Sorption Behavior of Hexabromocyclododecanes (HBCDs) on Weihe River Sediment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sorption Experiments
2.3. Analytical Methods
3. Results and Discussion
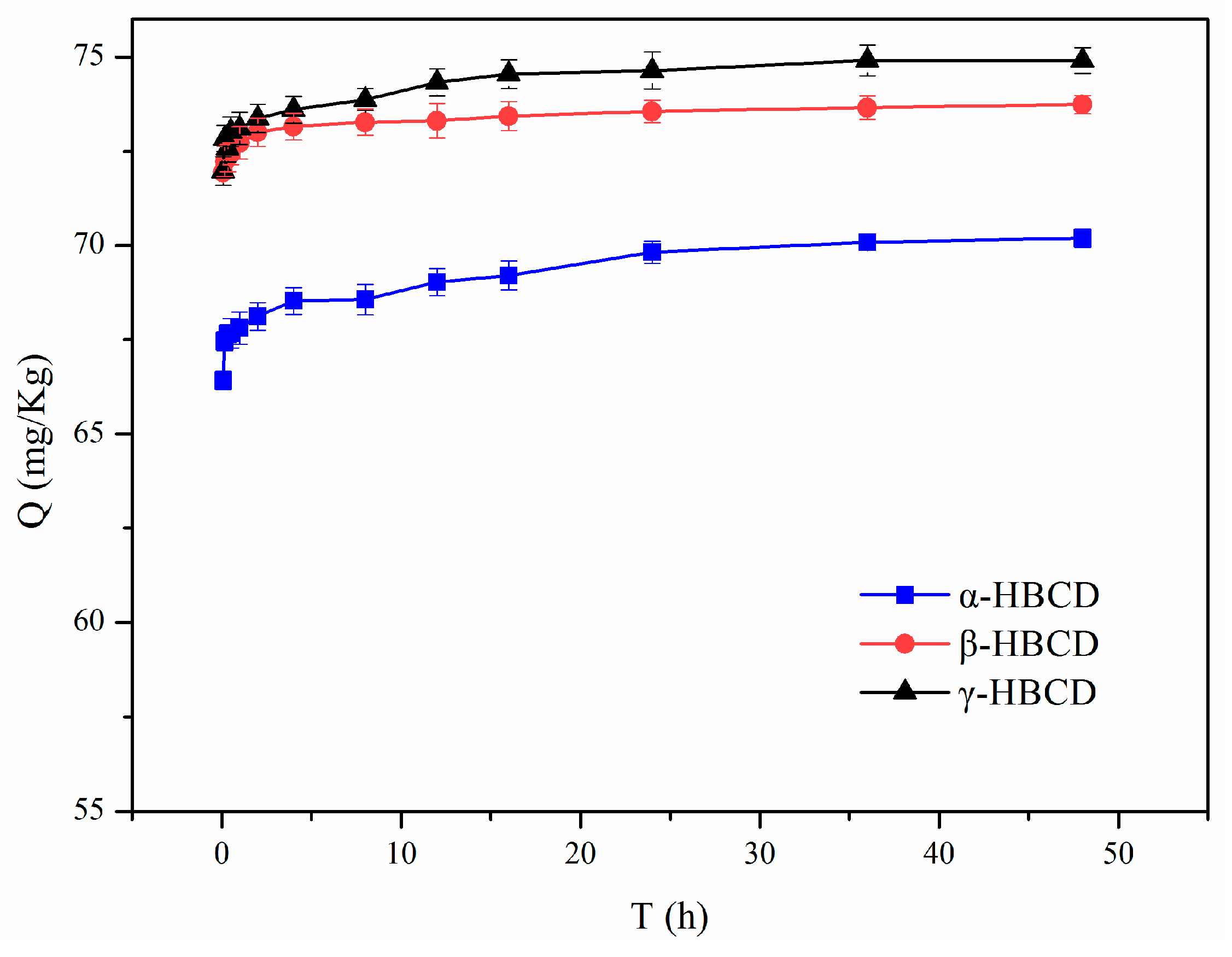
3.1. Sorption Kinetics
3.2. Sorption Isotherms
3.3. Sorption Thermodynamics
3.4. Effect of Temperature on HBCD Sorption
3.5. Effect of pH on HBCD Sorption
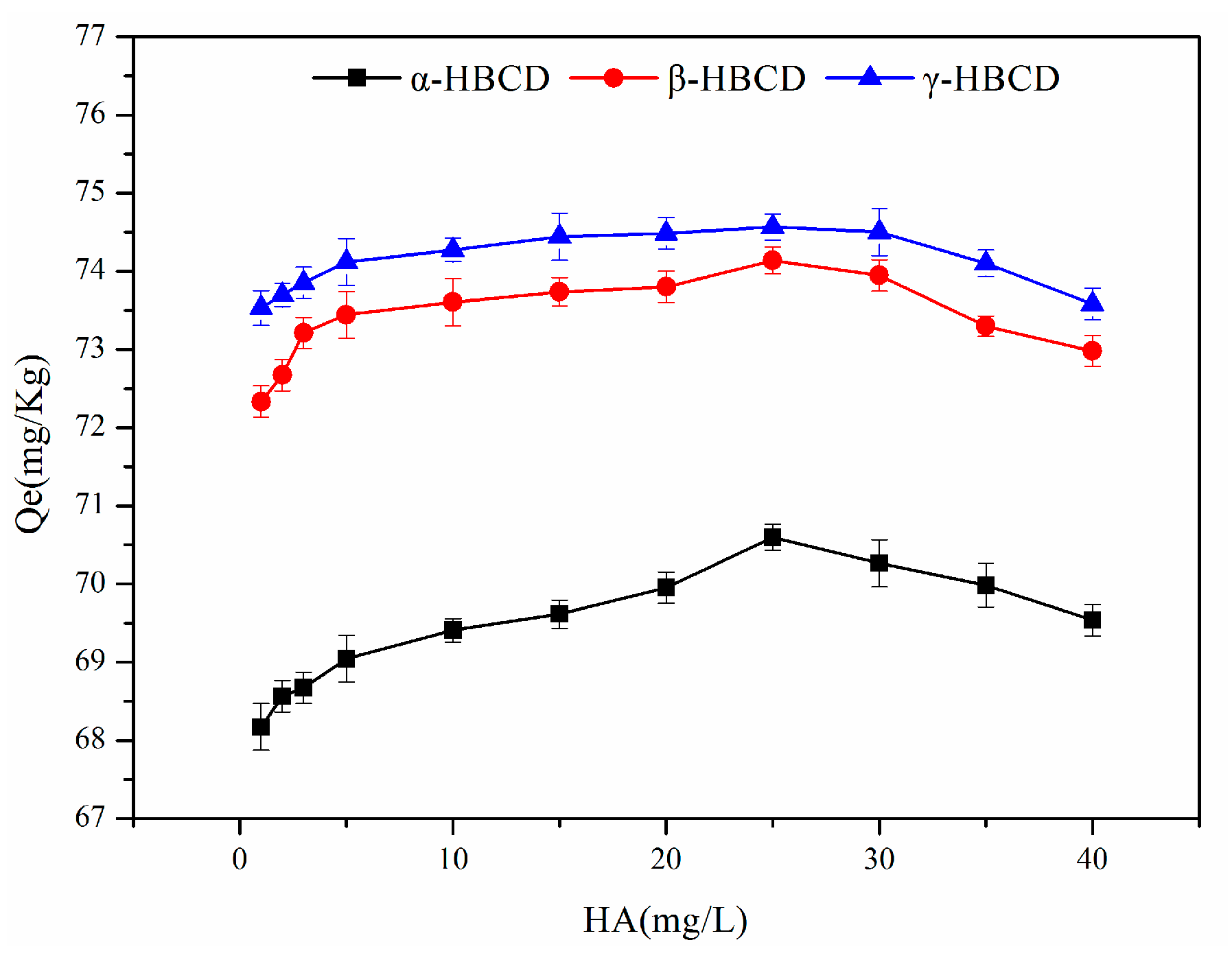
3.6. Effect of the HA Concentration on HBCD Sorption
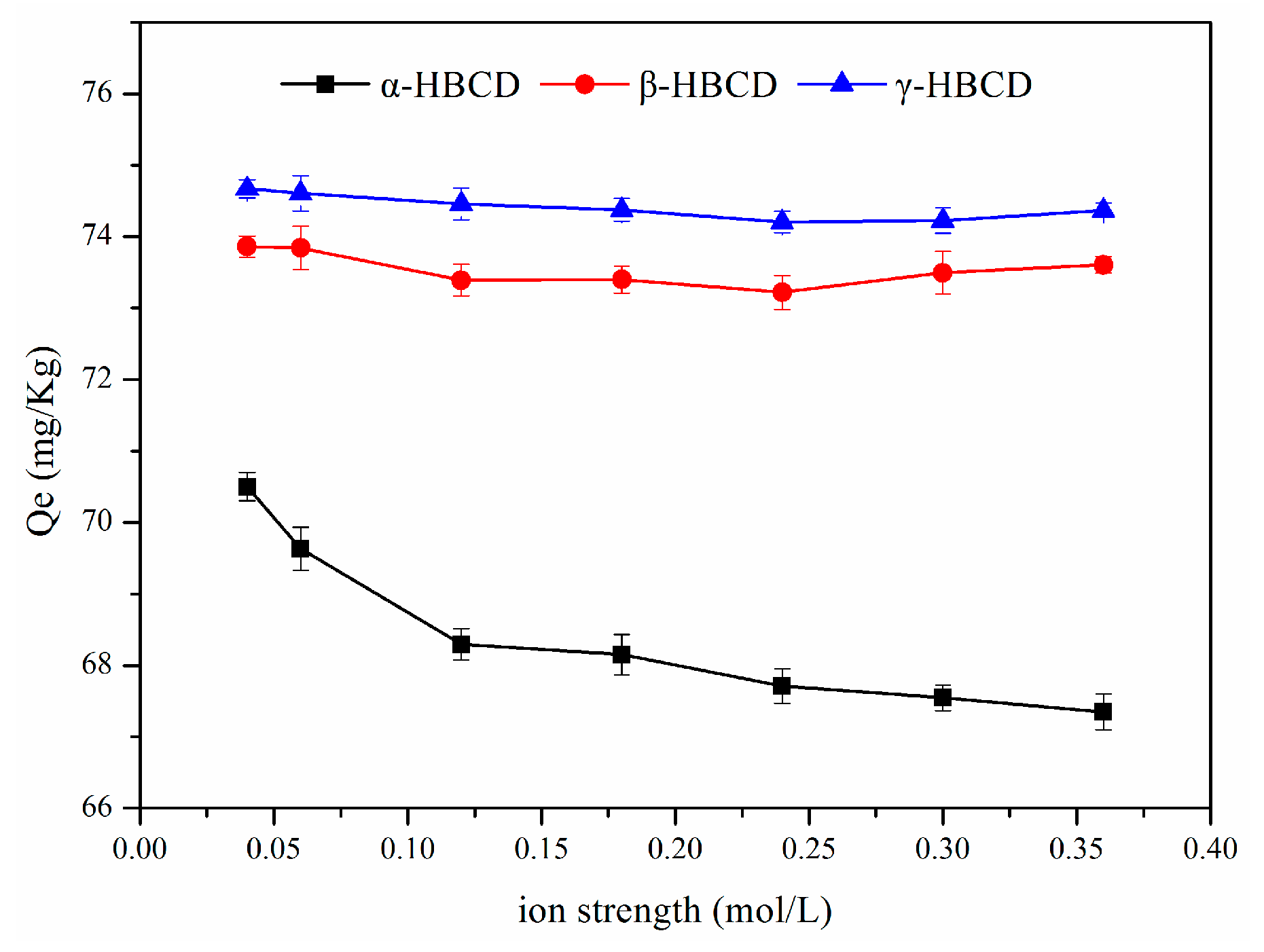
3.7. Effect of the Ionic Strength on HBCD Sorption
3.8. Sorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alaee, M.; Arias, P.; Sjödin, A.; Bergman, Å. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- Marvin, C.H.; Tomy, G.T.; Armitage, J.M.; Arnot, J.A.; McCarty, L.; Covaci, A.; Palace, V. Hexabromocyclododecane: Current understanding of chemistry, environmental fate and toxicology and implications for global management. Environ. Sci. Technol. 2011, 45, 8613–8623. [Google Scholar] [CrossRef]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Song, Y.K.; Hong, S.H. Hexabromocyclododecane in polystyrene based consumer products: An evidence of unregulated use. Chemosphere 2014, 110, 111–119. [Google Scholar] [CrossRef]
- Covaci, A.; Gerecke, A.C.; Law, R.J.; Voorspoels, S.; Kohler, M.; Heeb, N.V.; Leslie, H.; Allchin, C.R.; Boer, J.D. Hexabromocyclododecanes (HBCDs) in the Environment and Humans: A Review. Environ. Sci. Technol. 2006, 40, 3679–3688. [Google Scholar] [CrossRef] [PubMed]
- Law, R.J.; Kohler, M.; Heeb, N.V.; Gerecke, A.C.; Schmid, P.; Voorspoels, S.; Covaci, A.; Becher, G.; Janák, K.; Thomsen, C. Hexabromocyclododecane Challenges Scientists and Regulators. Environ. Sci. Technol. 2005, 1, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Choo, G.; Cho, H.S.; Park, K.; Shin, Y.J.; Oh, J.E. The occurrence and distribution of hexabromocyclododecanes in freshwater systems, focusing on tissue-specific bioaccumulation in crucian carp. Sci. Total Environ. 