Two-Dimensional Titanium Carbides (Ti3C2Tx) Functionalized by Poly(m-phenylenediamine) for Efficient Adsorption and Reduction of Hexavalent Chromium
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of Ti3C2Tx, Ti3C2Tx/PmPD, PmPD
2.3. Characterization
2.4. Batch Experiments
3. Results and Discussion
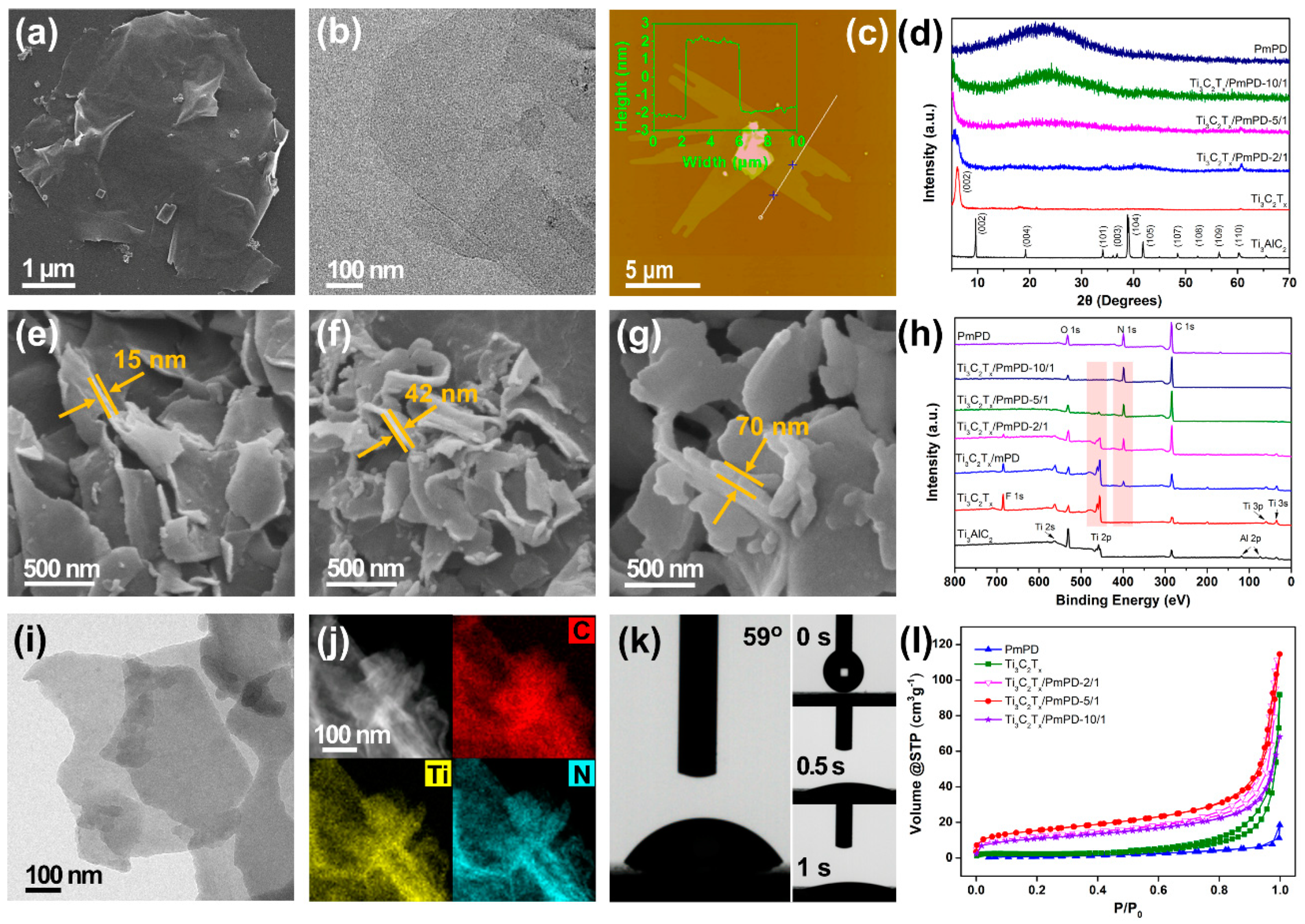
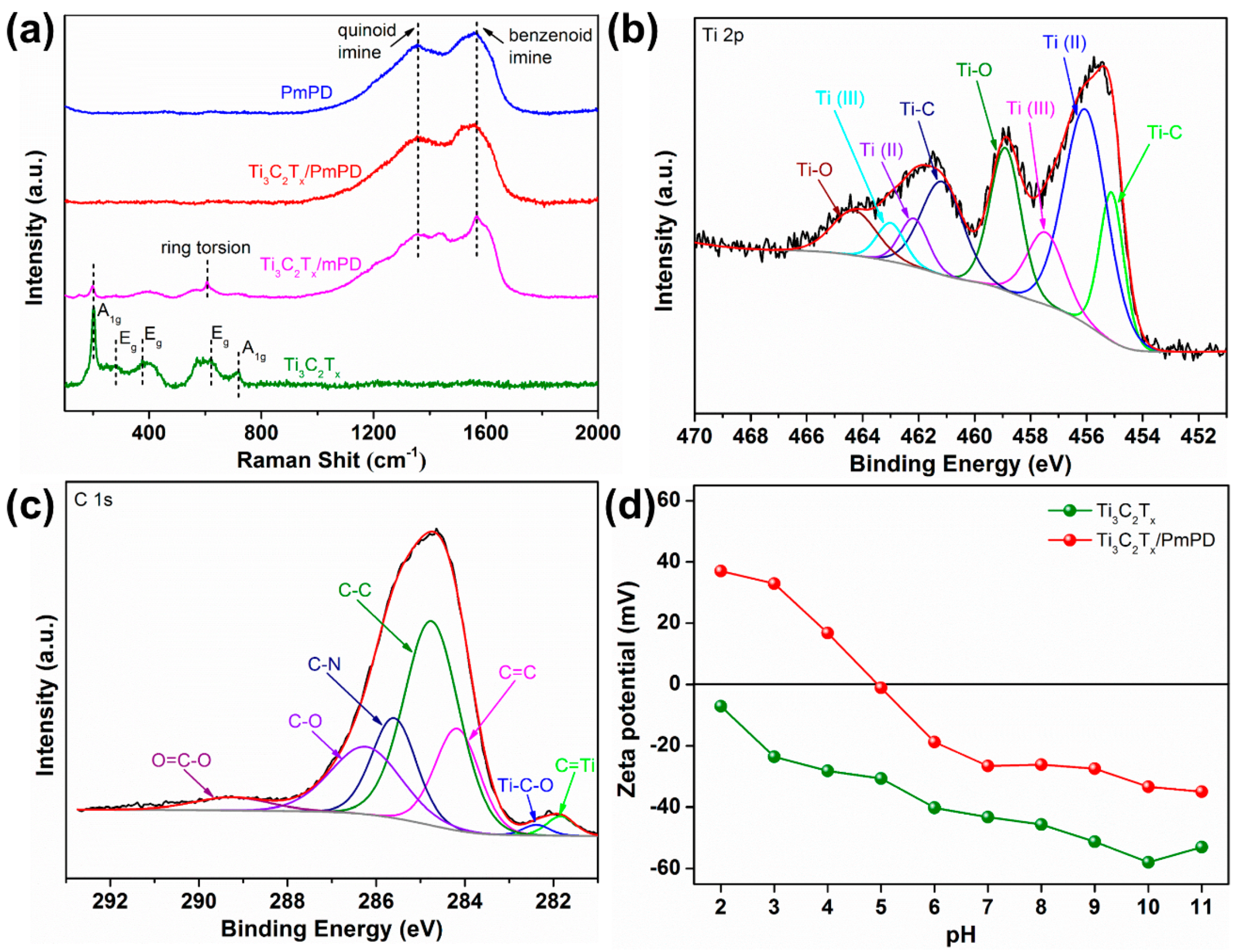
3.1. Material Characterization
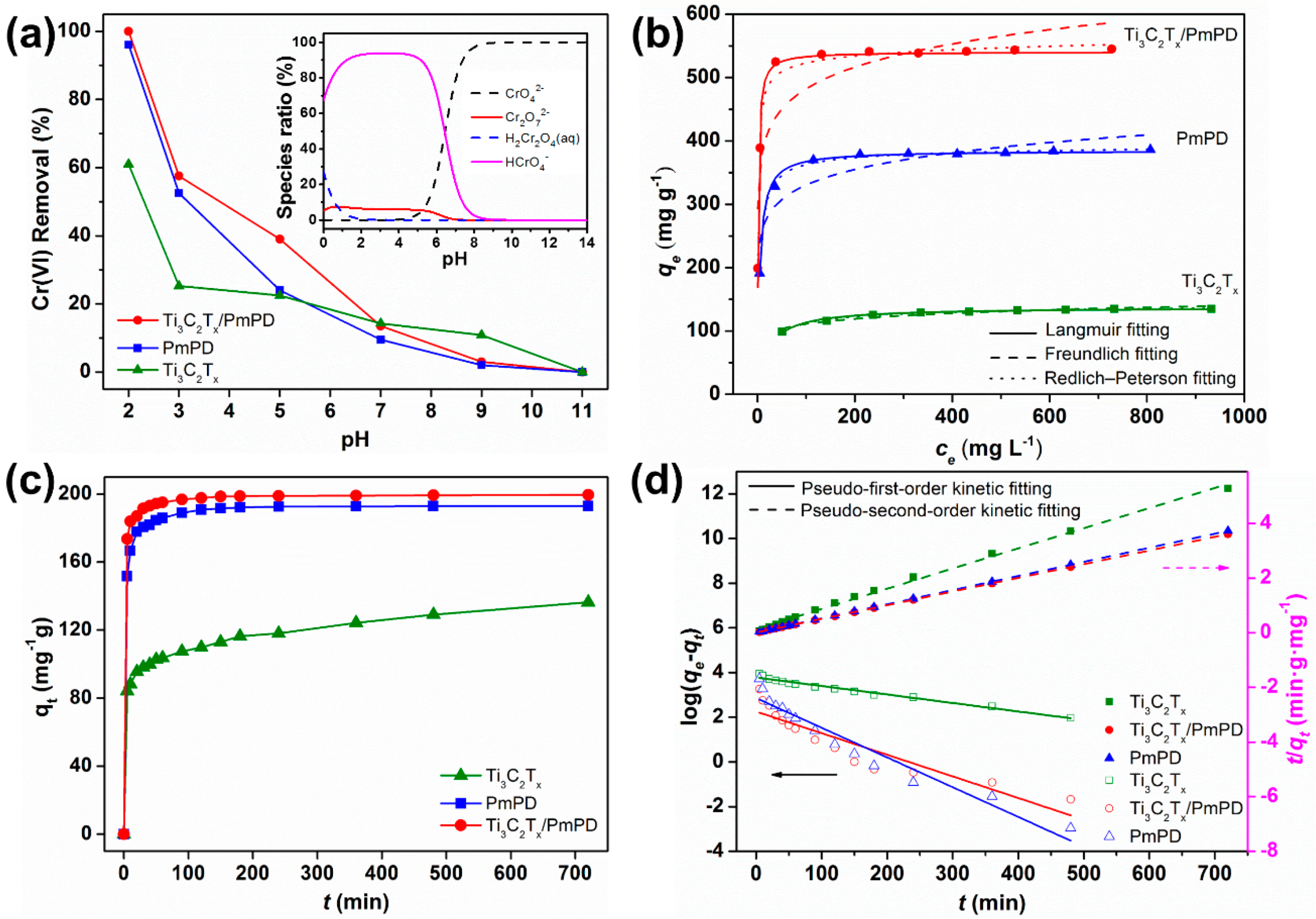
3.2. Adsorption Experiments
3.2.1. Effect of pH
3.2.2. Adsorption Isotherms
3.2.3. Adsorption Kinetics
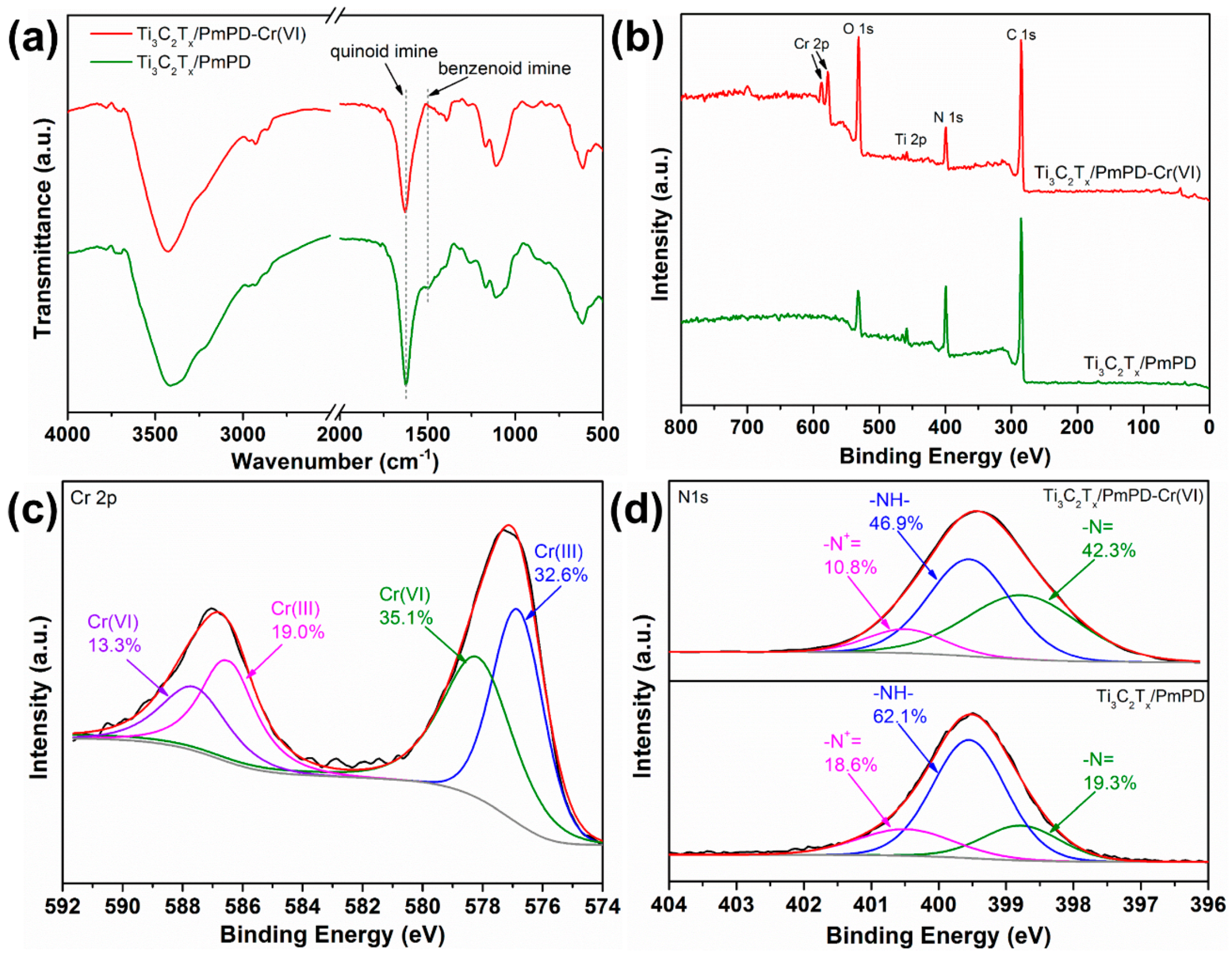
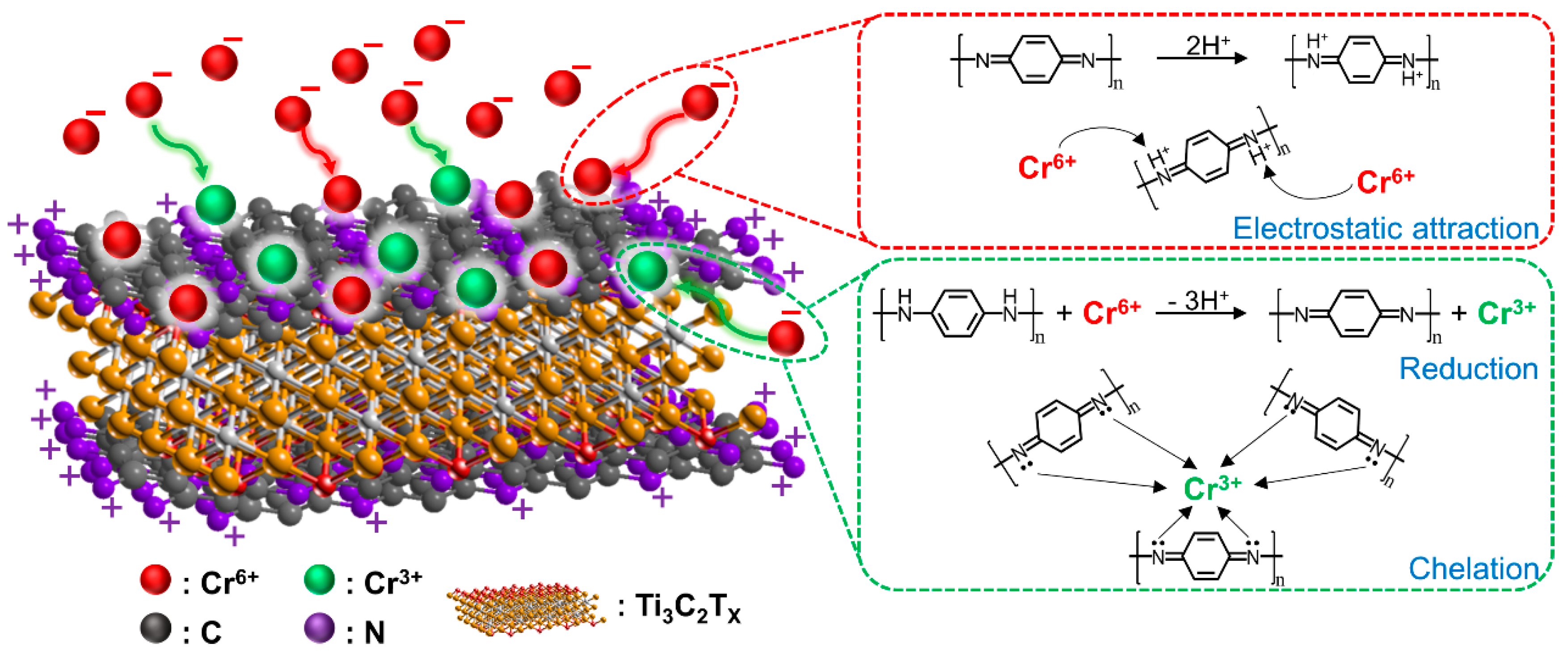
3.2.4. Adsorption Mechanism
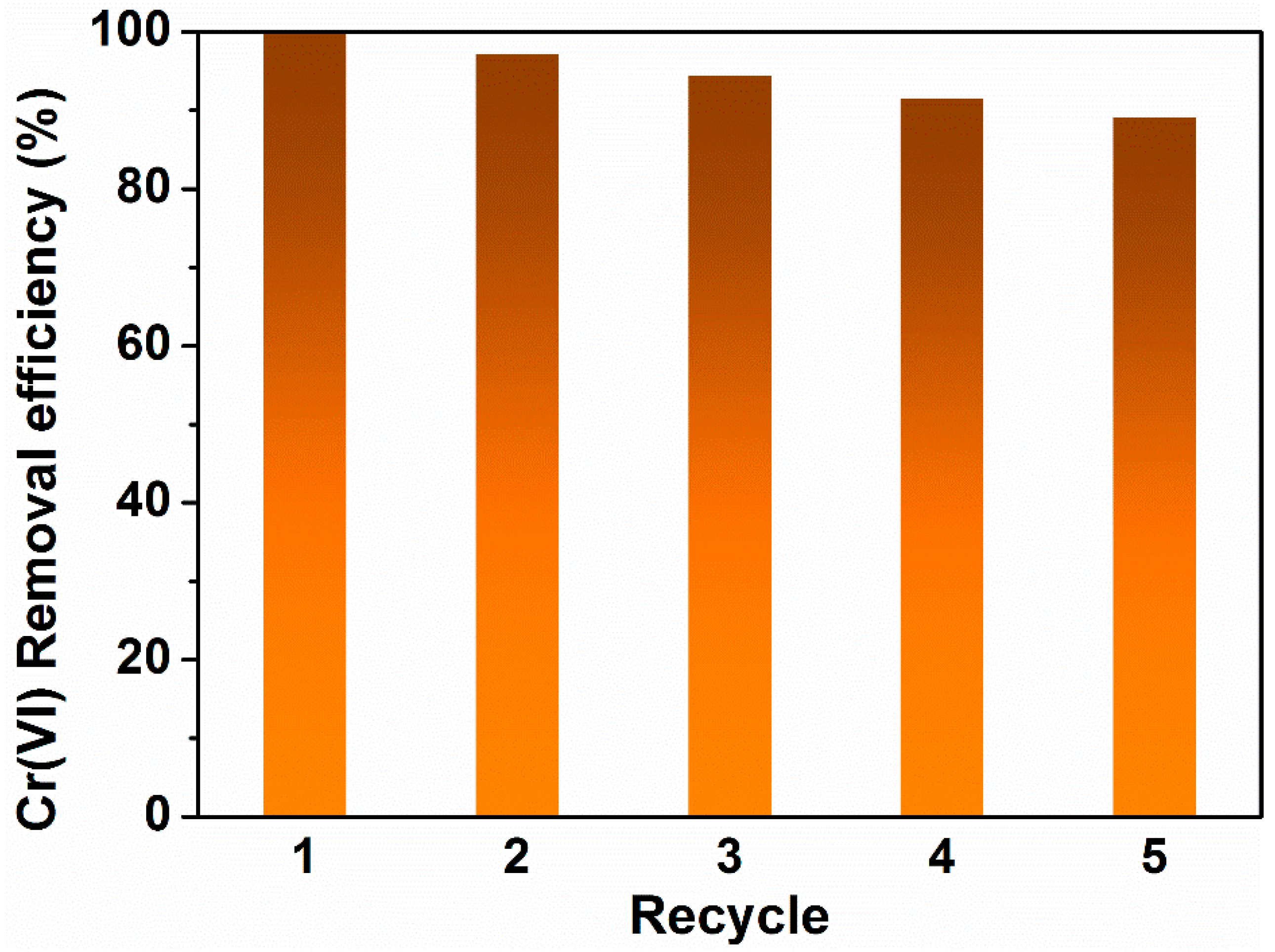
3.2.5. Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lv, D.; Zhou, J.S.; Cao, Z.; Xu, J.; Liu, Y.L.; Li, Y.Z.; Yang, K.L.; Lou, Z.M.; Lou, L.P.; Xu, X.H. Mechanism and influence factors of chromium(VI) removal by sulfide-modified nanoscale zerovalent iron. Chemosphere 2019, 224, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Song, M.M.; Zhou, M.; Ding, C.L.; Wang, Z.R.; Wang, Y.Y.; Yang, W.C.; Yang, Z.H.; Liao, Q.; Shi, Y. Response of Cupriavidus basilensis B-8 to CuO nanoparticles enhances Cr(VI) reduction. Sci. Total Environ. 2019, 688, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Y.; Ding, C.L.; Li, J.W.; Yang, Z.H.; Shi, Y. Multi-omics response of Pannonibacter phragmitetus BB to hexavalent chromium. Environ. Pollut. 2019, 249, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Estokova, A.; Palascakova, L.; Kanuchova, M. Study on Cr(VI) Leaching from Cement and Cement Composites. Int. J. Environ. Res. Public. Health 2018, 15, 824. [Google Scholar] [CrossRef]
- Serrà, A.; Gómez, E.; Philippe, L. Bioinspired ZnO-Based Solar Photocatalysts for the Efficient Decontamination of Persistent Organic Pollutants and Hexavalent Chromium in Wastewater. Catalysts 2019, 9, 974. [Google Scholar] [CrossRef]
