Comparative Study of the Composition of Sweat from Eccrine and Apocrine Sweat Glands during Exercise and in Heat
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Stimulated Sweating and Environmental Conditions
2.3. Sweat Collection
2.4. Experimental Design and Procedure
2.5. Sweat Preservation and Composition Analysis
2.6. Statistical Analysis
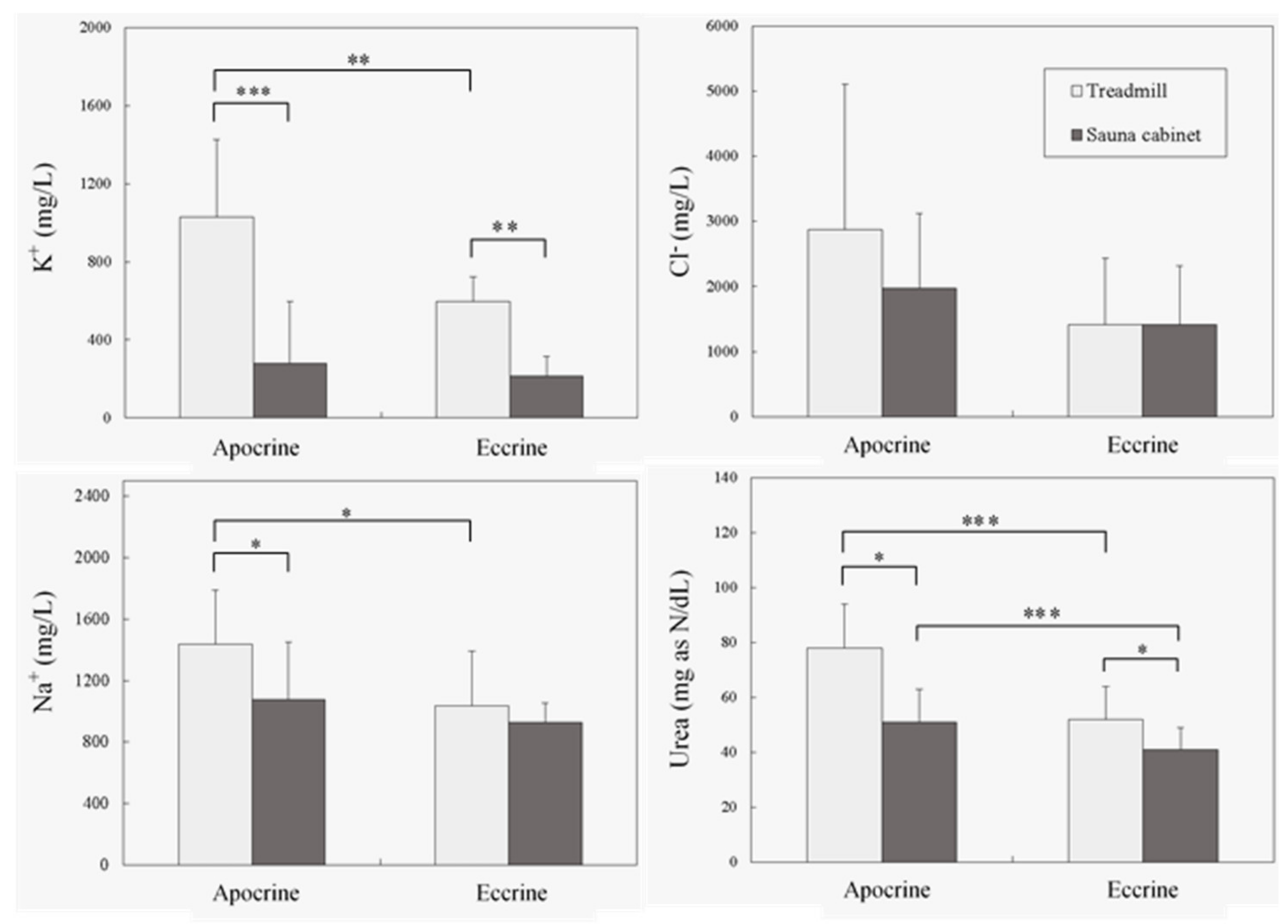
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilke, K.; Martin, A.; Terstegen, L.; Biel, S.S. A short history of sweat gland biology. Int. J. Cosmet. Sci. 2007, 29, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Laitano, O.; Bar-Or, O.; McDougall, D.; Heigenhauser, G.J.F. Effect of age and gender on sweat lactate and ammonia concentrations during exercise in the heat. Braz. J. Med. Biol. Res. 2007, 40, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Garden, J.W. Plasma and sweat histamine concentrations after heat exposure and physical exercise. J. Appl. Physiol. 1966, 21, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Fellmann, N.; Labbe, A.; Gachon, A.M.; Coudert, J. Thermal sweat lactate in cystic fibrosis and in normal children. Eur. J. Appl. Physiol. 1985, 54, 511–516. [Google Scholar] [CrossRef]
- Åstrand, P.O.; Rodahl, K. Textbook of Work Physiology; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Huang, C.T.; Chen, M.L.; Huang, L.L.; Mao, I.F. Uric acid and urea in human sweat. Chin. J. Physiol. 2002, 45, 109–116. [Google Scholar]
- Cone, E.J. New developments in biological measures of drug prevalence. NIDA Res. Monogr. 1997, 167, 108–129. [Google Scholar] [CrossRef]
- Dutkiewicz, E.P.; Lin, J.D.; Tseng, T.W.; Wang, Y.S.; Urban, P.L. Hydrogel micropatches for sampling and profiling skin metabolites. Anal. Chem. 2014, 86, 2337–2344. [Google Scholar] [CrossRef]
- Schiefferdecker, P. The skin glands of humans and mammals and their biological and racial anatomical significance and the muscularis sexualis. Zoologica 1922, 27, 1–154. [Google Scholar]
- Sato, K.; Sato, F. Sweat secretion by human axillary apoeccrine sweat gland in vitro. Am. J. Physiol. 1987, 252, R181–R187. [Google Scholar] [CrossRef]
