Tuberculosis-Related Hospitalizations in a Low-Incidence Country: A Retrospective Analysis in Two Italian Infectious Diseases Wards
Abstract
1. Introduction
2. Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| DST | drug susceptibility test |
| ECDC | European Center for Disease Prevention and Control |
| EPTB | extrapulmonary tuberculosis |
| ERS | European Respiratory Society |
| HIV | human immunodeficiency virus |
| IGRA | interferon-gamma release assay |
| IQR | interquartile range |
| MDR | multi-drug-resistant |
| MTB | Mycobacterium tuberculosis |
| NTM | nontuberculous mycobacteria |
| PTB | pulmonary tuberculosis |
| SD | standard deviation |
| TB | tuberculosis |
| USA | United States of America |
| XDR | extensively drug-resistant |
| WHO | World Health Organization |
References
- World Health Organization. Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/tb/publications/global_report/MainReport_18Sept2018.pdf?ua=1 (accessed on 1 November 2019).
- Hollo, V.; Beauté, J.; Ködmön, C.; van der Werf, M.J. Tuberculosis notification rate decreases faster in residents of native origin than in residents of foreign origin in the EU/EEA, 2010 to 2015. Euro Surveill. 2017, 22, 30486. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.J.; Tsang, C.A.; Pratt, R.H.; Price, S.F.; Langer, A.J. Tuberculosis—United States, 2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Cowger, T.L.; Wortham, J.M.; Burton, D.C. Epidemiology of tuberculosis among children and adolescents in the USA, 2007–2017: An analysis of national surveillance data. Lancet Public Health 2019, 4, e506–e516. [Google Scholar] [CrossRef]
- Lönnroth, K.; Mor, Z.; Erkens, C.; Bruchfeld, J.; Nathavitharana, R.R.; Van Der Werf, M.J.; Lange, C. Tuberculosis in migrants in low-incidence countries: Epidemiology and intervention entry points. Int. J. Tuberc. Lung Dis. 2017, 21, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.openpolis.it/numeri/gli-sbarchi-italia-negli-ultimi-10-anni/fonte: Ministero dell’Interno (accessed on 1 November 2019).
- Scotto, G.; Fazio, V.; Lo Muzio, L. Tuberculosis in the immigrant population in Italy: State-of-the-art review. Infez Med. 2017, 25, 199–209. [Google Scholar] [PubMed]
- Banta, J.E.; Ani, C.; Bvute, K.M.; Lloren, J.I.C.; Darnell, T.A. Pulmonary vs. extra-pulmonary tuberculosis hospitalizations in the US [1998–2014]. J. Infect. Public Health 2019. [Google Scholar] [CrossRef]
- eCDC. European Union Standards for Tuberculosis Care. Available online: https://ecdc.europa.eu/en/all-topics-ztuberculosisprevention-and-control/european-union-standards-tuberculosis-care (accessed on 1 November 2019).
- Compendium of WHO Guidelines and Associated Standards: Ensuring Optimum Delivery of the Cascade of Care for Patients with Tuberculosis, 2nd ed.; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/272644/9789241514101-eng.pdf?ua=1 (accessed on 1 November 2019).
