The Effect of Climate Change and the Snail-Schistosome Cycle in Transmission and Bio-Control of Schistosomiasis in Sub-Saharan Africa
Abstract
:1. Introduction
2. Impact of Climate Change on NTDs and Human Health in Sub-Saharan Africa
3. Snail as an Intermediate Host in the Transmission of Schistosomiasis
3.1. Distribution of Bulinus Species in Sub-Saharan Africa
3.2. Distribution of Biomphalaria Species in Sub-Saharan Africa
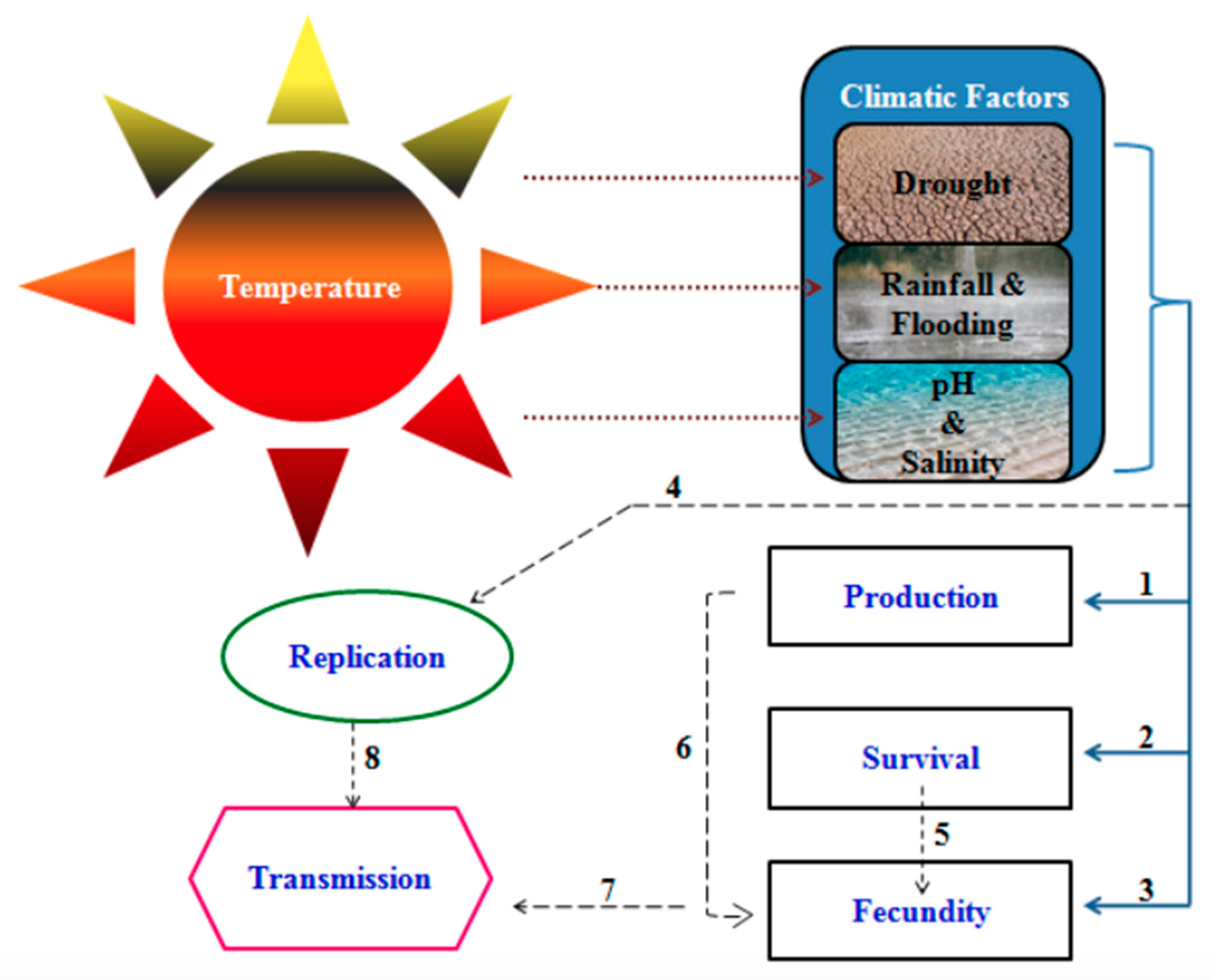
4. Impact of Climate Change on Schistosomiasis Transmission in Sub-Saharan Africa
4.1. Effect of Changes in Temperature on the Intermediate Host in Schistosomiasis Transmission
4.2. Effect of Changes in Rainfall on the Intermediate Host in Schistosomiasis Transmission
4.3. Effect of Flooding on the Intermediate Host in Schistosomiasis Transmission
4.4. Effect of Drought on the Intermediate Host in Schistosomiasis Transmission
4.5. Effect of pH and Conductivity on the Intermediate Host in Schistosomiasis Transmission
4.6. Effect of Salinity on the Intermediate Host in Schistosomiasis Transmission
5. Effects of Climate Change on the Geographic Habitat and Behaviour of Snail Species
6. Role of Mathematical Modelling in Disease Epidemiological Studies
7. Control Strategies for Schistosomiasis
8. Conclusions and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Kumaresan, J.; Sachs, S.E.; Sachs, J.D.; Savioli, L. Control of neglected tropical diseases. N. Eng. J. Med. 2007, 357, 1018–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotez, P.J.; Kamath, A. Neglected tropical diseases in sub-Saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Neg. Trop. Dis. 2009, 3, 412. [Google Scholar] [CrossRef] [PubMed]
- Oyinloye, B.; Adenowo, F.; Gxaba, N.; Kappo, A. The promise of antimicrobial peptides for treatment of human schistosomiasis. Curr. Drug Targets 2014, 15, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Adekiya, T.A.; Kappo, A.P.; Okosun, K.O. Temperature and rainfall impact on schistosomiasis. Glob. J. Pure Appl. Math. 2017, 13, 8453–8469. [Google Scholar]
- Adenowo, A.F.; Oyinloye, B.E.; Ogunyinka, B.I.; Kappo, A.P. Impact of human schistosomiasis in sub-Saharan Africa. Braz. J. Infect. Dis. 2015, 19, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention Report on Parasites-Schistosomiasis. Available online: https://www.cdc.gov/parasites/schistosomiasis/gen_info/faqs.html (accessed on 11 April 2018).