- Sato, K.; Kang, W.H.; Saga, K.; Sato, K.T. Biology of sweat glands and their disorders. I. Normal sweat gland function. J. Am. Acad. Dermatol. 1989, 20, 537–563. [Google Scholar] [CrossRef]
- Morgan, R.M.; Patterson, M.J.; Nimmo, M.A. Acute effects of dehydration on sweat composition in men during prolonged exercise in the heat. Acta. Physiol. 2004, 182, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Barnes, K.A.; Anderson, M.L.; Passe, D.H.; Stofan, J.R. Normative data for regional sweat sodium concentration and whole-body sweating rate in athletes. J. Sports Sci. 2016, 34, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Verde, T.; Shephard, R.; Corey, P.; Moore, R. Sweat composition in exercise and in heat. J. Appl. Physiol. 1982, 53, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Dill, D.B.; Hall, F.G.; Van Beaumont, W. Sweat chloride concentration: Sweat rate, metabolic rate, skin temperature, and age. J. Appl. Physiol. 1966, 21, 99–106. [Google Scholar] [CrossRef]
- Vairo, D.; Bruzzese, L.; Marlinge, M.; Fuster, L.; Adjriou, N.; Kipson, N.; Brunet, P.; Cautela, J.; Jammes, Y.; Mottola, G.; et al. Towards addressing the body electrolyte environment via sweat analysis: Pilocarpine iontophoresis supports assessment of plasma potassium concentration. Sci. Rep. 2017, 7, 11801. [Google Scholar] [CrossRef]
- Berenson, G.S.; Burch, G.E. The response of patients with congestive heart failure to a rapid elevation in atmospheric temperature and humidity. Am. J. Med. Sci. 1952, 223, 45–53. [Google Scholar] [CrossRef]
- Consolazio, C.F.; Matoush, L.O.; Nelson, R.A.; Hackler, L.R.; Preston, E.E. Relationship between calcium in sweat, calcium balance, and calcium requirements. J. Nutr. 1962, 78, 78–88. [Google Scholar] [CrossRef]
- Hussain, J.N.; Mantri, N.; Cohen, M.M. Working up a good sweat–the challenges of standardising sweat collection for metabolomics analysis. Clin. Biochem. Rev. 2017, 38, 13–34. [Google Scholar]
- Schwartz, S. 9 Showering Mistakes That Can Actually Hurt You. StethNews, Updated April 14, 2015. Available online: www.huffpost.com/entry/showering-mistakes-_n_7065204 (accessed on 30 March 2020).