2018, 635, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Cal, A.D.L.; Marsh, G.; Eljarrat, E.; Barceló, D. Transfer of hexabromocyclododecane from industrial effluents to sediments and biota: Case study in Cinca river (Spain). J. Hydrol. 2009, 369, 360–367. [Google Scholar] [CrossRef]
- United Nations Environment Programme. United Nations Environment Annual Repor; United Nations Environment Programme: Nairobi, Kenya, 2013. [Google Scholar]
- Zhang, Y.; Lu, Y.; Wang, P.; Li, Q.; Zhang, M.; Johnson, A.C. Transport of Hexabromocyclododecane (HBCD) into the soil, water and sediment from a large producer in China. Sci. Total. Environ. 2018, 610, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Law, R.J.; Covaci, A.; Harrad, S.; Herzke, D.; Abdallah, M.A.E.; Fernie, K.; Toms, L.-M.L.; Takigami, H. Levels and trends of PBDEs and HBCDs in the global environment: Status at the end of 2012. Environ. Int. 2014, 65, 147–158. [Google Scholar] [CrossRef]
- Drage, D.; Mueller, J.F.; Birch, G.; Eaglesham, G.; Hearn, L.K.; Harrad, S. Historical trends of PBDEs and HBCDs in sediment cores from Sydney estuary, Australia. Sci. Total Environ. 2015, 512, 177–184. [Google Scholar] [CrossRef]
- Cao, X.; Lu, Y.; Zhang, Y.; Khan, K.; Wang, C.; Baninla, Y. An overview of hexabromocyclododecane (HBCDs) in environmental media with focus on their potential risk and management in China. Environ. Pollut. 2018, 236, 283–295. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Uchea, C.; Chipman, J.K.; Harrad, S. Enantioselective biotransformation of hexabromocyclododecane by in vitro rat and trout hepatic sub-cellular fractions. Environ. Sci. Technol. 2014, 48, 2732–2740. [Google Scholar] [CrossRef]