- Bjorneboe, A. A comparison of the characteristics of two strains of Schistosoma intercalatum Fisher, 1934 in mice. J. Helminthol. 1978, 53, 195–203. [Google Scholar] [CrossRef]
- Ohmae, H.; Sinuon, M.; Kirinoki, M.; Matsumoto, J.; Chigusa, Y.; Socheat, D.; Matsuda, H. Schistosomiasis mekongi: From discovery to control. Parasitol. Int. 2004, 53, 135–142. [Google Scholar] [CrossRef]
- Wang, L.D.; Chen, H.G.; Guo, J.G.; Zeng, X.J.; Hong, X.L.; Xiong, J.J.; Wu, X.H.; Wang, X.H.; Wang, L.Y.; Xia, G.; et al. A strategy to control transmission of Schistosoma japonicum in China. N. Eng. J. Med. 2009, 360, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zheng, H.; Chen, X.; Zhang, L.; Wang, K.; Guo, J.; Huang, Z.; Zhang, B.; Huang, W.; Jin, K.; et al. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature 2009, 460, 345. [Google Scholar]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- McCreesh, N.; Arinaitwe, M.; Arineitwe, W.; Tukahebwa, E.; Booth, M. Effect of water temperature and population density on the population dynamics of Schistosoma mansoni intermediate host snails. Parasit. Vectors 2014, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- McCreesh, N.; Nikulin, G.; Booth, M. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit. Vectors 2015, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowel, C.; Fred, B.; Betson, M.; Sousa-Figueiredo, J.C.; Kabatereine, N.B.; Stothard, J.R. Environmental epidemiology of intestinal schistosomiasis in Uganda: Population dynamics of Biomphalaria (Gastropoda: Planorbidae) in Lake Albert and Lake Victoria with observations on natural infections with digenetic trematodes. BioMed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senghor, B.; Diaw, O.T.; Doucoure, S.; Seye, M.; Talla, I.; Diallo, A.; Bâ, C.T.; Sokhna, C. Study of the snail intermediate hosts of urogenital schistosomiasis in Niakhar, region of Fatick, West central Senegal. Parasit. Vectors 2015, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Longxing, Q.; Cui, J.; Huang, T.; Ye, F.; Jiang, L. Mathematical Model of Schistosomiasis under Flood in Anhui Province. Abstr. Appl. Anal. 2014, 7. [Google Scholar] [CrossRef]
- Xue, Z.; Gebremichael, M.; Ahmad, R.; Weldu, M.L.; Bagtzoglou, A.C. Impact of temperature and precipitation on propagation of intestinal schistosomiasis in an irrigated region in Ethiopia: Suitability of satellite datasets. Trop. Med. Int. Health 2011, 16, 1104–1111. [Google Scholar] [CrossRef]
- Ge, J.H.; Zhang, S.Q.; Wang, T.P.; Zhang, G.; Tao, C.; Lu, D.; Wang, Q.; Wu, W. Effects of flood on the prevalence of schistosomiasis in Anhui province in 1998. J. Trop. Dis. Parasitol. 2004, 2, 131–134. [Google Scholar]
- Mangal, T.D.; Paterson, S.; Fenton, A. Predicting the impact of long-term temperature changes on the epidemiology and control of schistosomiasis: A mechanistic model. PLoS ONE 2008, 3, e1438. [Google Scholar] [CrossRef] [Green Version]
- United Nations. Economic Commission for Africa. In Climate Change and Health in Africa Issues and Options; ClimDev Africa (Policy brief); © UN. ECA: Addis Ababa, Ethiopia, 2013; No. 12; p. 3. [Google Scholar]
- McMichael, A.J. Environmental and social influences on emerging infectious diseases: Past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1049–1058. [Google Scholar] [CrossRef] [Green Version]
- McMichael, A.J.; Woodruff, R.E.; Hales, S. Climate change and human health: Present and future risks. Lancet 2006, 367, 859–869. [Google Scholar] [CrossRef]
- McMichael, A.J.; Woodruff, R.E. Climate change and human health. In Encyclopedia of World Climatology; Springer: Haarlem, The Netherlands, 2005; pp. 209–213. [Google Scholar]
- Luque Fernández, M.Á.; Bauernfeind, A.; Jiménez, J.D.; Gil, C.L.; Omeiri, N.E.; Guibert, D.H. Influence of temperature and rainfall on the evolution of cholera epidemics in Lusaka, Zambia, 2003–2006: Analysis of a time series. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipp, E.K.; Huq, A.; Colwell, R.R. Effects of global climate on infectious disease: The cholera model. Clin. Microbiol. Rev. 2002, 15, 757–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockburn, T.A.; Cassanos, J.G. Epidemiology of endemic cholera. Public Health Rep. 1960, 75, 791–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendelsohn, J.; Dawson, T. Climate and cholera in KwaZulu-Natal, South Africa: The role of environmental factors and implications for epidemic preparedness. Int. J. Hyg. Environ. Healt. 2008, 211, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Trærup, S.L.; Ortiz, R.A.; Markandya, A. The costs of climate change: A study of cholera in Tanzania. Int. J. Environ. Res. Public Health 2011, 8, 4386–4405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parham, P.E.; Michael, E. Modeling the effects of weather and climate change on malaria transmission. Environ. Health Perspect. 2010, 118, 620. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, N.; Sonye, G.; Mogi, M.; Githeko, A.Y.G. The effects of climatic factors on the distribution and abundance of malaria vectors in Kenya. J. Med. Entomol. 2002, 39, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngarakana-Gwasira, E.T.; Bhunu, C.P.; Masocha, M.; Mashonjowa, E. Transmission dynamics of schistosomiasis in Zimbabwe: A mathematical and GIS Approach. Commun. Nonlinear Sci. Numer. Simul. 2016, 35, 137–147. [Google Scholar] [CrossRef]