- Geng, J.J.; Yin, Y.W.; Liang, Q.W.; Zhu, Z.J.; Luo, H.J. Polyethyleneimine cross-linked graphene oxide for removing hazardous hexavalent chromium: Adsorption performance and mechanism. Chem. Eng. J. 2019, 361, 1497–1510. [Google Scholar] [CrossRef]
- Park, M.; Park, J.; Kang, J.; Han, Y.S.; Jeong, H.Y. Removal of hexavalent chromium using mackinawite (FeS)-coated sand. J. Hazard. Mater. 2018, 360, 17–23. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of Hexavalent Chromium-Contaminated Water and Wastewater: A Review. Water Air Soil Pollut. 2008, 200, 59–77. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, J.J.; Li, R.H.; Park, J.; Meng, Y.; Zhou, B.Y.; Pensky, S.; Zhang, Z.Q. Enhanced sorption of hexavalent chromium [Cr(VI)] from aqueous solutions by diluted sulfuric acid-assisted MgO-coated biochar composite. Chemosphere 2018, 208, 408–416. [Google Scholar] [CrossRef]
- Jiang, D.N.; Huang, D.L.; Lai, C.; Xu, P.; Zeng, G.M.; Wan, J.; Tang, L.; Dong, H.R.; Huang, B.B.; Hu, T.J. Difunctional chitosan-stabilized Fe/Cu bimetallic nanoparticles for removal of hexavalent chromium wastewater. Sci. Total Environ. 2018, 644, 1181–1189. [Google Scholar] [CrossRef]
- El-Mehalmey, W.A.; Ibrahim, A.H.; Abugable, A.A.; Hassan, M.H.; Haikal, R.R.; Karakalos, S.G.; Zakid, O.; Alkordi, M.H. Metal-organic framework@silica as a stationary phase sorbent for rapid and cost-effective removal of hexavalent chromium. J. Mater. Chem. A 2018, 6, 2742–2751. [Google Scholar] [CrossRef]
- Li, L.L.; Feng, X.Q.; Han, R.P.; Zang, S.Q.; Yang, G. Cr(VI) removal via anion exchange on a silver-triazolate MOF. J. Hazard. Mater. 2017, 321, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.B.; Liu, S.N.; Zhang, W.Y.; Chen, Y.; Ouyang, D.; Han, L.; Yan, J.C.; Chen, M.F. Enhanced reduction and adsorption of hexavalent chromium by palladium and silicon rich biochar supported nanoscale zero-valent iron. J. Colloid Interf. Sci. 2019, 533, 428–436. [Google Scholar] [CrossRef]
