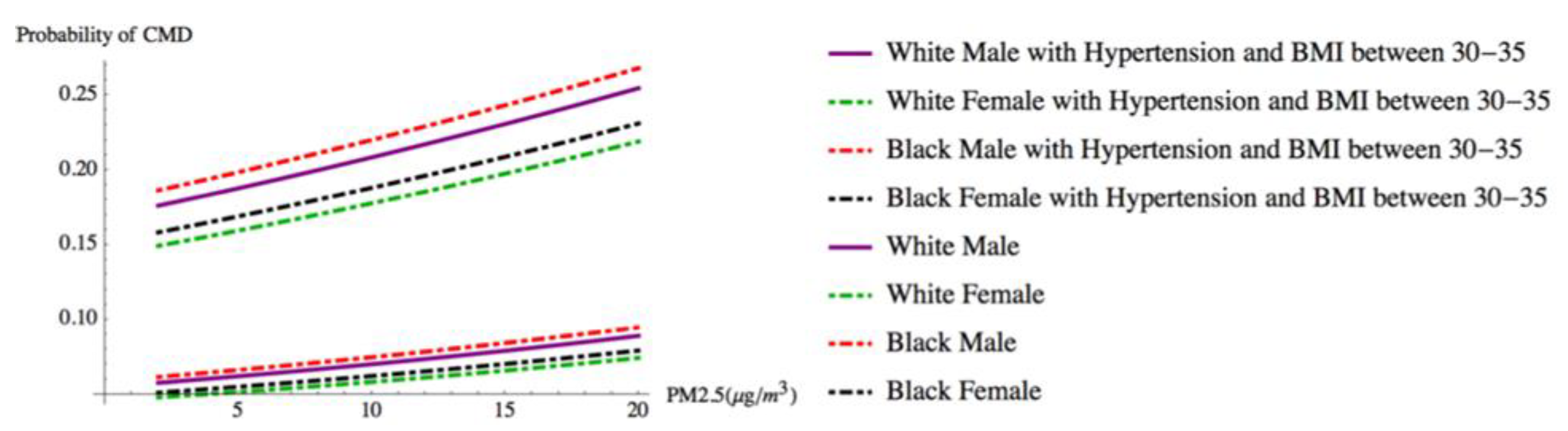
| 40 or higher | 1.061 | 0.1134 | 9.351 | 0 | 0.838 | 1.283 | 2.888 | 2.313 | 3.607 |
| 35–39 | 0.824 | 0.1051 | 7.846 | 0 | 0.618 | 1.03 | 2.280 | 1.856 | 2.801 |
| 30–34 | 0.597 | 0.0937 | 6.374 | 0 | 0.413 | 0.781 | 1.817 | 1.512 | 2.183 |
| 25–29 | 0.252 | 0.1101 | 2.291 | 0.022 | 0.036 | 0.468 | 1.287 | 1.037 | 1.597 |
| 18.5–24 | −0.015 | 0.1042 | −0.143 | 0.886 | −0.219 | 0.189 | 0.985 | 0.803 | 1.208 |
| Less than 18.5 | REF | - | - | - | - | - | - | - | - |
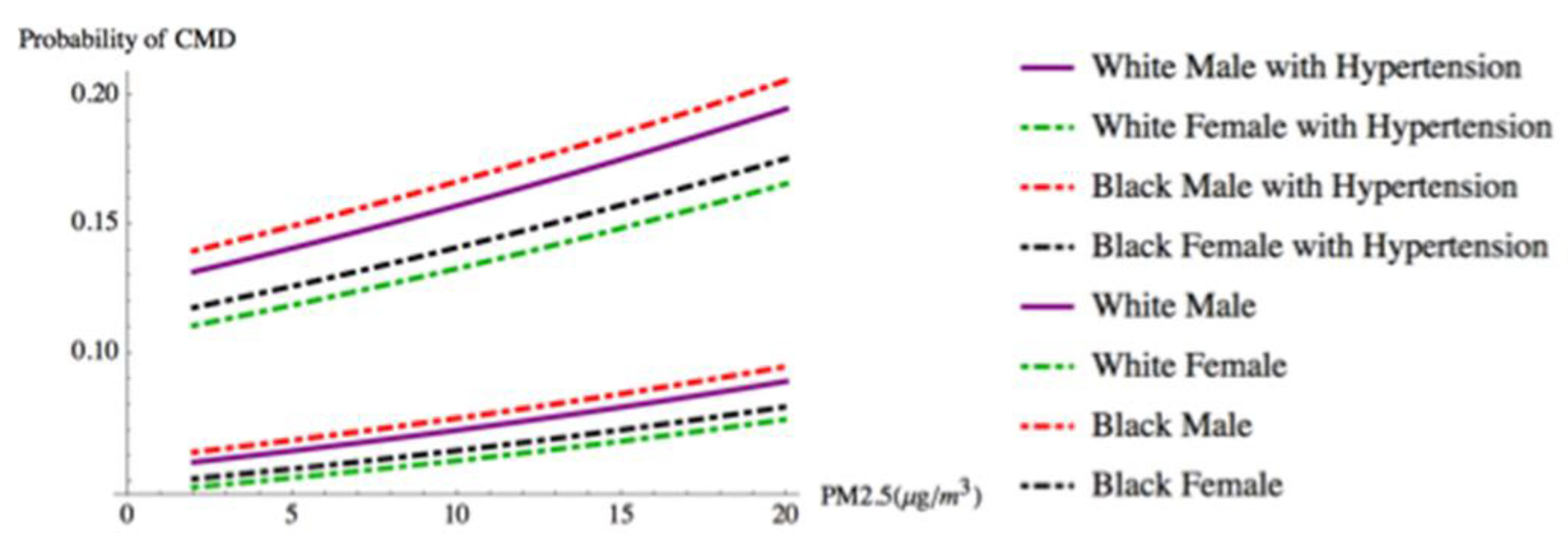
| Hypertension | |||||||||
| Yes | 0.907 | 0.0284 | 31.983 | 0 | 0.852 | 0.963 | 2.478 | 2.344 | 2.620 |
| No | REF | - | - | - | - | - | - | - | - |
| Hypercholesterol | |||||||||
| Yes | 0.959 | 0.0187 | 51.155 | 0 | 0.922 | 0.996 | 2.609 | 2.515 | 2.706 |
| No | REF | - | - | - | - | - | - | - | - |
| Employment | |||||||||
| Yes | −0.504 | 0.0222 | −22.681 | 0 | −0.548 | −0.461 | 0.604 | 0.578 | 0.631 |
| No | REF | - | - | - | - | - | - | - | - |
| Race | |||||||||
| Black | 0.069 | 0.0478 | 1.435 | 0.151 | −0.025 | 0.162 | 1.071 | 0.975 | 1.176 |
| White | REF | - | - | - | - | - | - | - | - |
| Smoking History | |||||||||
| Never Smoked | −0.017 | 0.0344 | −0.487 | 0.626 | −0.084 | 0.051 | 0.983 | 0.919 | 1.052 |
| Former Smoker | 0.19 | 0.0296 | 6.404 | 0 | 0.132 | 0.248 | 1.209 | 1.141 | 1.281 |
| Current Smoker | REF | - | - | - | - | - | - | - | - |
| PM2.5 | 0.026 | 0.0093 | 2.751 | 0.006 | 0.007 | 0.044 | 1.026 | 1.007 | 1.045 |
| Gender | |||||||||
| Female | −0.197 | 0.022 | −8.93 | 0 | −0.24 | −0.154 | 0.821 | 0.787 | 0.858 |
| Male | REF | - | - | - | - | - | - | - | - |