- Forbes, B.A.; Banaiee, N.; Beavis, K.G.; Brown-Elliott, B.A.; Della Latta, P.; Elliott, L.B.; Elliott, L.B.; Hall, G.S.; Hanna, B.; Perkins, M.D.; et al. Laboratory Detection and Identification of Mycobacteria; Approved Guideline, 1st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Gilbert, R.L.; Antoine, D.; French, C.E.; Abubakar, I.; Watson, J.M.; Jones, J.A. The impact of immigration on tuberculosis rates in the United Kingdom compared with other European countries. Int. J. Tuberc. Lung Dis. 2009, 13, 645–651. [Google Scholar]
- González-García, A.; Fortún, J.; Elorza Navas, E.; Martín-Dávila, P.; Tato, M.; Gómez-Mampaso, E.; Moreno, S. The changing epidemiology of tuberculosis in a Spanish tertiary hospital (1995–2013). Medicine (Baltim.) 2017, 96, e7219. [Google Scholar] [CrossRef]
- Baussano, I.; Mercadante, S.; Pareek, M.; Lalvani, A.; Bugiani, M. High Rates of Mycobacterium tuberculosis among Socially Marginalized Immigrants in Low-Incidence Area, 1991–2010, Italy. Emerg. Infect. Dis. 2013, 19, 1437–1445. [Google Scholar] [CrossRef]
- Ingrosso, L.; Vescio, F.; Giuliani, M.; Migliori, G.B.; Fattorini, L.; Severoni, S.; Rezza, G. Risk Factors for Tuberculosis in Foreign-Born People (FBP) in Italy: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e94728. [Google Scholar] [CrossRef][Green Version]
- Lombardi, G.; Dal Monte, P.; Denicolò, A.; Tadolini, M.; Martelli, G.; Bacchi Reggiani, M.L.; Viale, P.; Landini, M.P. Trend of microbiologically-confirmed tuberculosis in a low-incidence setting with high immigration rates. BMC Public Health 2014, 14, 340. [Google Scholar] [CrossRef] [PubMed]
- Odone, A.; Riccò, M.; Morandi, M.; Borrini, B.M.; Pasquarella, C.; Signorelli, C. Epidemiology of tuberculosis in a low-incidence Italian region with high immigration rates: Differences between not Italy-born and Italy-born TB cases. BMC Public Health 2011, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Odone, A.; Tillmann, T.; Sandgren, A.; Williams, G.; Rechel, B.; Ingleby, D.; Noori, T.; Mladovsky, P.; McKee, M. Tuberculosis among migrant populations in the European Union and the European Economic Area. Eur. J. Public Health 2015, 25, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Peto, H.M.; Pratt, R.H.; Harrington, T.A.; LoBue, P.A.; Armstrong, L.R. Epidemiology of Extrapulmonary Tuberculosis in the United States, 1993–2006. Clin. Infect. Dis. 2009, 49, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Villa, S.; Codecasa, L.R.; Faccini, M.; Pontello, M.M.; Ferrarese, M.; Castellotti, P.F.; Senatore, S.; Lamberti, A.; Mazzola, E.; Perno, C.F.; et al. Tuberculosis among asylum-seekers in Milan, Italy: Epidemiological analysis and evaluation of interventions. Eur. Respir. J. 2019, 54, 1900896. [Google Scholar] [CrossRef]
- Birchall Jenny; Part of the BRIDGE Team; Institute of Development Studies (IDS). Available online: https://www.rosavzw.be/digidocs/dd-001417_2016_Gender_Age_Migration_IDS.pdf (accessed on 1 November 2019).
- Casal, M.; Vaquero, M.; Rinder, H.; Tortoli, E.; Grosset, J.; Rush-Gerdes, S.; Gutierrez, J.; Jarlier, V. A case-control study for multidrug-resistant tuberculosis: Risk factors in four European countries. Microb. Drug Resist. 2005, 11, 62–67. [Google Scholar] [CrossRef]
- Fronti, E.; Vecchia, M.; Scudeller, L.; Praticò, L.; Marone, P.; Muzzi, A.; Minoli, L.; Seminari, E. Epidemiology of Mycobacterium tuberculosis infection in Pavia province, Lombardy, Northern Italy, 1998–2013. New Microbiol. 2016, 39, 264–268. [Google Scholar]
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2018–2016 Data. Available online: https://www.ecdc.europa.eu/sites/portal/files/documents/ecdc-tuberculosis-surveillance-monitoring-Europe-2018-19mar2018.pdf (accessed on 1 November 2019).
