Malaria Transmission around the Memve’ele Hydroelectric Dam in South Cameroon: A Combined Retrospective and Prospective Study, 2000–2016
Abstract
:1. Background
2. Methods
2.1. Study Design
2.2. Study Sites
2.3. Mosquito Collection and Morphological Identification
2.4. Molecular Identification
2.5. Determination of Plasmodium Falciparum Infectivity
2.6. Statistical Analysis
- The Human Biting Rate (HBR) which represents the average number of bites received per person per night;
- The Infection Rate (IR) that measured the proportion of mosquitoes positive for P. falciparum Circumsporozoite antigene;
- The Entomological Inoculation Rate (EIR), which is the number of infective bites received per person per night; EIR is calculated as the product of the HBR and IR.
2.7. Ethics Approval and Consent to Participate
3. Results
3.1. Composition and Abundance of Anopheline Fauna in Nyabessan and Olama
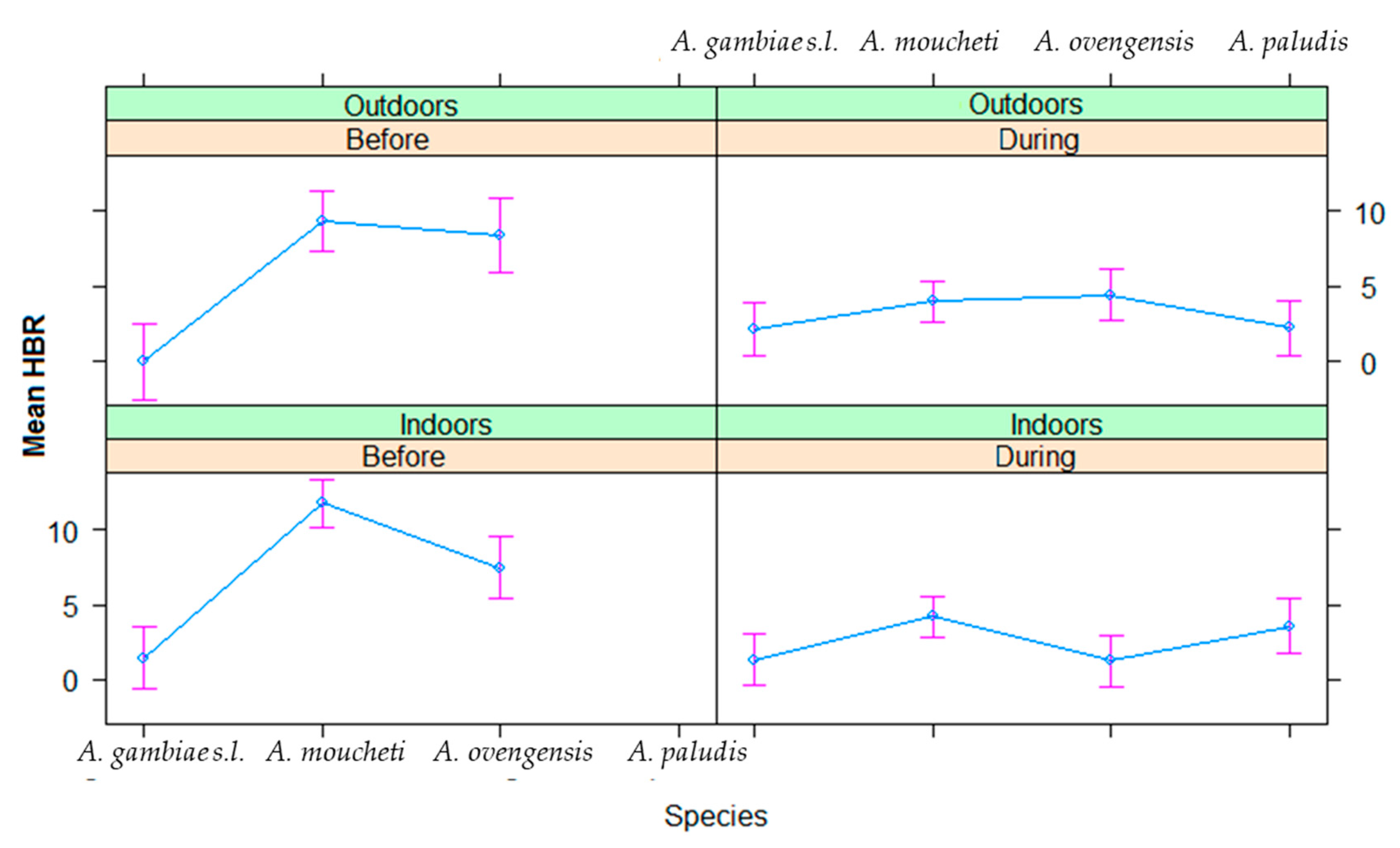
3.2. Night Biting Rates and Biting Cycles of Malaria Vector Populations, Indoors and Outdoors in Nyabessan and Olama