- Abiodun, G.J.; Maharaj, R.; Witbooi, P.; Okosun, K.O. Modelling the influence of temperature and rainfall on the population dynamics of Anopheles arabiensis. Malar. J. 2016, 15, 364. [Google Scholar] [CrossRef] [Green Version]
- Abdussalam, A.F.; Monaghan, A.J.; Steinhoff, D.F.; Dukic, V.M.; Hayden, M.H.; Hopson, T.M.; Thornes, J.E.; Leckebusch, G.C. The impact of climate change on meningitis in Northwest Nigeria: An assessment using CMIP5 climate model simulations. Weather Clim. Soc. 2014, 6, 371–379. [Google Scholar] [CrossRef]
- Codjoe, S.N.A.; Nabie, V.A. Climate change and cerebrospinal meningitis in the Ghanaian meningitis belt. Int. J. Environ. Res. Public Health. 2014, 11, 6923–6939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, K.A.; Blackburn, J.K. Overcoming barriers in evaluating outbreaks of diarrheal disease in resource poor settings: Assessment of recurrent outbreaks in Chobe District, Botswana. BMC Public Health 2013, 13, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, E.G. Control of Schistosoma japonicum snail intermediate host in riceland agroecosystems. In Vector-Borne Disease Control in Humans through Rice Agroecosystem Management; International Rice Research Institute: Los Baños, Laguna, Philippines, 1987; p. 203. [Google Scholar]
- Davis, G.M. Evolution of prosobranch snails transmitting Asian Schistosoma; coevolution with Schistosoma: A review. In Progress in Clinical Parasitology; Sun, T., Ed.; Springer: New York, NY, USA, 1993; pp. 145–204. [Google Scholar] [CrossRef]
- Ohmae, H.; Iwanaga, Y.; Nara, T.; Matsuda, H.; Yasuraoka, K. Biological characteristics and control of intermediate snail host of Schistosoma japonicum. Parasitol. Int. 2003, 52, 409–417. [Google Scholar] [CrossRef]
- Davis, G.M.; Carney, W.P. Description of Oncomelania hupensis lindoensis, first intermediate host of Schistosoma japonicum in Sulawesi (Celebes). In Proceedings of the Academy of Natural Sciences of Philadelphia; Academy of Natural Sciences: Philadelphia, PA, USA, 1973; Volume 125, pp. 1–34. [Google Scholar]
- Stunkard, H.W. Possible snail hosts of human schistosomes in the United States. J. Parasitol. 1946, 32, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Stunkard, H.W.; Shaw, C.R. The effect of dilution of sea water on the activity and longevity of certain marine cercariae, with descriptions of two new species. Biol. Bull. Woods Hole 1931, 61, 242–271. [Google Scholar] [CrossRef]
- Degarege, A.; Mekonnen, Z.; Levecke, B.; Legesse, M.; Negash, Y.; Vercruysse, J.; Erko, B. Prevalence of Schistosoma haematobium Infection among School-Age Children in Afar Area, Northeastern Ethiopia. PLoS ONE 2015, 10, e0133142. [Google Scholar] [CrossRef] [Green Version]
- Picquet, M.; Ernould, J.C.; Vercruysse, J.; Southgate, V.R.; Mbaye, A.; Sambou, B.; Niang, M.; Rollinson, D. The epidemiology of human schistosomiasis in the Senegal river basin. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 340–346. [Google Scholar] [CrossRef]
- Southgate, V.R. Schistosomiasis in the Senegal River Basin: Before and after the construction of the dams at Diama, Senegal and Manantali, Mali and future prospects. J. Helminthol. 1997, 71, 125–132. [Google Scholar] [CrossRef]
- Ngassam, R.K.; Kouninki, H.; Monglo, B.; Djekin, E.; Liang, S.; Tchuente, L.T. Identification and mapping of some potential transmission foci of schistosomasis in Maroua, Far North Region, Cameroon. Int. J. Innov. Appl. Stud. 2014, 7, 65. [Google Scholar]
- De Kock, K.N.; Wolmarans, C.T. Distribution and habitats of the Bulinus africanus species group, snail intermediate hosts of Schistosoma haematobium and S. mattheei in South Africa. Water SA 2005, 31, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Zein-Eddine, R.; Djuikwo-Teukeng, F.F.; Al-Jawhari, M.; Senghor, B.; Huyse, T.; Dreyfuss, G. Phylogeny of seven Bulinus species originating from endemic areas in three African countries, in relation to the human blood fluke Schistosoma haematobium. BMC Evol. Biol. 2014, 14, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majoros, G.; Fehér, Z.; Deli, T.; Földvári, G. Establishment of Biomphalaria tenagophila snails in Europe. Emerg. Infect. Dis. 2008, 14, 1812. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.S.; Costa, D.P.; Arruda, F. Competitive interactions between species of fresh-water snails: I. Laboratory. IC. Comparative survival of Biomphalaria glabrata and B. Straminea kept out of water. Memorias do Instituto Oswaldo Cruz 1985, 80, 155–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodruff, D.S.; Mulvey, M. Neotropical schistosomiasis: African affinities of the host snail Biomphalaria glabrata (Gastropoda: Planorbidae). Biol. J. Linnean Soc. 1997, 60, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Campbell, G.; Jones, C.S.; Lockyer, A.E.; Hughes, S.; Brown, D.; Noble, L.R.; Rollinson, D. Molecular evidence supports an African affinity of the Neotropical freshwater gastropod, Biomphalaria glabrata, Say 1818, an intermediate host for Schistosoma mansoni. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 2351–2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousif, F.; Ibrahim, A.; El Bardicy, S.N. Compatibility of Biomphalaria alexandrina, Biomphalaria glabrata and a hybrid of both to seven strains of Schistosoma mansoni from Egypt. J. Egyp. Soc. Parasitol. 1998, 28, 863–881. [Google Scholar]