- Kim, U.J.; Oh, J.E. Tetrabromobisphenol A and hexabromocyclododecane flame retardants in infant-mother paired serum samples, and their relationships with thyroid hormones and environmental factors. Environ. Pollut. 2014, 184, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Serchi, T.; Cambier, S.; Diepenbroek, C.; Renaut, J.; Van der Berg, J.H.; Kwadijk, C.; Gutleb, A.C.; Rijntjes, E.; Murk, A.J. Hexabromocyclododecane (HBCD) induced changes in the liver proteome of eu- and hypothyroid female rats. Toxicol. Lett. 2016, 245, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Zhu, J.Y.; Tang, L.; Liu, N.; Peng, B.Q.; Sun, R.; Xu, G. Hexabromocyclododecanes in surface sediments from Shanghai, China: Spatial distribution, seasonal variation and diastereoisomer-specific profiles. Chemosphere 2014, 111, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Kotani, K.; Managaki, S.; Masunaga, S. Levels and distribution of hexabromocyclododecane and its lower brominated derivative in Japanese riverine environment. Chemosphere 2014, 109, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Guo, C.; He, Y.; Li, L.; Meng, W. Levels and distribution of tetrabromobisphenol A and hexabromocyclododecane in Taihu Lake, China. Environ. Toxicol. Chem. 2013, 32, 2249–2255. [Google Scholar] [CrossRef]
- Li, B.; Yao, T.; Sun, H.; Zhang, Y.; Yang, J. Diastereomer- and enantiomer-specific accumulation, depuration, bioisomerization, and metabolism of hexabromocyclododecanes (HBCDs) in two ecologically different species of earthworms. Sci. Total Environ. 2016, 542, 427–434. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, H.; Yao, Y.; Wang, F.; Zhang, Y.; Liu, X. Fate and adverse effects of hexabromocyclododecane diastereoisomers (HBCDDs) in a soil-ryegrass pot system. Chemosphere 2017, 184, 452–459. [Google Scholar] [CrossRef]
- Li, B.; Zhu, H.; Sun, H.; Xu, J. Effects of the amendment of biochars and carbon nanotubes on the bioavailability of hexabromocyclododecanes (HBCDs) in soil to ecologically different species of earthworms. Environ. Pollut. 2017, 222, 191–200. [Google Scholar] [CrossRef]