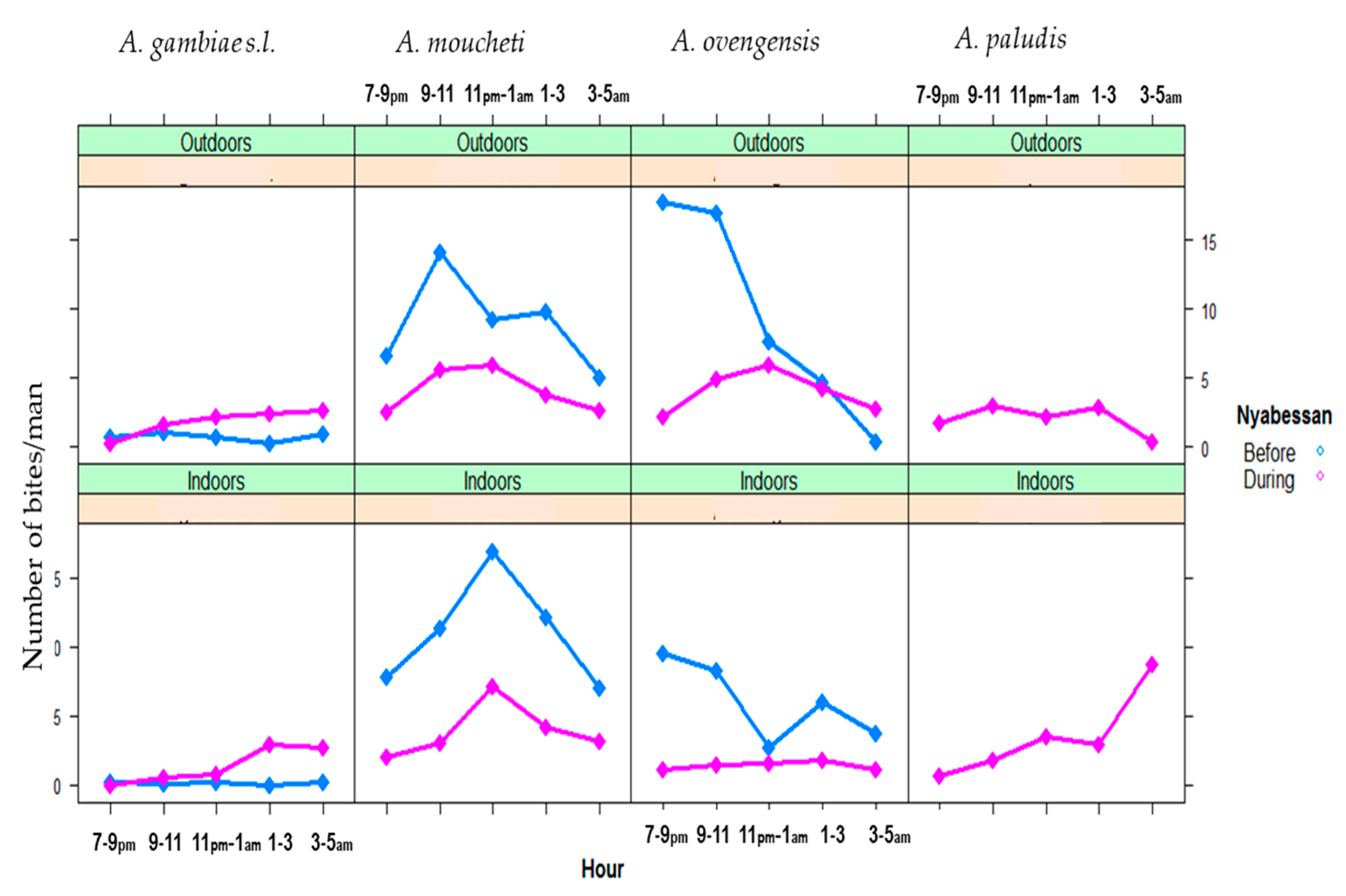
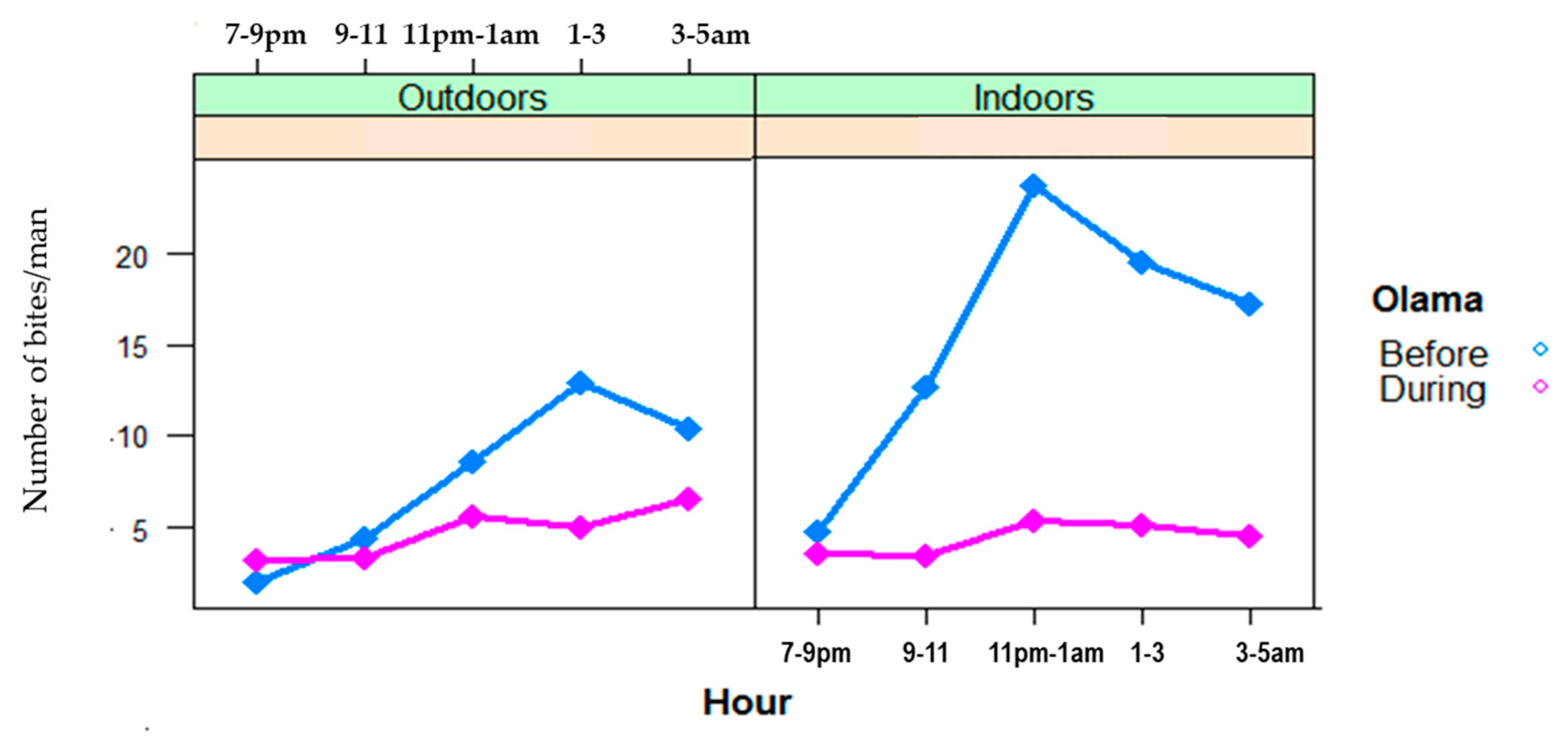
3.2.1. Night Biting Rates Indoors and Outdoors
3.2.2. Indoor and Outdoor Biting Cycles
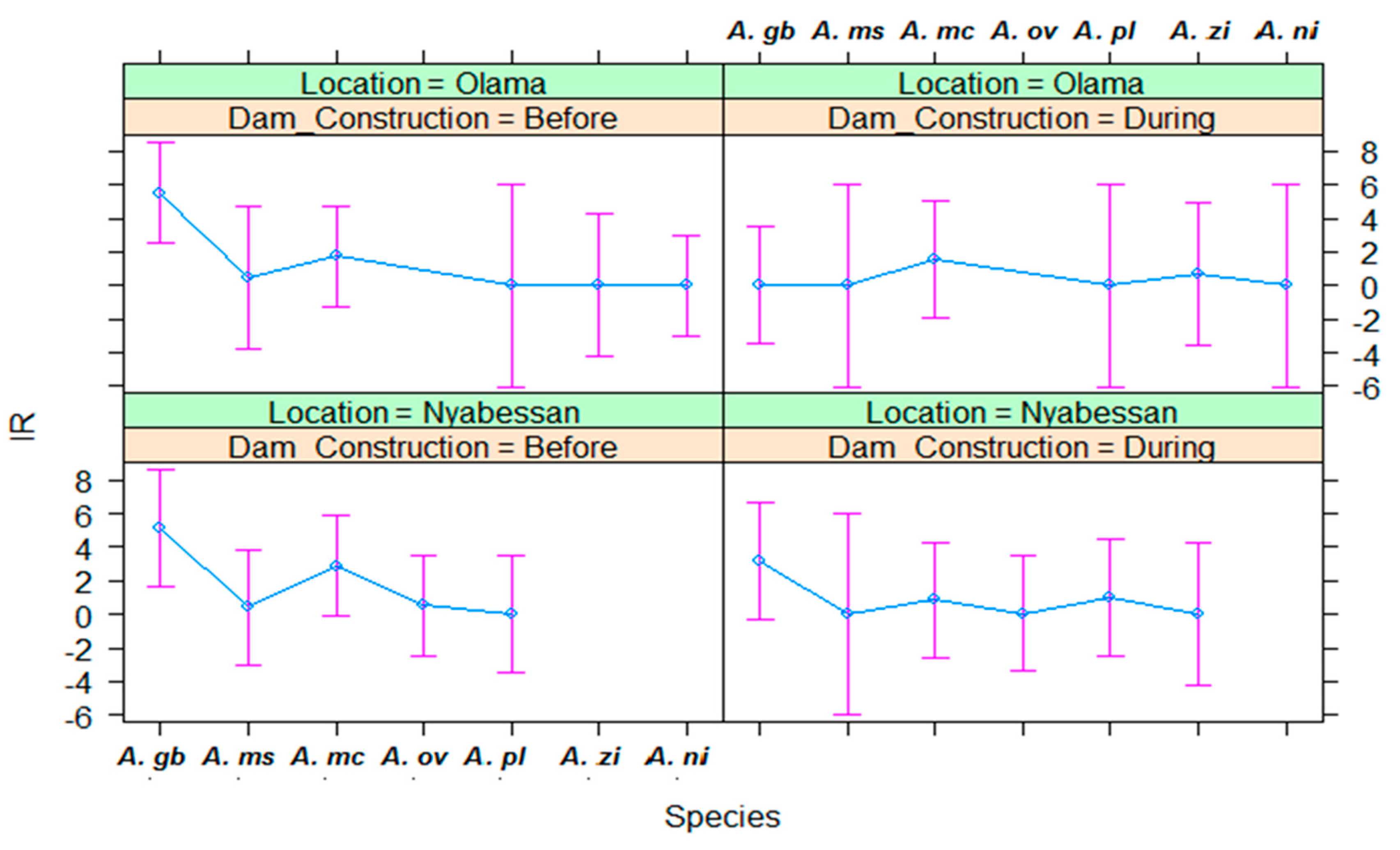
3.3. Infection Rates of Malaria Vectors in Nyabessan and Olama
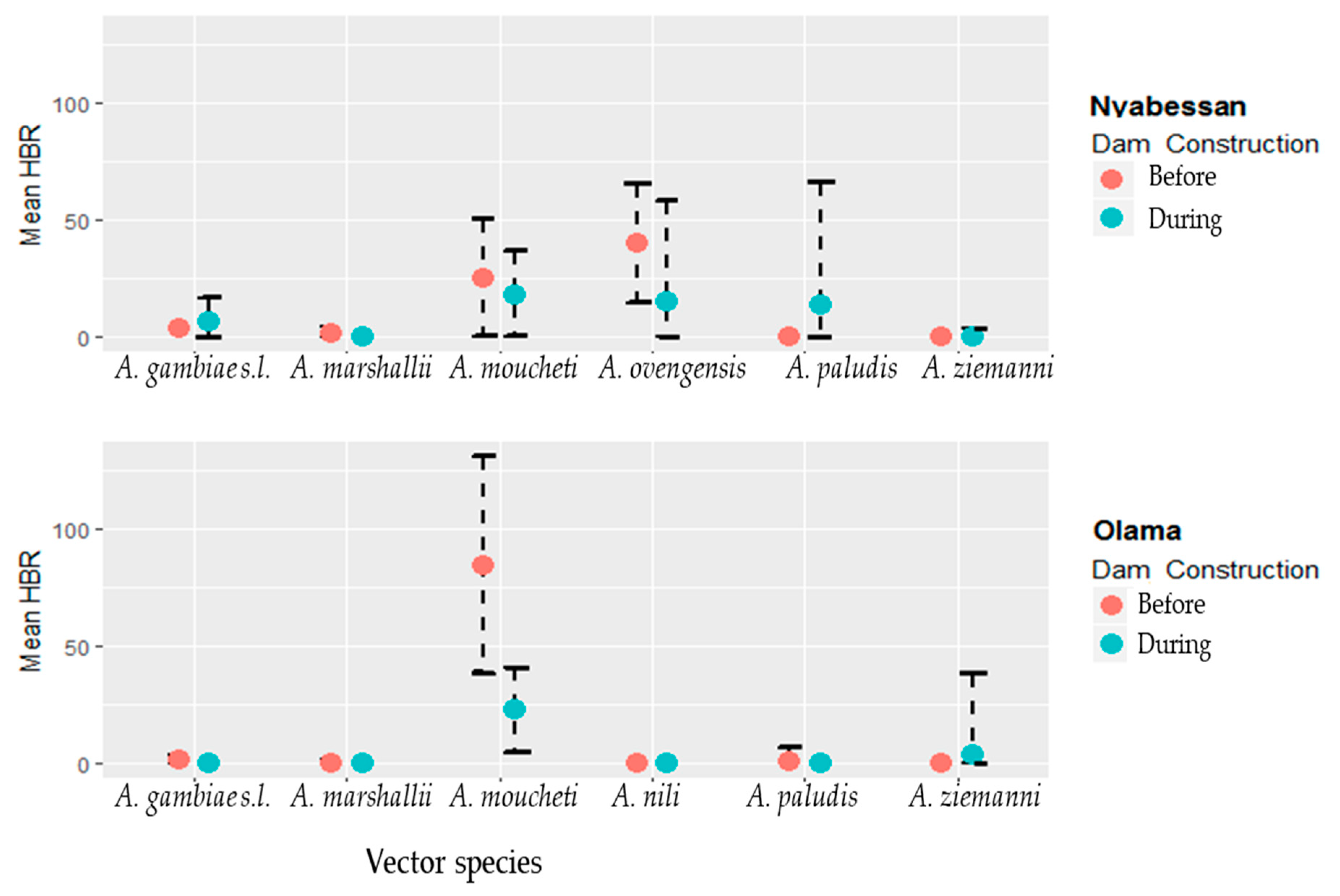
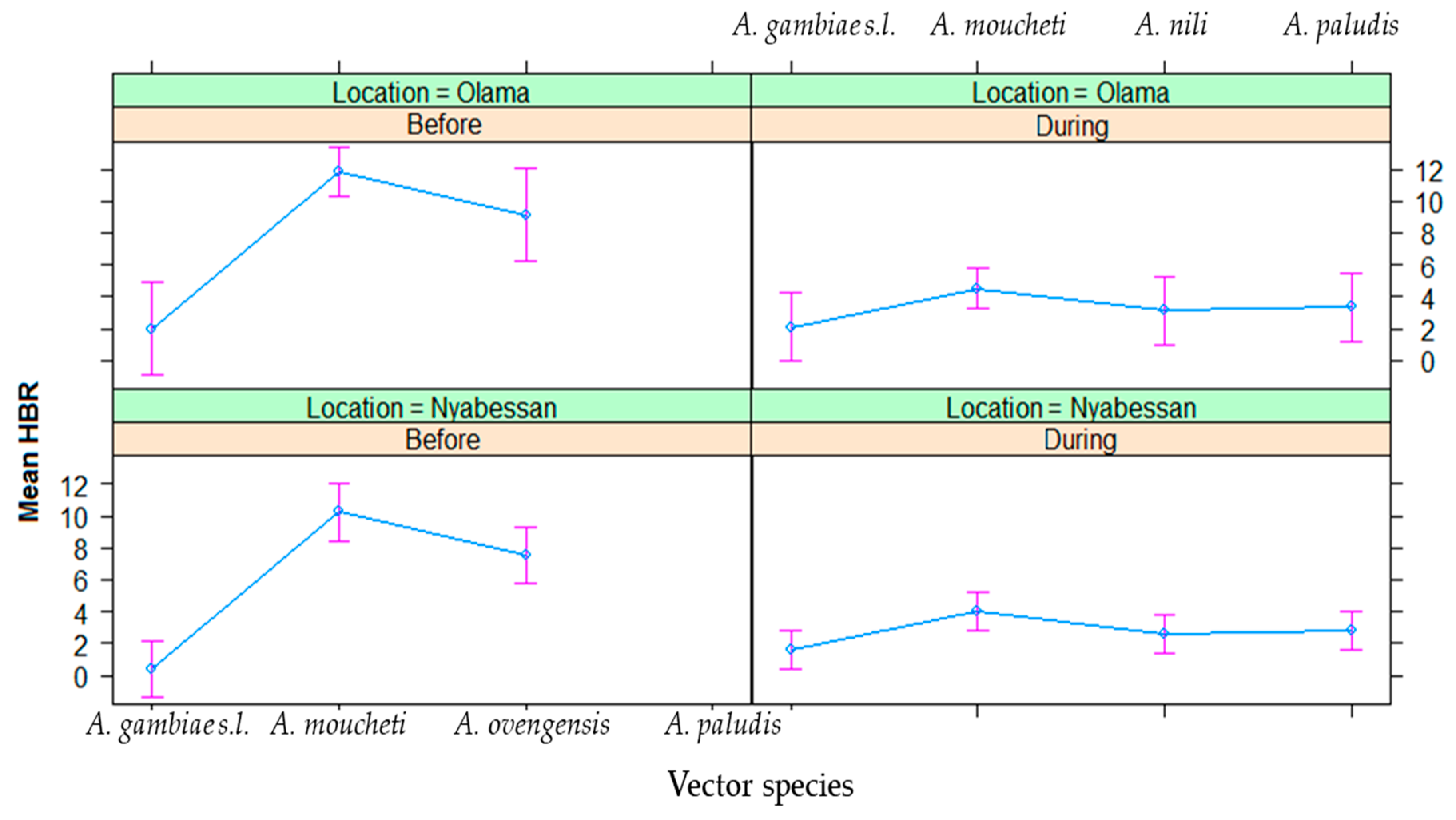
3.4. Entomological Inoculation Rates of Malaria Vectors in Nyabessan and Olama
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- MacCormack, C.P. Human ecology and behavior in malaria control in tropical Africa. Bull. World Health Organ. 1984, 62, 81–87. [Google Scholar]
- Parkes, M.; Panelli, R.; Weinstein, P. Converging paradigms for environmental health theory and practice. Environ. Health Perspect. 2003, 111, 669–675. [Google Scholar] [CrossRef] [PubMed]
- World Bank. The Water Resources Sector Strategy: An Overview; World Bank: Washington, DC, USA, 2004. [Google Scholar]