- Zhou, L.L.; Li, R.R.; Zhang, G.L.; Wang, D.F.; Cai, D.Q.; Wu, Z.Y. Zero-valent iron nanoparticles supported by functionalized waste rock wool for efficient removal of hexavalent chromium. Chem. Eng. J. 2018, 339, 85–96. [Google Scholar] [CrossRef]
- Samuel, M.S.; Subramaniyan, V.; Bhattacharya, J.; Chidambaram, R.; Qureshi, T.; Pradeep Singh, N.D. Ultrasonic-assisted synthesis of graphene oxide—Fungal hyphae: An efficient and reclaimable adsorbent for chromium(VI) removal from aqueous solution. Ultrason. Sonochem. 2018, 48, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.X.; Li, H.Y.; Xu, X.J.; Yu, H.W. Synthesis of reduced graphene oxide/NiO nanocomposites for the removal of Cr(VI) from aqueous water by adsorption. Microporo. Mesopor. Mat. 2018, 255, 7–14. [Google Scholar] [CrossRef]
- Vellaichamy, B.; Periakaruppan, P.; Nagulan, B. Reduction of Cr6+ from wastewater using a novel in situ-synthesized PANI/MnO2/TiO2 nanocomposite: Renewable, selective, stable, and synergistic catalysis. ACS Sustain. Chem. Eng. 2017, 5, 9313–9324. [Google Scholar] [CrossRef]
- Kera, N.H.; Bhaumik, M.; Pillay, K.; Ray, S.S.; Maity, A. Selective removal of toxic Cr(VI) from aqueous solution by adsorption combined with reduction at a magnetic nanocomposite surface. J. Colloid Interf. Sci. 2017, 503, 214–228. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Xiong, D.B.; Li, X.F.; Bai, Z.M.; Lu, S.G. Recent advances in layered Ti3C2TX MXene for electrochemical energy storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- VahidMohammadi, A.; Moncada, J.; Chen, H.; Kayali, E.; Orangi, J.; Carrero, C.A.; Beidaghi, M. Thick and freestanding MXene/PANI pseudocapacitive electrodes with ultrahigh specific capacitance. J. Mater. Chem. A 2018, 6, 22123–22133. [Google Scholar] [CrossRef]