- De Bruyne, G.; Aerts, J.M.; Vander Sloten, J.; Goffin, J.; Verpoest, I.; Berckmans, D. Transient sweat response of the human head during cycling. Int. J. Ind. Ergon. 2010, 40, 406–413. [Google Scholar] [CrossRef]
- Jadoon, S.; Karim, S.; Akram, M.R.; Kalsoom Khan, A.; Zia, M.A.; Siddiqi, A.R.; Murtaza, G. Recent developments in sweat analysis and its applications. Int. J. Anal. Chem. 2015, 164974. [Google Scholar] [CrossRef]
- Baker, L.B. Sweating rate and sweat sodium concentration in athletes: A review of methodology and intra/interindividual variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, N.; Fucci, N. The current status of sweat testing for drugs of abuse: A review. Curr. Med. Chem. 2013, 20, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Harker, M.; Coulson, H.; Fairweather, I.; Taylor, D.; Daykin, C.A. Study of metabolite composition of eccrine sweat from healthy male and female human subjects by 1 h NMR spectroscopy. Metabolomics 2006, 2, 105–112. [Google Scholar] [CrossRef]
- Kutyshenko, V.P.; Molchanov, M.; Beskaravayny, P.; Uversky, V.N.; Timchenko, M.A. Analyzing and mapping sweat metabolomics by high-resolution NMR spectroscopy. PLoS ONE 2011, 6, e28824. [Google Scholar] [CrossRef]
- Genuis, S.J.; Beesoon, S.; Lobo, R.A.; Birkholz, D. Human elimination of phthalate compounds: Blood, urine, and sweat (BUS) study. Sci. World J. 2012, 615068. [Google Scholar] [CrossRef]
- Mark, H.; Harding, C.R. Amino acid composition, including key derivatives of eccrine sweat: Potential biomarkers of certain atopic skin conditions. Int. J. Cosmet. Sci. 2013, 35, 163–168. [Google Scholar] [CrossRef]
- Sheng, J.; Qiu, W.; Xu, B.; Xu, H.; Tang, C. Monitoring of heavy metal levels in the major rivers and in residents’ blood in zhenjiang city, china, and assessment of heavy metal elimination via urine and sweat in humans. Environ. Sci. Pollut. Res. 2016, 23, 11034–11045. [Google Scholar] [CrossRef]
- Tang., S.; Yu, X.; Wu, C. Comparison of the levels of five heavy metals in human urine and sweat after strenuous exercise by ICP-MS. J. Appl. Math. Phys. 2016, 4, 183–188. [Google Scholar] [CrossRef]
- Ely, M.R.; Kenefick, R.W.; Cheuvront, S.N.; Chinevere, T.D.; Lacher, C.P.; Lukaski, H.C.; Montain, S.J. Surface contamination artificially elevates initial sweat mineral concentrations. J. Appl. Physiol. 2011, 110, 1534–1540. [Google Scholar] [CrossRef]
- Mackintosh, C.A.; Lidwell, O.M.; Towers, A.G.; Marples, R.R. The dimensions of skin fragments dispersed into the air during activity. Epidemiol. Infect. 1978, 81, 471–480. [Google Scholar] [CrossRef]
- Groscurth, P. Anatomy of Sweat Glands. In Hyperhidrosis and Botulinum Toxin in Dermatology; Karger Publishers: Basel, Switzerland, 2002; Volume 30, pp. 1–9. [Google Scholar]
- Al-Tamer, Y.Y.; Hadi, E.A. Sweat urea, uric acid and creatinine concentrations in uraemic patients. Urol. Res. 1997, 25, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.P.; Polliack, A.A.; Bader, D.L. The analysis of metabolites in human sweat: Analytical methods and potential application to investigation of pressure ischaemia of soft tissues. Ann. Clin. Biochem. 1994, 31, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, A.; Watanabe, H.; Kobayashi, M.; Chiba, M.; Inaba, Y.; Kimura, N.; Ito, T. Concentrations of trace elements in sweat during sauna bathing. Tohoku J. Exp. Med. 2001, 195, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.A.; Machado-Moreira, C.A. Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extreme Physiol. Med. 2013, 2, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, B.M.; Schummer, C.; Rodrigues, S.B.; Wennig, R. Determination of the volume of sweat accumulated in a sweatpatch using sodium and potassium as internal reference. J. Chromatogr. B 2007, 852, 333–337. [Google Scholar] [CrossRef]
- Sato, K. The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev. Physiol. Biochem. Pharmacol. 1977, 79, 51–131. [Google Scholar] [CrossRef]
- Weschler, L.B. Sweat electrolyte concentrations obtained from within occlusive coverings are falsely high because sweat itself leaches skin electrolytes. J. Appl. Physiol. 2008, 105, 1376–1377. [Google Scholar] [CrossRef][Green Version]