- Sotgiu, G.; Falzon, D.; Hollo, V.; Ködmön, C.; Lefebvre, N.; Dadu, A.; van der Werf, M. Determinants of site of tuberculosis disease: An analysis of European surveillance data from 2003 to 2014. PLoS ONE 2017, 12, e0186499. [Google Scholar] [CrossRef]
- Ade, S.; Harries, A.D.; Trébucq, A.; Ade, G.; Agodokpessi, G.; Adjonou, C.; Azon, S.; Anagonou, S. National profile and treatment outcomes of patients with extrapulmonary tuberculosis in Bénin. PLoS ONE 2014, 9, e95603. [Google Scholar] [CrossRef]
- Tesgaye, F.; Defar, A.; Beyene, T.; Shafi, O.; Klinkenberg, E.; Howe, R. Documentation and treatment outcomes of smear-negative and extra-pulmonary tuberculosis in Ethiopia. Public Health Action 2014, 4 (Suppl. S3), S25–S30. [Google Scholar] [CrossRef]
- Ohene, S.A.; Bakker, M.I.; Ojo, J.; Toonstra, A.; Awudi, D.; Klatser, P. Extra-pulmonary tuberculosis: A retrospective study of patients in Accra, Ghana. PLoS ONE 2019, 14, e0209650. [Google Scholar] [CrossRef] [PubMed]
- Pollett, S.; Banner, P.; O’Sullivan, M.V.; Ralph, A.P. Epidemiology, Diagnosis and Management of Extra-Pulmonary Tuberculosis in a Low-Prevalence Country: A Four Year Retrospective Study in an Australian Tertiary Infectious Diseases Unit. PLoS ONE 2016, 11, e0149372. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.M.; Singh, J.; Diana, S.C.; Singh, S. Molecular characterization of Mycobacterium tuberculosis isolates from North Indian patients with extrapulmonary tuberculosis. Tuberculosis (Edinb) 2013, 93, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Vinhas, S.A.; Reis-Santos, B.; Palaci, M.; Peres, R.L.; Aguiar, P.P.; Correa