- Williams, S.N.; Hunter, P.J. The distribution of Bulinus and Biomphalaria in Khartoum and Blue Nile Provinces, Sudan. Bull. World Health Organ. 1968, 39, 949. [Google Scholar]
- Hostettmann, K. On the use of plants and plant-derived compounds for the control of schistosomiasis. Naturwissenschaften 1984, 71, 247–251. [Google Scholar] [CrossRef]
- Mohamed, A.H.; El-Din, A.T.S.; Mohamed, A.M.; Habib, M.R. The relationship between genetic variability and the susceptibility of Biomphalaria alexandrina snails to Schistosoma mansoni infection. Memorias do Instituto Oswaldo Cruz 2012, 107, 326–337. [Google Scholar] [CrossRef] [Green Version]
- Mutuku, M.W.; Dweni, C.K.; Mwangi, M.; Kinuthia, J.M.; Mwangi, I.N.; Maina, G.M.; Agola, L.E. Field-derived Schistosoma mansoni and Biomphalaria pfeifferi in Kenya: A compatible association characterized by lack of strong local adaptation, and presence of some snails able to persistently produce cercariae for over a year. Parasites Vectors 2014, 7, 533. [Google Scholar] [CrossRef]
- Stensgaard, A.S.; Utzinger, J.; Vounatsou, P.; Hürlimann, E.; Schur, N.; Saarnak, C.F.; Simoonga, C. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: Does climate matter? Acta Trop. 2013, 128, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Monde, C.; Syampungani, S.; van den Brink, P.J. Natural and human induced factors influencing the abundance of Schistosoma host snails in Zambia. Environ. Monit. Asst. 2016, 188, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kock, K.N.; Van Eeden, J.A. Life table studies on freshwater snails. The effect of constant temperature on the population dynamics of Biomphalaria pfeifferi (Krauss). Wetenskaplike Bydraes van die PU vir CHO Reeks B. Natuurwetenskappe 1981, 107, 1–17. [Google Scholar]
- Poulin, R. Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 2006, 132, 143–151. [Google Scholar] [CrossRef]
- McCreesh, N.; Booth, M. Challenges in predicting the effects of climate change on Schistosoma mansoni and Schistosoma haematobium transmission potential. Trends Parasitol. 2013, 29, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Joubert, P.H.; Pretorius, S.J.; DeKock, K.N.; Vaneeden, J.A. Survival of Bulinus-Africanus (Krauss), Bulinus-globosus (Morelet) and Biomphalaria-pfeifferi (Krauss) at constant high-temperatures. S. Afr. J. Zool. 1986, 21, 8588. [Google Scholar]
- McCreesh, N.; Booth, M. The effect of simulating different intermediate host snail species on the link between water temperature and schistosomiasis risk. PLoS ONE 2014, 9, e87892. [Google Scholar] [CrossRef] [Green Version]
- Falade, M.O.; Otarigho, B. Survival potential, fecundity and fertility of Biomphalaria pfeifferi (Krauss, 1848) during acclimatization in the laboratory. Zool. Ecol. 2013, 23, 157–161. [Google Scholar] [CrossRef]
- Kubiriza, G.K.; Madsen, H.; Likongwe, J.S.; Stauffer, J.R., Jr.; Kang’Ombe, J.; Kapute, F. Effect of temperature on growth, survival and reproduction of Bulinus nyassanus (Smith, 1877) (Mollusca: Gastropoda) from Lake Malawi. Afr. Zool. 2010, 45, 315–320. [Google Scholar] [CrossRef]
- O’Keefe, J.H. Population biology of the freshwater snail Bulinus globosus on the Kenya coast: I. Population fluctuations in relation to climate. J. Appl. Ecol. 1985, 22, 73–84. [Google Scholar]
- Woolhouse, M.E.J.; Chandiwana, S.K. Population dynamics model for Bulinus globosus, intermediate host for Schistosoma haematobium, in river habitats. Acta Trop. 1990, 47, 151–160. [Google Scholar] [CrossRef]
- Appleton, C.C.; Eriksson, I.M. The influence of fluctuating above-optimal temperature regimes on the fecundity of Biomphalaria pfeifferi (Mollusca: Planorbidae). Trans. R. Soc. Tropi. Med. Hyg. 1984, 78, 49–54. [Google Scholar] [CrossRef]
- Codjoe, S.N.A.; Larbi, R.T. Climate change/variability and schistosomiasis transmission in Ga district, Ghana. Clim. Dev. 2016, 8, 58–71. [Google Scholar] [CrossRef]
- Martens, W.J.M.; Jetten, T.H.; Rotmans, J.; Niessen, L.W. Climate change and vector-borne diseases: A global modelling perspective. Glob. Environ. Chang. 1995, 5, 195–209. [Google Scholar] [CrossRef]
- Ernould, J.C.; Ba, K.; Sellin, B. The impact of the local water-development programme on the abundance of the intermediate hosts of schistosomiasis in three villages of the Senegal River delta. Ann. Trop. Med. Parasitol. 1999, 93, 135–145. [Google Scholar]