- New Partnership for Africa’s Development (NEPAD); Comprehensive Africa Agriculture Development Program (CAADP); South Africa NEPAD; MidrandGillies, M.T.; Coetzee, M. A Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region), 55th ed.; Publication of the South African Institute for Medical Research: Johannesburg, South Africa, 1987. [Google Scholar]
- Ripert, C.; Samé-Ekobo, A.; Palmer, D.; Enyong, P. Évaluation des répercussions sur les endémies parasitaires (malaria, schistosomiase, onchocercose, dracunculose) de la construction de 57 barrages dans les Monts Mandara (Nord-Cameroun). Bull. Soc. Pathol. Exot. 1979, 72, 324–339. [Google Scholar]
- Petr, T. Tropical man-made lakes: Their ecological impact. Arch. Hydrobiol. 1978, 81, 368–385. [Google Scholar]
- Philippon, B.; Mouchet, J. Répercussion des aménagements hydrauliques à usage agricole sur l’épidémiologie des maladies à vecteurs en Afrique intertropicale. Paris, Cahiers du CENECA. Coll. Intern. Doc. 1976, 3, 14. [Google Scholar]
- Finkelman, J.; Arata, A.A. Vector-borne diseases associated with development projects. In Selected Working Papers for Third, Fourth, Fifth and Sixth PEEM Meeting; Secretariat du TEAE; WHO: Genève, Switzerland, 1987. [Google Scholar]
- Wijeyaratne, P.M. Method of forecasting the vector-borne disease implication in the development of different types of water resources projects: Vector aspects. In Selected Working Papers for Third, Fourth, Fifth and Sixth PEEM Meeting; Secretariat du TEAE; WHO: Genève, Switzerland, 1987. [Google Scholar]
- Bos, R. Water resources development policies, environmental management and human health. Parasitol. Today 1990, 6, 173–174. [Google Scholar] [CrossRef]
- Hunter, J.M.; Rey, L.; Chu, K.Y.; Adekolu-John, L.; Mon, K.L. Parasitic Diseases in Water Resources Development. The Need for Inter Sectorial Negotiation; WHO: Genève, Switzerland, 1993; 152p. [Google Scholar]
- Atangana, S.; Charlois, M.; Foumbi, J.; Ripert, C.; Samé-Ekobo, A. Incidence des barrages sur la santé publique au Cameroun. Afr. Méd. 1980, 19, 141–149. [Google Scholar]