Ribeiro, F.K.; Marques, H.S.; do Valle Dettoni, V.; Johnson, J.L.; et al. Extrapulmonary tuberculosis: Mycobacterium tuberculosis strains and host risk factors in a large urban setting in Brazil. PLoS ONE 2013, 8, e74517. [Google Scholar] [CrossRef] [PubMed]
| Variables | Overall n = 166 | Italian (n = 52) | Foreign-Born (n = 114) | p-Value & | |
|---|---|---|---|---|---|
| Year of diagnosis, n (%) | 2013 | 17 (10.2) | 3 (5.8) | 14 (12.3) | 0.29 |
| 2014 | 20 (12.1) | 6 (11.5) | 14 (12.3) | ||
| 2015 | 34 (20.5) | 13 (25.0) | 21 (18.4) | ||
| 2016 | 58 (34.9) | 22 (42.3) | 36 (31.6) | ||
| 2017 | 37 (22.3) | 8 (15.4) | 29 (25.4) | ||
| Median (IQR) age, years | 37 (26–55) | 71.5 (44.5–80.0) | 30 (24–40) | <0.0001 | |
| Age >65 years | 31 (18.7) | 31 (59.62) | 0 (0.0) | <0.0001 | |
| Age group, n (%) | 0–24 | 31 (18.7) | 2 (3.9) | 29 (25.4) | 0.001 |
| 25–44 | 74 (44.6) | 11 (21.2) | 63 (55.3) | <0.0001 | |
| 45–64 | 30 (18.1) | 8 (15.4) | 22 (19.3) | 0.55 | |
| 65–79 | 17 (10.2) | 17 (32.7) | 0 (0.0) | <0.0001 | |
| ≥80 | 14 (8.4) | 14 (26.9) | 0 (0.0) | <0.0001 | |
| Males, n (%) | 118 (71.1) | 32 (61.5) | 86 (75.4) | 0.07 | |
| Immunodepression, n (%) | 45 (27.1) | 25 (48.1) | 20 (17.5) | <0.0001 | |
| Causes of immunodepression, n (%) | HIV positivity | 14 (31.1) | 4 (16.0) | 10 (50.0) | 0.01 |
| Hematological diseases | 6 (13.3) | 6 (24.0) | 0 (0.0) | 0.02 | |
| Alcohol | 4 (8.9) | 1 (4.0) | 3 (15.0) | 0.20 | |
| Diabetes mellitus | 4 (8.9) | 2 (8.0) | 2 (10.0) | 0.82 | |
| Solid tumor | 4 (8.9) | 4 (16.0) | 0 (0.0) | 0.06 | |