| Random Effect Covariance | Estimate | Std. Error | Z | p-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Var (Intercept) | 0.017 | 0.008 | 1.984 | 0.047 | 0.006 | 0.045 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juarez, P.D.; Tabatabai, M.; Burciaga Valdez, R.; Hood, D.B.; Im, W.; Mouton, C.; Colen, C.; Al-Hamdan, M.Z.; Matthews-Juarez, P.; Lichtveld, M.Y.; et al. The Effects of Social, Personal, and Behavioral Risk Factors and PM2.5 on Cardio-Metabolic Disparities in a Cohort of Community Health Center Patients. Int. J. Environ. Res. Public Health 2020, 17, 3561. https://doi.org/10.3390/ijerph17103561
Juarez PD, Tabatabai M, Burciaga Valdez R, Hood DB, Im W, Mouton C, Colen C, Al-Hamdan MZ, Matthews-Juarez P, Lichtveld MY, et al. The Effects of Social, Personal, and Behavioral Risk Factors and PM2.5 on Cardio-Metabolic Disparities in a Cohort of Community Health Center Patients. International Journal of Environmental Research and Public Health. 2020; 17(10):3561. https://doi.org/10.3390/ijerph17103561
Chicago/Turabian StyleJuarez, Paul D., Mohammad Tabatabai, Robert Burciaga Valdez, Darryl B. Hood, Wansoo Im, Charles Mouton, Cynthia Colen, Mohammad Z. Al-Hamdan, Patricia Matthews-Juarez, Maureen Y. Lichtveld, and et al. 2020. "The Effects of Social, Personal, and Behavioral Risk Factors and PM2.5 on Cardio-Metabolic Disparities in a Cohort of Community Health Center Patients" International Journal of Environmental Research and Public Health 17, no. 10: 3561. https://doi.org/10.3390/ijerph17103561
APA StyleJuarez, P. D., Tabatabai, M., Burciaga Valdez, R., Hood, D. B., Im, W., Mouton, C., Colen, C., Al-Hamdan, M. Z., Matthews-Juarez, P., Lichtveld, M. Y., Sarpong, D., Ramesh, A., Langston, M. A., Rogers, G. L., Phillips, C. A., Reichard, J. F., Donneyong, M. M., & Blot, W. (2020). The Effects of Social, Personal, and Behavioral Risk Factors and PM2.5 on Cardio-Metabolic Disparities in a Cohort of Community Health Center Patients. International Journal of Environmental Research and Public Health, 17(10), 3561. https://doi.org/10.3390/ijerph17103561