- Guo, M.; Liu, C.B.; Zhang, Z.Z.; Zhou, J.; Tang, Y.H.; Luo, S.L. Flexible Ti3C2TX@Al electrodes with ultrahigh areal capacitance: In situ regulation of interlayer conductivity and spacing. Adv. Funct. Mater. 2018, 28, 1803196. [Google Scholar] [CrossRef]
- Shahzad, A.; Nawaz, M.; Moztahida, M.; Tahir, K.; Kim, J.; Lim, Y.; Kim, B.; Jang, J.; Lee, D.S. Exfoliation of titanium aluminum carbide (211 MAX phase) to form nanofibers and two-dimensional nanosheets and their application in aqueous-phase cadmium sequestration. ACS Appl. Mater. Interfaces 2019, 11, 19156–19166. [Google Scholar] [CrossRef]
- Mu, W.J.; Du, S.Z.; Li, X.L.; Yu, Q.H.; Wei, H.Y.; Yang, Y.C.; Peng, S.M. Removal of radioactive palladium based on novel 2D titanium carbides. Chem. Eng. J. 2019, 358, 283–290. [Google Scholar] [CrossRef]
- Wang, L.; Song, H.; Yuan, L.Y.; Li, Z.; Zhang, Y.J.; Gibson, J.K.; Zheng, L.R.; Chai, Z.F.; Shi, W.Q. Efficient U(VI) reduction and sequestration by Ti2CTX MXene. Environ. Sci. Technol. 2018, 52, 10748–10756. [Google Scholar] [CrossRef]
- Wang, L.; Song, H.; Yuan, L.Y.; Li, Z.J.; Zhang, P.; Gibson, J.K.; Zheng, L.; Wang, H.Q.; Chai, Z.F.; Shi, W.Q. Effective removal of anionic Re(VII) by surface-modified Ti2CTx MXene nanocomposites: Implications for Tc(VII) sequestration. Environ. Sci. Technol. 2019, 53, 3739–3747. [Google Scholar] [CrossRef]
- Jhon, Y.I.; Koo, J.; Anasori, B.; Seo, M.; Lee, J.H.; Gogotsi, Y.; Jhon, Y.M. Metallic MXene saturable absorber for femtosecond mode-locked lasers. Adv. Mater. 2017, 29, 1702496. [Google Scholar] [CrossRef]
- Xu, Y.X.; Shi, G.Q.; Duan, X.F. Self-assembled three-dimensional graphene macrostructures: Synthesis and applications in supercapacitors. Acc. Chem. Res. 2015, 48, 1666–1675. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.Z.; Ji, Y.; Liu, Q.; Cheng, L.; Guan, K.; Zhang, M.; Liu, G.; Xiong, J.; Yang, J.; et al. 2D MXene nanofilms with tunable gas transport channels. Adv. Funct. Mater. 2018, 28, 1801511. [Google Scholar] [CrossRef]