- Diaz, J.H. The influence of global warming on natural disasters and their public health outcomes. Am. J. Disaster Med. 2007, 2, 33–42. [Google Scholar] [CrossRef]
- Cissé, G.; Koné, B.; Bâ, H.; Mbaye, I.; Koba, K.; Utzinger, J.; Tanner, M. Ecohealth and climate change: Adaptation to flooding events in riverside secondary cities, West Africa. In Resilient Cities; Springer: Dordrecht, The Netherlands, 2011; pp. 55–67. [Google Scholar]
- Wu, X.H.; Zhang, S.Q.; Xu, X.J.; Huang, Y.X.; Steinmann, P.; Utzinger, J.; Wang, T.-P.; Xu, J.; Zheng, J.; Zhou, X.-N. Effect of floods on the transmission of schistosomiasis in the Yangtze River valley, People’s Republic of China. Parasitol. Int. 2008, 57, 271–276. [Google Scholar] [CrossRef]
- Epstein, P.R.; Sharp, D. Health and Climate Change; Yale School Of Forestry and Environmental Studies Publications Series: New Haven, CT, USA, 1993. [Google Scholar]
- Stanke, C.; Kerac, M.; Prudhomme, C.; Medlock, J.; Murray, V. Health effects of drought: A systematic review of the evidence. PLoS Curr. 2013, 5. [Google Scholar] [CrossRef] [Green Version]
- Jokinen, E.H. The aestivation pattern of a population of Lymnaea elodes (Say) (Gastropoda: Lymnaeidae). Am. Midl. Nat. 1978, 100, 43–53. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Prevalence of schistosome infections within molluscan populations: Observed patterns and theoretical predictions. Parasitology 1979, 79, 63–94. [Google Scholar] [CrossRef]
- Zein, A.Z. Spontaneous reduction in Schistosoma mansoni infection in endemic communities of the lake Tana basin, northwestern Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 656–658. [Google Scholar] [CrossRef]
- Pugh, R.N.H.; Gilles, H.M. Malumfashi endemic diseases project. III. Urinary schistosomiasis: A longitudinal study. Ann. Trop. Med. Parasitol. 1978, 72, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Chandiwana, S.K.; Makaza, D.; Taputaira, A. Variations in the incidence of schistosomiasis in the highveld regions of Zimbabwe. Trop. Med. Parasitol. 1987, 39, 313–319. [Google Scholar]
- Webbe, G. The transmission of Schistosoma haematobium in an area of Lake Province, Tanganyika. Bull. World Health Organ. 1962, 27, 59–85. [Google Scholar] [PubMed]
- Caldeira, K.; Wickett, M.E. Oceanography: Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W. The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Can. J. Fish Aquat. Sci. 2001, 58, 18–29. [Google Scholar] [CrossRef]
- Savitz, J.; Harrould-Kolieb, E. The oceans’ acid test: Can our reefs be saved? Front. Ecol. Environ. 2008, 6, 515. [Google Scholar] [CrossRef]
- Lannig, G.; Eilers, S.; Pörtner, H.O.; Sokolova, I.M.; Bock, C. Impact of ocean acidification on energy metabolism of oyster, Crassostrea gigas—Changes in metabolic pathways and thermal response. Mar. Drugs 2010, 8, 2318–2339. [Google Scholar] [CrossRef] [Green Version]
- Koprivnikar, J.; Lim, D.; Fu, C.; Brack, S.H.M. Effects of temperature, salinity, and pH on the survival and activity of marine cercariae. Parasitol. Res. 2010, 106, 1167–1177. [Google Scholar] [CrossRef]
- Marie, M.A.; El-Deeb, F.A.; Hasheesh, W.S.; Mohamed, R.A.; Sayed, S.S. Impact of Seasonal Water Quality and Trophic Levels on the Distribution of Various Freshwater Snails in Four Egyptian Governorates. Appl. Ecol. Environ. Sci. 2015, 3, 117–126. [Google Scholar]
- Ntonifor, H.N.; Ajayi, J.A. Studies on the ecology and distribution of some medically important freshwater snail species in Bauchi State, Nigeria. Int. J. Biol. Chem. Sci. 2007, 1, 121–127. [Google Scholar] [CrossRef]
- Kazibwe, F.; Makanga, B.; Rubaire-Akiiki, C.; Ouma, J.; Kariuki, C.; Kabatereine, N.B.; Booth, M.; Vennervald, B.J.; Sturrock, R.F.; Stothard, J.R. Ecology of Biomphalaria (Gastropoda: Planorbidae) in Lake Albert, Western Uganda: Snail distribution, infection with schistosomes and temporal associations with environmental dynamics. Hydrobiology 2006, 568, 433–444. [Google Scholar] [CrossRef]
- Mahmoud, K.A. The Feeding Ecology of the Snail Intermediate Hosts of Schistosomiasis in Egypt. Ph.D. Thesis, Faculty of Science, Cairo University, Cairo, Egypt, 1994. [Google Scholar]
- El- Khayat, H.M.M.; Mostafa, B.B.; Mahmoud, K.M.A.; El-Said, K.M.; Ismail, N.M.M. The association between freshwater snails, macrophytes and water quality in different water courses in Egypt. New Egy. J. Med. 2009, 40, 381–392. [Google Scholar]
- Ramasamy, R.; Surendran, S.N. Possible impact of rising sea levels on vector-borne infectious diseases. BMC Infect. Dis. 2011, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Mouritsen, K.N. The Hydrobia ulvae–Maritrema subdolum association: Influence of temperature, salinity, light, water-pressure and secondary host exudates on cercarial emergence and longevity. J. Helminthol. 2002, 76, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Widmann, M. Impact of Large-Scale Environmental Features Changes on Host-Parasite Interaction in Marine and Freshwater Ecosystems. Biosci. Master Rev. 2013, 1–9. Available online: http://biologie.ens-lyon.fr/ressources/bibliographies/pdf/m1-11-12-biosci-reviews-widmann-m-2c-m.pdf?lang=fr (accessed on 25 December 2019).