- Ripert, C.; Samé-Ekobo, A.; Tribouley, J.; Becker, M.; Solle, J.; Kouinche, A.; Haumont, M.; Raccurt, C. Étude épidémiologique du paludisme dans la région du futur lac de retenue de Bini (Adamaoua), Cameroun. Bull. Liais. Doc. Oceac 1991, 97, 40–44. [Google Scholar]
- Ripert, C.; Samé-Ekobo, A.; Tribouley, J.; Becker, M.; Salle, J.; Kouinche, A.; Haumont, M.; Raccurt, C. Étude épidémiologique de la bilharziose intestinale et de la nécatorose dans la région du futur lac de retenue de Bini (Adamaoua), Cameroun. Bull. Liais. Doc. Oceac 1991, 97, 62–66. [Google Scholar]
- Ripert, C.; Samé-Ekobo, A.; Roche, B.; Couprié, B. Les Foyers de Dracunculose des Monts Mandara. Distribution Géographique, Physionomie des Sites de Transmission et Techniques D’éradication; Cahiers de l’IMPM; Institut de Recherches Médicales et D’études Des Plantes Médicinales: Yaoundé, Cameroon, 1987; pp. 4–5. [Google Scholar]
- World Health Organization. World Malaria Report 2018; WHO: Genève, Switzerland, 2018; 166p, Available online: https://www.who.int/malaria/publications/world-malaria-report-2018/en/ (accessed on 23 January 2019).