- Yu, W.T.; Zhang, L.Y.; Wang, H.Y.; Chai, L.Y. Adsorption of Cr(VI) using synthetic poly(m-phenylenediamine). J. Hazard. Mater. 2013, 260, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Ren, L.L.; Jin, L.F.; Huang, L.; He, Y.J.; Tang, J.W.; Yang, W.C.; Wang, H.Y. In-situ functionalization of poly(m-phenylenediamine) nanoparticles on bacterial cellulose for chromium removal. Chem. Eng. J. 2018, 344, 441–452. [Google Scholar] [CrossRef]
- Li, X.G.; Huang, M.R.; Duan, W. Novel multifunctional polymers from aromatic diamines by oxidative polymerizations. Chem. Mater. 2002, 102, 2925–3030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zha, X.J.; Pu, J.H.; Bai, L.; Bao, R.Y.; Liu, Z.Y.; Yang, M.B.; Yang, W. Macroporous three-dimensional MXene architectures for highly efficient solar steam generation. J. Mater. Chem. A 2019, 7, 10446–10455. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, L.; Peng, J.; Zhai, M.L.; Shi, W.Q. Efficient thorium(IV) removal by two-dimensional Ti2CTx MXene from aqueous solution. Chem. Eng. J. 2019, 366, 192–199. [Google Scholar] [CrossRef]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D yitanium carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Xie, J.W.; Zhang, M.C.; Zhou, Q.; Liu, F.Q. Insight into the adsorption of PPCPs by porous adsorbents: Effect of the properties of adsorbents and adsorbates. Environ. Pollut. 2016, 214, 524–531. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/graphene films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation stability of colloidal two-dimensional titanium carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Jin, L.F.; Huang, L.; Ren, L.L.; He, Y.J.; Tang, J.W.; Wang, S.; Yang, W.C.; Wang, H.Y.; Chai, L.Y. Preparation of stable and high-efficient poly(m-phenylenediamine)/reduced graphene oxide composites for hexavalent chromium removal. J. Mater. Sci. 2019, 54, 383–395. [Google Scholar] [CrossRef]