- Koprivnikar, J.; Poulin, R. Interspecific and intraspecific variation in cercariae release. J. Parasitol. 2009, 95, 14–19. [Google Scholar] [CrossRef]
- Lei, F.; Poulin, R. Effects of salinity on multiplication and transmission of an intertidal trematode parasite. Mar. Biol. 2011, 158, 995–1003. [Google Scholar] [CrossRef]
- Thompson, J.H. Host-parasite relationships of Schlstosoma mansoni. Exp. Parasitol. 1954, 3, 140–160. [Google Scholar] [CrossRef]
- Paraense, W.L. The schistosome vectors in the Americas. Memórias do Instituto Oswaldo Cruz 2010, 96, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Muirhead-Thompson, R.C. The ecology of vector snail habitats and mosquito breeding-places. The experimental approach to basic problems. Bull. World Health Organ. 1958, 19, 637–659. [Google Scholar]
- Mostafa, O.M. Effect of salinity and drought on the survival of Biomphalaria arabica, the intermediate host of Schistosoma mansoni in Saudi Arabia. Egy. Acad. J. Biol. Sci. B Zool. 2009, 1, 1–6. [Google Scholar] [CrossRef]
- Neto, L.; Batista, O.; Gomes, E.C.; Junior, O.; Andrade, R.; Reis, D.L.; Souza-Santos, R.; Bocanegra, S.; Barbosa, C.S. Biological and environmental factors associated with risk of schistosomiasis mansoni transmission in Porto de Galinhas, Pernambuco State. Brazil. Cadernos de Saude Publica 2013, 29, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quayle, L.M.; Appleton, C.C.; Dickens, C.W.S. The Impact of River Flow Regulation and Manipulation on the Invertebrate Hosts of Malaria, Bilharzia and Liver Fluke Disease; Water Research Commission: Pretoria, South Africa, 2010. [Google Scholar]
- Shiff, C.J. The influence of temperature on the vertical movement of Bulinus (Physopsis) globosus in the laboratory and in the field. S. Afr. J. Sci. 1966, 62, 210. [Google Scholar]
- De Kock, K.N.; Wolmarans, C.T.; Du Preez, L.H. Freshwater mollusc diversity in the Kruger National Park: A comparison between a period of prolonged drought and a period of exceptionally high rainfall. Koedoe 2002, 45, 1–11. [Google Scholar] [CrossRef]
- Brown, D.S. Freshwater Snails of Africa and Their Medical Importance; CRC Press: Boca Raton, FL, USA; Taylor and Francis: London, UK, 1994. [Google Scholar] [CrossRef]
- Appleton, C.C.; Stiles, G. Geology and geomorphology in relation to the distribution of snail intermediate hosts of bilharzia in South Africa. Ann. Trop. Med. Parasitol. 1976, 70, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Brown, D. Freshwater molluscs. In Biogeography and Ecology of Southern Africa; Werger, M.J.A., Ed.; Junk: The Haque, The Netherlands, 1978; pp. 1153–1180. [Google Scholar]
- Pretorius, S.J.; van Eeden, J.A.; de Kock, K.N.; Joubert, P.H. Mark-recapture studies on Bulinus (Physopsis) africanus (Krauss) (Mollusca, Pulmonata). Malacologia 1982, 22, 93–102. [Google Scholar]
- Donnelly, F.A.; Appleton, C.C.; Schutte, C.H.J. The influence of salinity on the cercariae of three species of Schistosoma. Int. J. Parasitol. 1984, 14, 13–21. [Google Scholar] [CrossRef]
- Jennings, A.C. Effect of the Total Dissolved Salts in Water on the Biology of the Freshwater Snail Biomphalaria Pfeifferi. Potchefstroomse Universiteit vir CHO. 1973. Available online: http://agris.fao.org/agris-search/search.do?recordID=US201300625806 (accessed on 25 December 2019).