- Jeong, G.H.; Hwang, N.R.; Hwang, E.H.; Lee, B.C.; Yoon, J. Hexabromocyclododecanes in crucian carp and sediment from the major rivers in Korea. Sci. Total Environ. 2014, 470–471, 1471–1478. [Google Scholar] [CrossRef]
- Al-Odaini, N.A.; Shim, W.J.; Han, G.M.; Jang, M.; Hong, S.H. Enrichment of hexabromocyclododecanes in coastal sediments near aquaculture areas and a wastewater treatment plant in a semi-enclosed bay in South Korea. Sci. Total Environ. 2015, 505, 290–298. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, Y.; Mao, L.; Feng, Z.; Yu, H. Sorption behavior of tetrabromobisphenol A in two soils with different characteristics. J. Hazard. Mater. 2008, 160, 456–461. [Google Scholar] [CrossRef]
- Li, F.; Jin, J.; Tan, D.; Wang, L.; Geng, N.; Cao, R.; Gao, Y.; Chen, J. Hexabromocyclododecane and tetrabromobisphenol A in sediments and paddy soils from Liaohe River Basin, China: Levels, distribution and mass inventory. J. Environ. Sci. 2016, 48, 209–217. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Q.; Miao, X.; Huang, X.; Li, B.; Su, G.; Zheng, M. Determination of hexabromocyclododecanes in sediments from the Haihe River in China by an optimized HPLC-MS-MS method. J. Environ. Sci. 2017, 55, 174–183. [Google Scholar] [CrossRef]
- Huang, W.; Schlautman, M.; Weber, W.J. A Distributed Reactivity Model for Sorption by Soils and Sediments. 5. The Influence of Near-Surface Characteristics in Mineral Domains. Environ. Sci. Technol. 1996, 20, 2993–3000. [Google Scholar] [CrossRef]
- Weber, W.J.; Huang, W. A Distributed Reactivity Model for Sorption by Soils and Sediments. 4. Intraparticle Heterogeneity and Phase-Distribution Relationships under Nonequilibrium Conditions. Environ. Sci. Technol. 1996, 30, 881–888. [Google Scholar] [CrossRef]
- Pignatello, J.; Xing, B. Mechanisms of Slow Sorption of Organic Chemicals to Natural Particles. Environ. Sci. Technol. 1996, 30, 1–11. [Google Scholar] [CrossRef]