- Han, F.; Luo, S.J.; Xie, L.Y.; Zhu, J.J.; Wei, W.; Chen, X.; Liu, F.W.; Chen, W.; Zhao, J.L.; Dong, L.; et al. Boosting the yield of MXene 2D sheets via a facile hydrothermal-assisted intercalation. ACS Appl. Mater. Interfaces 2019, 11, 8443–8452. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.B.; Sun, R.H.; Liu, Y.F.; Liu, Z.S.; Zhou, A.G.; Yu, Z.Z. Hydrophobic, flexible, and lightweight MXene foams for high-performance electromagnetic-interference shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; He, Y.J.; Chai, L.Y.; Lei, H.; Yang, W.C.; Hou, L.J.; Yuan, T.; Jin, L.F.; Tang, C.J.; Luo, J. Highly-dispersed Fe2O3@C electrode materials for Pb2+ removal by capacitive deionization. Carbon 2019, 153, 12–20. [Google Scholar] [CrossRef]
- Zhou, B.; Huang, D.Y.; Wu, J.S.; Zhu, Q.H.; Zhu, H.H. Horizontal and Vertical Distributions of Chromium in a Chromate Production District of South Central China. Int. J. Environ. Res. Public Health 2018, 15, 571. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, C.L.; Tan, X.L.; Xu, H.; Zhu, K.R. Polyaniline-modified 3D-flower-like molybdenum disulfide composite for efficient adsorption/photocatalytic reduction of Cr(VI). J. Colloid Interf. Sci. 2016, 476, 62–70. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.Y.; Li, C.F.; Yang, W.C.; Song, T.T.; Tang, C.J.; Meng, Y.; Dai, S.; Wang, H.Y.; Chai, L.Y.; et al. Synthesis of core-shell magnetic Fe3O4@poly(m-Phenylenediamine) particles for chromium reduction and adsorption. Environ. Sci. Technol. 2015, 49, 5654–5662. [Google Scholar] [CrossRef]
- Li, N.; Yue, Q.Y.; Gao, B.Y.; Xu, X.; Kan, Y.J.; Zhao, P. Magnetic graphene oxide functionalized by poly dimethyl diallyl ammonium chloride for efficient removal of Cr(VI). J. Taiwan Inst. Chem. E 2018, 91, 499–506. [Google Scholar] [CrossRef]