- NjanNloga, A.; Vincent, R.; Toto, J.C.; Carnevale, P. Anopheles moucheti, vecteur principal du paludisme au sud-Cameroun. Bull. Liais. Doc. Oceac 1993, 26, 63–67. [Google Scholar]
- Fontenille, D.; Simard, F. Unravelling complexities in human malaria transmission dynamics in Africa through a comprehensive knowledge of vector populations. Comp. Immunol. Microbiol. Inf. Dis. 2004, 27, 357–375. [Google Scholar] [CrossRef]
- Coluzzi, M.; Sabatini, A.; Di Deco, M.A. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 483–497. [Google Scholar] [CrossRef]
- Benelli, G.; Beier, J.C. Current vector control challenges in the fight against malaria. Act. Trop. 2017, 174, 91–96. [Google Scholar] [CrossRef]
- Molyneux, D. Patterns of change in vector-borne diseases. Ann. Trop. Med. Parasitol. 1997, 91, 827–839. [Google Scholar]
- International Committee on Large Dams (ICOLD). World Register of Dams; ICOLD: Paris, France, 1998. [Google Scholar]
- Degoutte Gérard (ENGREF). Small Dams, Guidelines for Design, Construction and Monitoring. Cemagref Editions and Engref (France), with French Committee of Large Dams; Translated from French Original Book “Petits Barrages, Recommandations Pour la Conception, le Réalization et le Suivi”, 1997 Cemagref Editions and Engref (France), with French Committee of Large Dams; Coordination Gérard Degoutte: Aix-En-Provence Area, France, 2002; 173p, ISBN 2-85362-448-X. [Google Scholar]
- Keiser, J.; De Castro, M.; Maltese, M.; Bos, R.; Tanner, M.; Singer, B.; Utzinger, J. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. Am. J. Trop. Med. Hyg. 2005, 72, 392–406. [Google Scholar] [CrossRef]
- Sanchez-Ribas, J.; Parra-Henao, G.; Guimarães, A.E. Impact of dams and irrigation schemes in anopheline (Diptera: Culicidae) bionomics and malaria epidemiology. Rev. Inst. Med. Trop. Sao Paulo 2012, 54, 179–191. [Google Scholar] [CrossRef]
- Kibret, S.; Lautze, J.; McCartney, M.; Wilson, G.; Nhamo, L. Malaria impact of large dams in sub-Saharan Africa: Maps, estimates and predictions. Malar. J. 2015, 14, 339. [Google Scholar] [CrossRef]
- Jobin, W. Dams and Disease; E & FN Spon: New York, NY, USA, 1999. [Google Scholar]
- Sleigh, A.C.; Jackson, S. Dams development and health: A missed opportunity. Lancet 2001, 357, 570–571. [Google Scholar] [CrossRef]
- WHO Health Impact Assessment: First Inter-Ministerial Conference on Health and Conference in Africa, Healthy Security through Healthy Environments; WHO: Geneva, Switzerland, 2008.
- Kibret, S.; Lautze, J.; McCartney, M.; Nhamo, L.; Wilson, G.G. Malaria and large dams in sub-Saharan Africa: Future impacts in a changing climate. Malar. J. 2016, 15, 448. [Google Scholar] [CrossRef]
- Atangana, S.; Foumbi, J.; Charlois, M.; Ambroise-Thomoas, P.; Ripert, C. Epidemiological study of onchocerciasis and malaria in Bamendjin area (Cameroon) malacolgic fauna and risks of schistosomiasis introduction. Méd. Trop. 1979, 39, 537–543. [Google Scholar]
- Gillies, M.T.; Coetzee, M. A Supplement to the Anophelinae of Africa South of the Sahara; Publications of the South African Institute for Medical Research: Johannesburg, South Africa, 1987; p. 55. [Google Scholar]
- Gillies, M.T.; De Meillon, B. The Anophelinae of Africa South of the Sahara, 54th ed.; Publication of the South African Institute for Medical Research: Johannesburg, South Africa, 1968. [Google Scholar]
- Collins, F.H.; Mendez, M.A.; Razmussen, M.O.; Mehaffey, P.C.; Besansky, N.J.; Finnerty, V.A. Ribosomal RNA gene probe differentiates member species of Anopheles gambiae complex. Am. J. Trop. Med. Hyg. 1987, 37, 37–41. [Google Scholar] [CrossRef]
- Santolamazza, F.; Mancini, E.; Simard, F.; Qi, Y.; Tu, Z.; Della Torre, A. Insertion polymorphisms of SINE 200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 2008, 7, 163. [Google Scholar] [CrossRef]
- Burkot, T.R.; Williams, J.L.; Schneider, J. Identification of Plasmodium falciparum infected mosquitoes by a double antibody enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 1984, 33, 783–788. [Google Scholar] [CrossRef]
- Wirtz, R.A.; Burkot, T.R.; Graves, P.M.; Andre, R.G. Field evaluation of enzyme-linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J. Med. Entomol. 1987, 24, 433–437. [Google Scholar] [CrossRef]
- Hervy, J.F.; Le Goff, G.; Geoffroy, B.; Herve, J.P.; Manga, L.; Brunhes, J. Les Anophèles de la Région Afro Tropicale; IRD Orstom: Paris, France, 1998.