| Langmuir Model Parameter | Freundlich Model Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm (mg/Kg) | KL | R2 | RSS/dof | RL | KF | n | R2 | RSS/dof | |
| α-HBCD | 443.56 | 6.23 | 0.9867 | 32.05 | 0.243 | 1233.43 | 0.83 | 0.9805 | 41.53 |
| β-HBCD | 614.29 | 19.54 | 0.9766 | 62.72 | 0.093 | 5424.61 | 0.87 | 0.9737 | 62.73 |
| γ-HBCD | 1146.37 | 91.41 | 0.9486 | 141.89 | 0.021 | 71,870.33 | 0.96 | 0.9388 | 150.08 |
| T(K) | K | ΔG (kJ·mol−1) | ΔH (kJ·mol−1) | ΔS (kJ·mol−1) | |
|---|---|---|---|---|---|
| α-HBCD | 298 | 3127.58 | −19.94 | −49.08 | −0.0975 |
| 308 | 2341.07 | −19.22 | |||
| 318 | 1426.58 | −17.99 | |||
| β-HBCD | 298 | 25286.73 | −25.12 | −64.87 | −0.1335 |
| 308 | 14274.37 | −23.70 | |||
| 318 | 8598.41 | −22.45 | |||
| γ-HBCD | 298 | 293639.20 | −31.19 | −75.40 | −0.1480 |
| 308 | 183989.34 | −30.03 | |||
| 318 | 88950.41 | −28.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yuan, X.; Yang, S. Sorption Behavior of Hexabromocyclododecanes (HBCDs) on Weihe River Sediment. Int. J. Environ. Res. Public Health 2020, 17, 247. https://doi.org/10.3390/ijerph17010247
Wang X, Yuan X, Yang S. Sorption Behavior of Hexabromocyclododecanes (HBCDs) on Weihe River Sediment. International Journal of Environmental Research and Public Health. 2020; 17(1):247. https://doi.org/10.3390/ijerph17010247
Chicago/Turabian StyleWang, Xueli, Xiaoyu Yuan, and Shengke Yang. 2020. "Sorption Behavior of Hexabromocyclododecanes (HBCDs) on Weihe River Sediment" International Journal of Environmental Research and Public Health 17, no. 1: 247. https://doi.org/10.3390/ijerph17010247
APA StyleWang, X., Yuan, X., & Yang, S. (2020). Sorption Behavior of Hexabromocyclododecanes (HBCDs) on Weihe River Sediment. International Journal of Environmental Research and Public Health, 17(1), 247. https://doi.org/10.3390/ijerph17010247