- Sakulthaew, C.; Chokejaroenrat, C.; Poapolathep, A.; Satapanajaru, T.; Poapolathep, S. Hexavalent chromium adsorption from aqueous solution using carbon nano-onions (CNOs). Chemosphere 2017, 184, 1168–1174. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, L.; Li, A.M. Eucalyptus sawdust derived biochar generated by combining the hydrothermal carbonization and low concentration KOH modification for hexavalent chromium removal. J. Environ. Manag. 2018, 206, 989–998. [Google Scholar] [CrossRef]
- Zou, G.D.; Guo, J.X.; Peng, Q.M.; Zhou, A.G.; Zhang, Q.R.; Liu, B.Z. Synthesis of urchin-like rutile titania carbon nanocomposites by iron-facilitated phase transformation of MXene for environmental remediation. J. Mater. Chem. A 2016, 4, 489–499. [Google Scholar] [CrossRef]
- Ying, Y.L.; Liu, Y.; Wang, X.Y.; Mao, Y.Y.; Cao, W.; Hu, P.; Peng, X.S. Two-dimensional titanium carbide for efficiently reductive removal of highly toxic chromium(VI) from water. ACS Appl. Mater. Interfaces 2015, 7, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.Q.; Gu, M.M.; Jin, W.; Zhou, J.B. CTAB-functionalized C@SiO2 double-shelled hollow microspheres with enhanced and selective adsorption performance for Cr(VI). J. Alloy Compd. 2019, 777, 1304–1312. [Google Scholar] [CrossRef]
- Li, X.G.; Ma, X.L.; Sun, J.; Huang, M.R. Powerful reactive sorption of silver(I) and mercury(II) onto poly(o-phenylenediamine) microparticles. Langmuir 2009, 25, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.F.; Chai, L.Y.; Ren, L.L.; Jiang, Y.X.; Yang, W.C.; Wang, S.; Liao, Q.; Wang, H.Y.; Zhang, L.Y. Enhanced adsorption-coupled reduction of hexavalent chromium by 2D poly(m-phenylenediamine)-functionalized reduction graphene oxide. Environ. Sci. Pollut. Res. 2019, 26, 31099–31110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Wang, Y.Y.; Peng, B.; Yu, W.T.; Wang, H.Y.; Wang, T.; Deng, B.W.; Chai, L.Y.; Zhang, K.; Wang, J.X. Preparation of a macroscopic, robust carbon-fiber monolith from filamentous fungi and its application in Li–S batteries. Green Chem. 2014, 16, 3926. [Google Scholar] [CrossRef]