- Awono-Ambene, H.P.; Kengne, P.; Simard, F.; Antonio-Nkondjio, C.; Fontenille, D. Description and bionomics of Anopheles (Cellia) ovengensis (Diptera: Culicidae), a new malaria vector species of the Anopheles nili group from south Cameroon. J. Med. Entomol. 2004, 41, 561–568. [Google Scholar] [CrossRef]
- van der Hoek, W.; Amerasinghe, F.P.; Konradsen, F.; Amerasinghe, P.H. Characteristics of malaria vector breeding habitats in Sri Lanka: Relevance for environmental management. Southeast Asian J. Trop. Med. Pub. Health 1998, 29, 168–172. [Google Scholar]
- Mouchet, J.; Gariou, J. Anopheles moucheti au Cameroun. Cah. ORSTOM Sér. Entomolméd. Parasitol. 1966, 4, 71–81. [Google Scholar]
- Antonio-Nkondjio, C.; Simard, F.; Awono-Ambene, P.; Ngassam, P.; Toto, J.; Tchuinkam, T.; Fontenille, D. Malaria vectors and urbanization in the equatorial forest region of south Cameroon. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 347–354. [Google Scholar] [CrossRef]
- Ijumba, J.N.; Shenton, F.C.; Clarke, S.E.; Mosha, F.W.; Lindsay, S.W. Irrigated crop production is associated with less malaria than traditional agricultural practices in Tanzania. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 476–480. [Google Scholar] [CrossRef]
- Adam, J.P. Note faunistique et biologique sur les anophèles de la région de Yaoundé et la transmission du paludisme en zone forestière du sud Cameroun. Bull. Soc. Pathol. Exot. 1956, 49, 210–220. [Google Scholar]
- Antonio-Nkondjio, C.; Awono-Ambene, H.P.; Toto, J.C.; Meunier, J.Y.; Zebaze-Kemleu, S.; Nyambam, R.; Wondji, C.S.; Tchuinkam, T.; Fontenille, D. High malaria transmission intensity in a village close to Yaounde, the capital city of Cameroon. J. Med. Entomol. 2002, 39, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Manga, L.; Toto, J.C.; Carnevale, P. Malaria vectors and transmission in an area deforested for a new international airport in southern Cameroon. Ann. Soc. Bel. Med. Trop. 1995, 75, 43–49. [Google Scholar]
- Languillon, J.; Mouchet, J.; Rivola, E.; Rateau, J. Contribution à l’étude de l’épidémiologie du paludisme dans la région forestière du Sud-Cameroun. Méd. Trop. 1956, 16, 247–379. [Google Scholar]
- Antonio-Nkondjio, C.; Ndo, C.; Costantini, C.; Awono-Ambene, H.; Fontenille, D.; Simard, F. Distribution and larval habitat characterization of Anopheles nili and An. moucheti along river networks in south Cameroon. Acta Trop. 2009, 112, 270–276. [Google Scholar] [CrossRef]
- Kyalo, D.; Amratia, P.; Mundia, C.W.; Mbogo, C.M.; Coetzee, M.; Snow, R.W. A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898–2016. Wellcome Open Res. 2017, 2, 57. [Google Scholar] [CrossRef]
- Ayala, D.; Costantini, C.; Ose, K.; Kamdem, G.C.; Antonio-Nkondjio, C.; Agbor, J.P.; Awono-Ambene, P.; Fontenille, D.; Simard, F. Habitat suitability and ecological niche profile of major malaria vectors in Cameroon. Malar. J. 2009, 8, 307. [Google Scholar] [CrossRef]
- Pajot, F.X.; Serges, L.G. Notes sur Ia biologie d’Anopheles hargreavesi (Evans), 1927, et d’Anopeles paludis de Yaounde (Cameroun), le long du fleuve Nyong. Cah. ORSTOM Ent. Med. Parasitol. 1964, 2, 3–15. [Google Scholar]
- Karch, S.; Mouchet, J. Anopheles paludis: Vecteur important du paludisme au Zaïre. Bull. Soc. Pathol. Exot. 1992, 85, 388–389. [Google Scholar]
- Boussès, P.; Granouillac, B. Les Anophèles de la Région Afro Tropicale. 2011. Available online: http://bioinfo-web.mpl.ird.fr/identiciels/anopheles/html/index.html (accessed on 23 January 2019).