| Malnutrition | 4 (8.9) | 1 (4.0) | 3 (15.0) | 0.20 | |
| Autoimmune disease | 3 (6.7) | 3 (12.0) | 0 (0.0) | 0.11 | |
| Chronic renal failure | 2 (4.4) | 1 (4.0) | 1 (5.0) | 0.87 | |
| Other diseases | 4 (8.9) | 3 (12.0) | 1 (5.0) | 0.41 | |
| Comorbidity *, n (%) | 39 (23.5) | 23 (44.2) | 16 (14.0) | <0.0001 | |
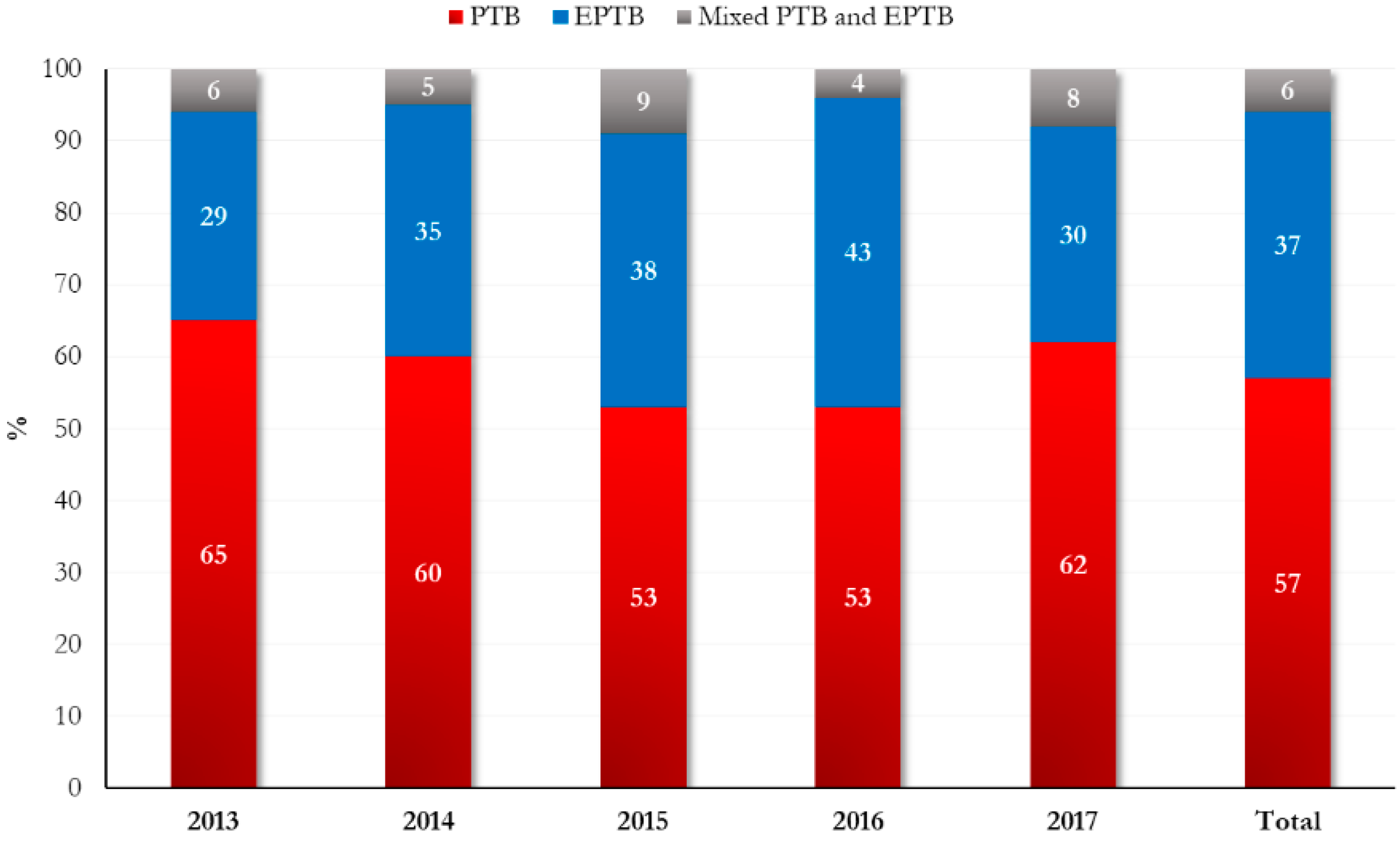
| TB form, n (%) | PTB ** | 95 (57.2) | 32 (61.5) | 63 (55.3) | 0.66 |
| EPTB *** | 61 (36.8) | 18 (34.6) | 43 (37.7) | ||
| PTB and EPTB | 10 (6.0) | 2 (3.9) | 8 (7.0) | ||
| TB drug resistance, n (%) | 16 (13.0) | 1 (2.9) | 15 (17.1) | 0.04 | |
| Variables | Pulmonary (n = 95) | Extrapulmonary (n = 61) | p-Value | |
|---|---|---|---|---|
| Year of diagnosis, n (%) | 2013 | 11 (11.6) | 5 (8.2) | 0.74 |
| 2014 | 12 (12.6) | 7 (11.5) | ||
| 2015 | 18 (19.0) | 13 (21.3) | ||
| 2016 | 31 (32.6) | 25 (41.0) | ||
| 2017 | 23 (24.2) | 11 (18.0) | ||
| Median (IQR) age, years | 39 (29–58) | 32 (24–55) | 0.07 | |
| Age >65 years | 19 (20.0) | 12 (19.7) | 0.96 | |
| Age group, n (%) | 0–24 | 10 (10.5) | 16 (26.2) | 0.13 |
| 25–44 | 48 (50.5) | 23 (37.7) | ||
| 45–64 | 18 (19.0) | 10 (16.4) | ||
| 65–79 | 10 (10.5) | 7 (11.5) | ||
| ≥80 | 9 (9.5) | 5 (8.2) | ||
| Males, n (%) | 65 (68.4) | 45 (73.8) | 0.48 | |
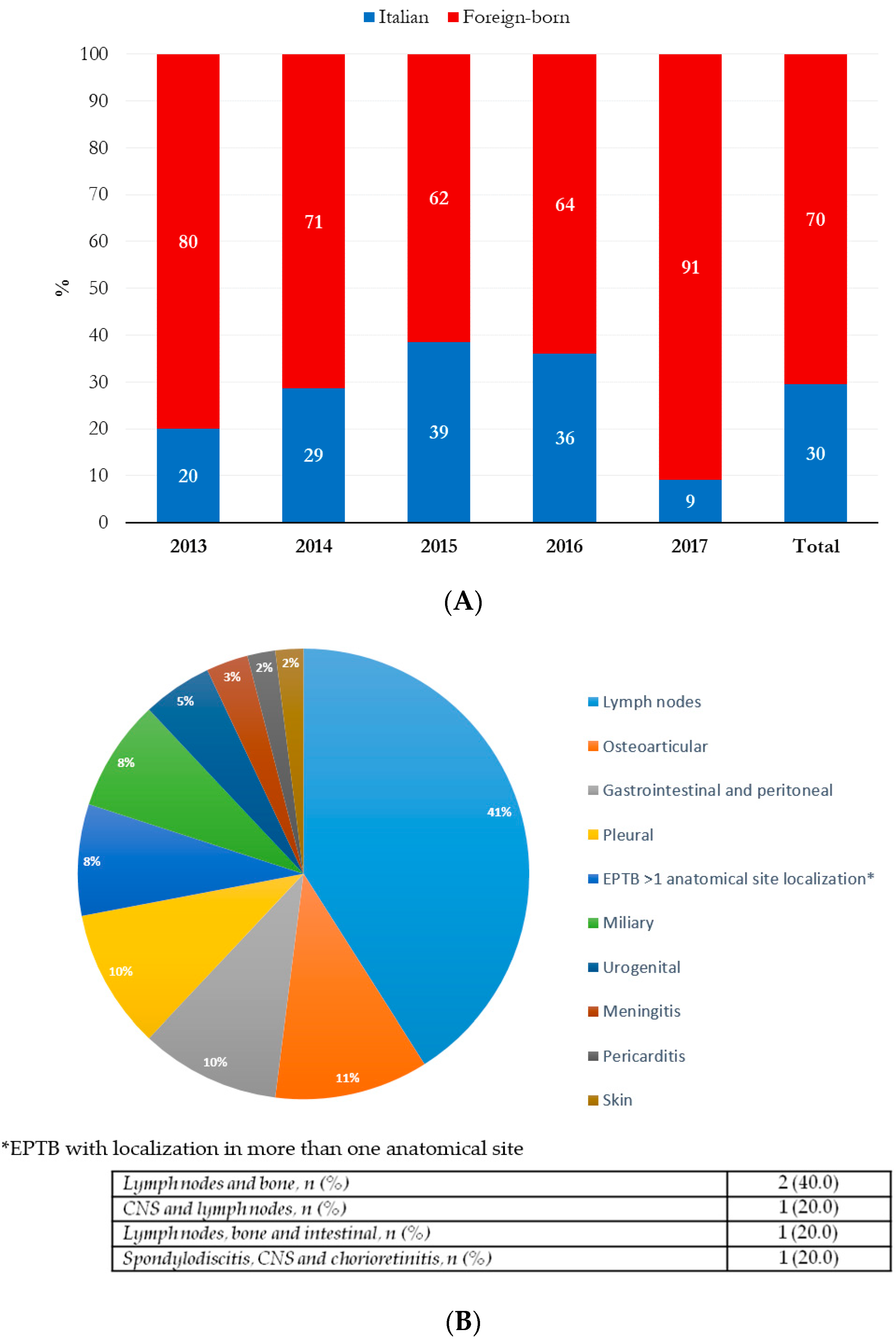
| Geographical area of origin, n (%) | Italy | 32 (33.7) | 18 (29.5) | 0.59 |
| Africa | 19 (20.0) | 24 (39.3) | 0.01 | |
| Europe | 35 (36.8) | 7 (11.5) | 0.001 | |
| Other countries * | 9 (9.5) | 12 (19.7) | 0.07 | |
| Immunodepression, n (%) | 17 (17.9) | 23 (37.7) | 0.006 | |