- Heeg, J. A note on the effects of drastic changes in total dissolved solids on the aquatic pulmonate snail Bulinus (Physopsis) africanus Krauss. J. Limnol. Soc. S. Afr. 1975, 1, 29–32. [Google Scholar]
- Donnelly, F.A.; Appleton, C.C.; Schutte, C.H.J. Thobae influence of salinity on certain aspects of the biology of Bulinus (Physopsis) africanus. Int. J. Parasitol. 1983, 13, 539–545. [Google Scholar] [CrossRef]
- Appleton, C.C.; Ellery, W.N.; Byskov, J.; Mogkweetsinyana, S.S. Epidemic transmission of intestinal schistosomiasis in the seasonal part of the Okavango Delta, Botswana. Ann. Trop. Med. Parasitol. 2008, 102, 611–623. [Google Scholar] [CrossRef]
- Willems, J.C.; Polderman, J.W. Introduction to Mathematical Systems Theory: A Behavioral Approach; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2013; Volume 26. [Google Scholar]
- Wolkenhauer, O.; Auffray, C.; Jaster, R.; Steinhoff, G.; Dammann, O. The road from systems biology to systems medicine. Pediatric Res. 2013, 73, 502–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, S.; Baxter, N.T.; Huffnagle, G.B.; Petrosino, J.F.; Schloss, P.D. Mathe- matical modeling of primary succession of murine intestinal microbiota. Proc. Natl. Acad. Sci. USA 2014, 111, 439–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacciabue, P.C. Modelling and Simulation of Human Behaviour in System Control; Springer Science and Business Media: London, UK, 2013. [Google Scholar]
- Hughes, B.P.; Newstead, S.; Anund, A.; Shu, C.C.; Falkmer, T. A review of models relevant to road safety. Accid. Anal. Prev. 2015, 74, 250–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, R.M. Uses and abuses of mathematics in biology. Science 2004, 303, 790–793. [Google Scholar] [CrossRef] [Green Version]
- Funk, S.; Salathé, M.; Jansen, V.A. Modelling the influence of human behaviour on the spread of infectious diseases: A review. J. R. Soc. Interface 2010, 7, 1247–1256. [Google Scholar] [CrossRef]
- Huppert, A.; Katriel, G. Mathematical modelling and prediction in infectious disease epidemiology. Clin. Microbiol. Infect. 2013, 19, 999–1005. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.M.; Sleigh, A.C.; Li, Y.; Feng, Z.; Davis, G.M.; Chen, H.; Ross, A.G.; Bergquist, R.; McManus, D.P. Mathematical modelling of schistosomiasis japonica: Comparison of control strategies in the People’s Republic of China. Acta Trop. 2002, 82, 253–262. [Google Scholar] [CrossRef]
- Da’Dara, A.A.; Li, Y.S.; Xiong, T.; Zhou, J.; Williams, G.M.; McManus, D.P.; Feng, Z.; Xin, L.Y.; Gray, D.J.; Harn, D.A. DNA-based vaccines protect against zoonotic schistosomiasis in water buffalo. Vaccine 2008, 26, 3617–3625. [Google Scholar] [CrossRef] [Green Version]
- Gray, D.J.; Li, Y.S.; Williams, G.M.; Zhao, Z.Y.; Harn, D.A.; Li, S.M.; Ren, M.Y.; Feng, Z.; Guo, F.Y.; Guo, J.G.; et al. A multi-component integrated approach for the elimination of schistosomiasis in the People’s Republic of China: Design and baseline results of a 4-year cluster-randomised intervention trial. Int. J. Parasitol. 2014, 44, 659–668. [Google Scholar] [CrossRef]
- Li, E.Y.; Gurarie, D.; Lo, N.C.; Zhu, X.; King, C.H. Improving public health control of schistosomiasis with a modified WHO strategy: A model-based comparison study. Lancet Glob. Health 2019, 7, e1414–e1422. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.J.; Bergquist, R. Potential impact of climate change on schistosomiasis: A global assessment attempt. Trop. Med. Inf. Dis. 2018, 3, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, U.B.; Stendel, M.; Midzi, N.; Mduluza, T.; Soko, W.; Stensgaard, A.S.; Vennervald, B.J.; Mukaratirwa, S.; Kristensen, T.K. Modelling climate change impact on the spatial distribution of fresh water snails hosting trematodes in Zimbabwe. Parasit. Vectors 2014, 7, 536. [Google Scholar] [CrossRef] [PubMed]
- Kalinda, C.; Chimbari, M.J.; Grant, W.E.; Wang, H.H.; Odhiambo, J.N.; Mukaratirwa, S. Simulation of population dynamics of Bulinus globosus: Effects of environmental temperature on production of Schistosoma haematobium cercariae. PLoS Negl. Trop. Dis. 2018, 12, e0006651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marti, H. Field observations on the population dynamics of Bulinusglobosus, the intermediate host of Schistosoma haematobium in the Ifakara area, Tanzania. J. Parasitol. 1986, 72, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Utzinger, J.; Sun, L.P.; Hong, Q.B.; Vounatsou, P.; Tanner, M.; Zhou, X.N. Effect of temperature on the development of Schistosoma japonicum within Oncomelaniahupensis, and hibernation of O. hupensis. Parasitol. Res. 2007, 100, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, J.; Mayombana, C.; Mez, K.; Tanner, M. Evaluation of chemical and physical-morphological factors as potential determinants of Biomphalariapfeifferi (Krauss, 1848) distribution. Memrias do Instituto Oswaldo Cruz 1997, 92, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, M.A. Strategic control of schistosome intermediate host. Asian J. Epidemiol. 2010, 3, 123–140. [Google Scholar] [CrossRef]
- Mantawy, M.M.; Mahmoud, A.H. Effect of Allium cepa and Allium sativum feeding on glucose, glycogen, protein bands profile and phenol oxidase activity in Biomphalariaalexandrina. J. Egypt. Soc. Parasitol. 2002, 32, 271–283. [Google Scholar]