- Quinton, P.M. Effects of some ion transport inhibitors on secretion and reabsorption in intact and perfused single human sweat glands. Pflügers Archiv 1981, 391, 309–313. [Google Scholar] [CrossRef]
- Sato, K. The mechanism of eccrine sweat secretion. In Perspectives in Exercise Science and Sports Medicine. Exercise, Heat, and Thermoregulation; Gisolfi, C.V., Lamb, D.R., Nadel, E.R., Eds.; Brown & Benchmark: Dubuque, IA, USA, 1993. [Google Scholar]
- Reddy, M.M.; Quinton, P.M. Rapid regulation of electrolyte absorption in sweat duct. J. Membr. Biol. 1994, 140, 57–67. [Google Scholar] [CrossRef]
- Huxley, H. The mechanism of muscular contraction. Science 1969, 164, 1356–1366. [Google Scholar] [CrossRef]
- Costill, D.L. Water and electrolyte requirements during exercise. Clin. Sports Med. 1984, 3, 639–648. [Google Scholar] [PubMed]
- Buono, M.J.; Claros, R.; DeBoer, T.; Wong, J. Na+ secretion rate increases proportionally more than the Na+ reabsorption rate with increases in sweat rate. J. Appl. Physiol. 2008, 105, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Tanaka, T.; Fujioka, H.; Yoshihara, S.; Ochi, T.; Kuroiwa, A. Differences in composition of sweat induced by thermal exposure and by running exercise. Clin. Cardiol. 1988, 11, 707–709. [Google Scholar] [CrossRef]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Povedano, M.M.; Calderón-Santiago, M.; de Castro, M.L.; Priego-Capote, F. Metabolomics analysis of human sweat collected after moderate exercise. Talanta 2018, 177, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, N.; Salava, A.; Auvinen, P.; Lauerma, A. Skin biomes. Curr. Allergy Asthma Rep. 2016, 16, 1–7. [Google Scholar] [CrossRef][Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
| Items | Mean | Standard Deviation | Range |
|---|---|---|---|
| Age (years) | 22.2 | 0.4 | 22.0–23.4 |
| Stature (cm) | 171.9 | 7.8 | 161.5–179.1 |
| Body weight (kg) | 68.9 | 8.6 | 60.5–81.3 |
| Resting heart rate (beats/min) | 73.2 | 3.6 | 67–81 |
| Maximal peak expiratory flow (l) | 9.4 | 0.6 | 8.9–10.3 |
| Exercise frequency (times/week) | 2.8 | 1.1 | 2–4 |
| Compositions | DF | SS | MS | F | Significance | Power | |
|---|---|---|---|---|---|---|---|
| Sweating condition | Urea | 1 | 2109 | 2109 | 13.08 | P < 0.005 | 0.930 |
| Na+ | 1 | 1013937 | 1013937 | 14.88 | P < 0.001 | 0.956 | |
| Cl− | 1 | 1193942 | 1193942 | 0.59 | P = 0.452 | 0.114 | |
| K+ | 1 | 1920438 | 1920438 | 27.07 | P < 0.001 | 0.999 | |
| Sweat gland | Urea | 1 | 1926 | 1926 | 11.95 | P < 0.005 | 0.908 |
| Na+ | 1 | 450182 | 450182 | 6.61 | P < 0.05 | 0.786 | |
| Cl− | 1 | 6073222 | 6073222 | 2.99 | P = 0.990 | 0.377 | |
| K+ | 1 | 365313 | 365313 | 5.15 | P < 0.05 | 0.714 | |
| Sweating condition × Sweat gland | Urea | 1 | 376 | 376 | 2.33 | P = 0.142 | 0.307 |
| Na+ | 1 | 87483 | 87483 | 1.28 | P = 0.271 | 0.190 | |
| Cl− | 1 | 1200195 | 1200195 | 0.59 | P = 0.450 | 0.114 | |
| K+ | 1 | 206276 | 206276 | 2.91 | P = 0.104 | 0.368 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Kuan, W.-H.; Liu, C.-L. Comparative Study of the Composition of Sweat from Eccrine and Apocrine Sweat Glands during Exercise and in Heat. Int. J. Environ. Res. Public Health 2020, 17, 3377. https://doi.org/10.3390/ijerph17103377
Chen Y-L, Kuan W-H, Liu C-L. Comparative Study of the Composition of Sweat from Eccrine and Apocrine Sweat Glands during Exercise and in Heat. International Journal of Environmental Research and Public Health. 2020; 17(10):3377. https://doi.org/10.3390/ijerph17103377
Chicago/Turabian StyleChen, Yi-Lang, Wen-Hui Kuan, and Chao-Lin Liu. 2020. "Comparative Study of the Composition of Sweat from Eccrine and Apocrine Sweat Glands during Exercise and in Heat" International Journal of Environmental Research and Public Health 17, no. 10: 3377. https://doi.org/10.3390/ijerph17103377
APA StyleChen, Y.-L., Kuan, W.-H., & Liu, C.-L. (2020). Comparative Study of the Composition of Sweat from Eccrine and Apocrine Sweat Glands during Exercise and in Heat. International Journal of Environmental Research and Public Health, 17(10), 3377. https://doi.org/10.3390/ijerph17103377