| Causes of immunodepression, n (%) | HIV positivity | 3 (17.7) | 10 (43.5) | 0.18 |
| Hematological diseases | 1 (5.9) | 5 (21.7) | ||
| Alcohol | 4 (23.5) | 0 (0.0) | ||
| Diabetes mellitus | 2 (11.8) | 2 (8.7) | ||
| Solid tumor | 2 (11.8) | 2 (8.7) | ||
| Malnutrition | 0 (0.0) | 1 (4.4) | ||
| Autoimmune disease | 2 (11.8) | 1 (4.4) | ||
| Chronic renal failure | 1 (5.9) | 1 (4.4) | ||
| Other diseases | 2 (11.8) | 1 (4.4) | ||
| Comorbidity &, n (%) | 16 (16.8) | 21 (34.4) | 0.01 | |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Age, years | 1.0 (1.0–1.0) | 0.21 | 1.0 (1.0–1.0) | 0.98 | |
| Age >65 years | 1.0 (0.4–2.2) | 0.96 | - | - | |
| Age groups | 0.9 (0.7–1.1) | 0.29 | - | - | |
| Males | 1.3 (0.6–2.7) | 0.48 | 1.3 (0.6–2.9) | 0.58 | |
| Foreign-born | 1.2 (0.6–2.4) | 0.59 | - | - | |
| Geographical area of origin | Italy | 0.8 (0.4–1.7) | 0.59 | - | - |
| Africa | 2.6 (1.3–5.3) | 0.009 | 0.8 (0.3–2.6) | 0.76 | |
| Eastern Europe | 0.2 (0.1–0.5) | 0.001 | - | - | |
| Other countries * | 2.3 (0.9–5.9) | 0.07 | - | - | |
| European origin | 0.3 (0.2–0.7) | <0.0001 | 0.2 (0.1–0.6) | 0.004 | |
| HIV positivity | 6.0 (1.6–22.8) | 0.008 | 2.3 (0.4–12.1) | 0.33 | |
| Hematological diseases | 8.4 (1.0–73.7) | 0.06 | - | - | |
| Other causes of immunodepression | 1.0 (0.4–2.5) | 0.91 | - | - | |