- Mello-Silva, C.C.; Vasconcellos, M.C.D.; Pinheiro, J.; Rodrigues, M.D.L.D.A. Physiological changes in Biomphalaria glabrata Say, 1818 (Pulmonata: Planor-bidae) caused by sub-lethal concentrations of the latex of Euphorbia splendens var. hislopii NEB (Euphorbiaceae). Memrias do Instituto Oswaldo Cruz 2006, 101, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Kloos, H.; Passos, L.K.J.; LoVerde, P.; Oliveira, R.C.; Gazzinelli, A. Dis- tribution and Schistosoma mansoni infection of Biomphalaria glabrata in different habitats in a rural area in the Jequitinhonha Valley, Minas Gerais, Brazil: Envi-ronmental and epidemiological aspects. Memrias do Instituto Oswaldo Cruz 2004, 99, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Coelho, P.M.Z.; Carvalho, O.D.S.; Andrade, Z.D.A.; Martins-Sousa, R.L.; Rosa, F.M.; Barbosa, L.; Pereira, C.A.J.; Caldeira, R.L.; Jannotti-Passos, L.K.; Godard, A.L.B.; et al. Biomphalariatenagophila/Schistosoma mansoni in-teraction: Premises for a new approach to biological control of schistosomiasis. Mem-rias do Instituto Oswaldo Cruz 2004, 99, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.B.; de Jong-Brink, M.; Li, K.W.; Sassen, M.M.; Spijker, S.; van Elk, R.; Buijs, S.; van Minnen, J.; van Kesteren, R.E. Granularin, a novel molluscan opsonin comprising a single vWF type C domain is up-regulated during parasitation. FASEB J. 2004, 18, 845–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da, S.D.; Spada, R.G.M.; Sobral-Hamaguchi, S.S.; Abdel-Hamid, Z.; Zuim, N.R.B.; Zanotti-Magalhes, E.M.; Magalhes, L.A.; Ribeiro-Paes, J.T. Biomphalariatenagophila: Genetic variability within intermediate snail hosts susceptible and resistant to Schistosoma mansoni infection. Parasite 2004, 11, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makinde, O.D.; Okosun, K.O. Impact of chemo-therapy on optimal control of malaria disease with infected immigrants. BioSystems 2011, 104, 32–41. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, Z.; Milner, F. A schistosomiasis model with an age- structure in human hosts and its application to treatment strategies. Math. Biosci. 2007, 205, 83–107. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Spear, R.C.; Seto, E.; Hubbard, A.; Qiu, D. A multi-group model of Schistosoma japonicum transmission dynamics and control: Model calibration and control prediction. Trop. Med. Int. Health 2005, 10, 263–278. [Google Scholar] [CrossRef]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Kew, O.M.; Cáceres, V.M.; Sutter, R.W.; Thompson, K.M. A dynamic model of poliomyelitis outbreaks: Learning from the past to help inform the future. Am. J. Epidemiol. 2005, 162, 358–372. [Google Scholar] [CrossRef]
- Eubank, S.; Guclu, H.; Kumar, V.A.; Marathe, M.V.; Srinivasan, A.; Toroczkai, Z.; Wang, N. Modelling disease outbreaks in realistic urban social networks. Nature 2004, 429, 180–184. [Google Scholar] [CrossRef]
- Duncan, S.R.; Scott, S.; Duncan, C.J. Modelling the different smallpox epidemics in England. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 346, 407–419. [Google Scholar]
- Legrand, J.; Viboud, C.; Boelle, P.Y.; Valleron, A.J.; Flahault, A. Modelling responses to a smallpox epidemic taking into account uncertainty. Epidemiol. Infect. 2004, 132, 19–25. [Google Scholar] [CrossRef]
- White, P.J.; Garnett, G.P. Mathematical modelling of the epidemiology of tuberculosis. In Modelling Parasite Transmission and Control; Michael, E., Spear, R.C., Eds.; Advances in Experimental Medicine and Biology: New York, NY, USA, 2010; Volume 673, pp. 127–140. [Google Scholar]
- Hargrove, J.W.; Ouifki, R.; Kajunguri, D.; Vale, G.A.; Torr, S.J. Modeling the control of trypanosomiasis using trypanocides or insecticide-treated livestock. PLoS Negl. Trop. Dis. 2012, 6, e1615. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Almeida, A.; Mendes, T.; de Oliveira, R.N.; Corrêa, S.D.A.P.; Allegretti, S.M.; Belo, S.; Tomás, A.; Anibal, F.D.F.; Carrilho, E.; Afonso, A. Morphological characteristics of Schistosoma mansoni PZQ-resistant and-susceptible strains are different in presence of praziquantel. Front Microbiol. 2016, 7, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aruleba, R.T.; Adekiya, T.A.; Oyinloye, B.E.; Masamba, P.; Mbatha, L.S.; Pretorius, A.; Kappo, A.P. PZQ therapy: How close are we in the development of effective alternative anti-schistosomal drugs? Infect. Disord. Drug Targets 2018. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adekiya, T.A.; Aruleba, R.T.; Oyinloye, B.E.; Okosun, K.O.; Kappo, A.P. The Effect of Climate Change and the Snail-Schistosome Cycle in Transmission and Bio-Control of Schistosomiasis in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2020, 17, 181. https://doi.org/10.3390/ijerph17010181
Adekiya TA, Aruleba RT, Oyinloye BE, Okosun KO, Kappo AP. The Effect of Climate Change and the Snail-Schistosome Cycle in Transmission and Bio-Control of Schistosomiasis in Sub-Saharan Africa. International Journal of Environmental Research and Public Health. 2020; 17(1):181. https://doi.org/10.3390/ijerph17010181
Chicago/Turabian StyleAdekiya, Tayo Alex, Raphael Taiwo Aruleba, Babatunji Emmanuel Oyinloye, Kazeem Oare Okosun, and Abidemi Paul Kappo. 2020. "The Effect of Climate Change and the Snail-Schistosome Cycle in Transmission and Bio-Control of Schistosomiasis in Sub-Saharan Africa" International Journal of Environmental Research and Public Health 17, no. 1: 181. https://doi.org/10.3390/ijerph17010181
APA StyleAdekiya, T. A., Aruleba, R. T., Oyinloye, B. E., Okosun, K. O., & Kappo, A. P. (2020). The Effect of Climate Change and the Snail-Schistosome Cycle in Transmission and Bio-Control of Schistosomiasis in Sub-Saharan Africa. International Journal of Environmental Research and Public Health, 17(1), 181. https://doi.org/10.3390/ijerph17010181