| Composites | SBET (m2 g−1) | Pore Volume (cm−3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| Ti3C2Tx | 10.42 | 0.14 | 27.25 |
| Ti3C2Tx/PmPD-2/1 | 43.74 | 0.17 | 7.86 |
| Ti3C2Tx/PmPD-5/1 | 55.93 | 0.18 | 6.34 |
| Ti3C2Tx/PmPD-10/1 | 38.99 | 0.11 | 5.40 |
| PmPD | 2.44 | 0.029 | 23.54 |
| Adsorbents | Qm (mg g−1) | pH | References |
|---|---|---|---|
| PDMDAAC | 95.2 | 2 | [48] |
| carbon nano-onions | 23.5 | 3 | [49] |
| Biochar | 45.88 | 2 | [50] |
| Fe@GA beads | 33.9 | 3 | [14] |
| nZVIRS700-Pd | 117.1 | 3 | [13] |
| Modified MXene | 225 | 6 | [51] |
| MXene | 250 | 2 | [52] |
| Ti3C2Tx/PmPD | 540.47 | 2 | this work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Chai, L.; Yang, W.; Wang, H.; Zhang, L. Two-Dimensional Titanium Carbides (Ti3C2Tx) Functionalized by Poly(m-phenylenediamine) for Efficient Adsorption and Reduction of Hexavalent Chromium. Int. J. Environ. Res. Public Health 2020, 17, 167. https://doi.org/10.3390/ijerph17010167
Jin L, Chai L, Yang W, Wang H, Zhang L. Two-Dimensional Titanium Carbides (Ti3C2Tx) Functionalized by Poly(m-phenylenediamine) for Efficient Adsorption and Reduction of Hexavalent Chromium. International Journal of Environmental Research and Public Health. 2020; 17(1):167. https://doi.org/10.3390/ijerph17010167
Chicago/Turabian StyleJin, Linfeng, Liyuan Chai, Weichun Yang, Haiying Wang, and Liyuan Zhang. 2020. "Two-Dimensional Titanium Carbides (Ti3C2Tx) Functionalized by Poly(m-phenylenediamine) for Efficient Adsorption and Reduction of Hexavalent Chromium" International Journal of Environmental Research and Public Health 17, no. 1: 167. https://doi.org/10.3390/ijerph17010167
APA StyleJin, L., Chai, L., Yang, W., Wang, H., & Zhang, L. (2020). Two-Dimensional Titanium Carbides (Ti3C2Tx) Functionalized by Poly(m-phenylenediamine) for Efficient Adsorption and Reduction of Hexavalent Chromium. International Journal of Environmental Research and Public Health, 17(1), 167. https://doi.org/10.3390/ijerph17010167