| Comorbidity & | 2.5 (1.2–5.3) | 0.01 | 3.4 (1.1–10.4) | 0.03 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campogiani, L.; Compagno, M.; Coppola, L.; Malagnino, V.; Maffongelli, G.; Saraca, L.M.; Francisci, D.; Baldelli, F.; Fontana, C.; Grelli, S.; et al. Tuberculosis-Related Hospitalizations in a Low-Incidence Country: A Retrospective Analysis in Two Italian Infectious Diseases Wards. Int. J. Environ. Res. Public Health 2020, 17, 124. https://doi.org/10.3390/ijerph17010124
Campogiani L, Compagno M, Coppola L, Malagnino V, Maffongelli G, Saraca LM, Francisci D, Baldelli F, Fontana C, Grelli S, et al. Tuberculosis-Related Hospitalizations in a Low-Incidence Country: A Retrospective Analysis in Two Italian Infectious Diseases Wards. International Journal of Environmental Research and Public Health. 2020; 17(1):124. https://doi.org/10.3390/ijerph17010124
Chicago/Turabian StyleCampogiani, Laura, Mirko Compagno, Luigi Coppola, Vincenzo Malagnino, Gaetano Maffongelli, Lavinia Maria Saraca, Daniela Francisci, Franco Baldelli, Carla Fontana, Sandro Grelli, and et al. 2020. "Tuberculosis-Related Hospitalizations in a Low-Incidence Country: A Retrospective Analysis in Two Italian Infectious Diseases Wards" International Journal of Environmental Research and Public Health 17, no. 1: 124. https://doi.org/10.3390/ijerph17010124
APA StyleCampogiani, L., Compagno, M., Coppola, L., Malagnino, V., Maffongelli, G., Saraca, L. M., Francisci, D., Baldelli, F., Fontana, C., Grelli, S., Andreoni, M., Sotgiu, G., Saderi, L., & Sarmati, L. (2020). Tuberculosis-Related Hospitalizations in a Low-Incidence Country: A Retrospective Analysis in Two Italian Infectious Diseases Wards. International Journal of Environmental Research and Public Health, 17(1), 124. https://doi.org/10.3390/ijerph17010124