- Fondjo, E.; Robert, V.; Le Goff, G.; Toto, J.C.; Carnevale, P. Le paludisme urbain à Yaoundé (Cameroun). 2 — Etude entomologique dans deux quartiers peu urbanisés. Bull. Soc. Pathol. Exot. 1992, 85, 57–63. [Google Scholar]
- Manga, L.; Robert, V.; Messi, J.; Desfontaine, M.; Carnevale, P. Le paludisme urbain à Yaoundé, Cameroun. Etude entomologique dans deux quartiers centraux. Mém. Soc. Belge Ent. 1992, 35, 155–162. [Google Scholar]
- Robert, V.; Gazin, P.; Ouedraogo, V.; Carnevale, P. Le paludisme urbain à Bobo-Dioulasso (Burkina Faso). Etude entomologique de la transmission. Cah. ORSTOM Ser. Ent. Med. Parasitol. 1986, 24, 121–128. [Google Scholar]
- Trape, J.F.; Zoulani, A. Malaria and urbanization in Central Africa: The example of Brazzaville. Part II: Results of entomological surveys and epidemiological analysis. Trans. R. Soc. Trop. Med. Hyg. 1987, 81 (Suppl. 2), 10–18. [Google Scholar] [CrossRef]
- Wondji, C.; Simard, F.; Petrarca, V.; Etang, J.; Santolamazza, F.; Torre, A.D.; Fontenille, D. Species and Populations of the Anopheles gambiae Complex in Cameroon with Special Emphasis on Chromosomal and Molecular Forms of Anopheles gambiae s.s. J. Med. Entomol. 2005, 42, 998–1005. [Google Scholar] [CrossRef]
- Kamdem, C.; Tene Fossog, B.; Simard, F.; Etouna, J.; Ndo, C.; Kengne, P.; Bousses, P.; Etoa, F.X.; Awono-Ambene, P.; Fontenille, D.; et al. Anthropogenic Habitat Disturbance and Ecological Divergence between Incipient Species of the Malaria Mosquito Anopheles gambiae. PLoS ONE 2012, 7, e39453. [Google Scholar] [CrossRef] [PubMed]
- Kudom, A.A. Larval ecology of Anopheles coluzzii in Cape Coast, Ghana: Water quality, nature of habitat and implication for larval control. Malar. J. 2015, 14, 447. [Google Scholar] [CrossRef] [PubMed]
- Tabue, R.N.; Nem, T.; Atangana, J.; Bigoga, J.D.; Patchoke, S.; Tchouine, F. Anopheles ziemanni a locally important malaria vector in Ndop health district, North West region of Cameroon. Parasit. Vectors 2014, 7, 262. [Google Scholar] [CrossRef]

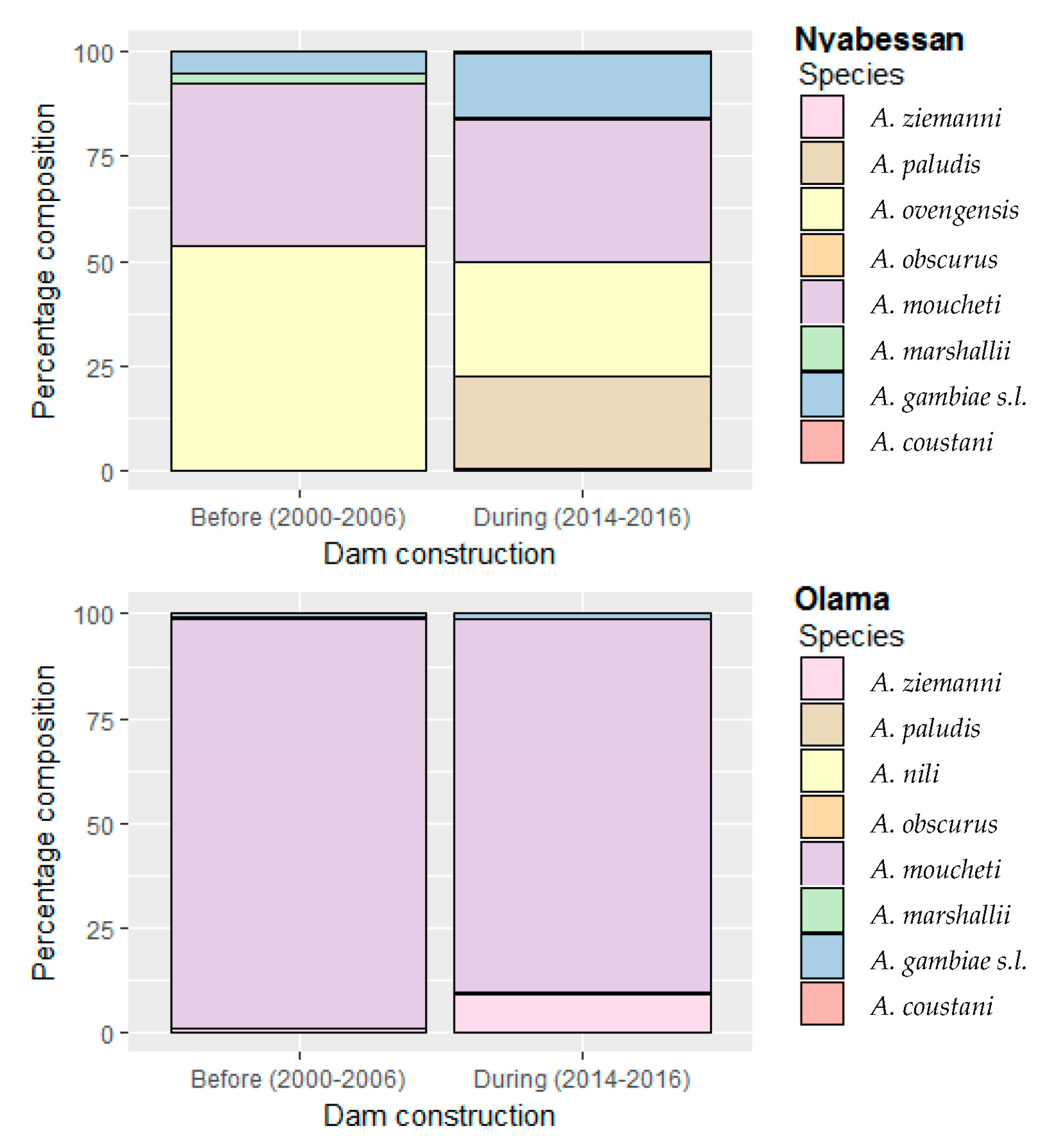

| Study Site | Species Distribution before Dam Construction | Species Distribution during Dam Construction | p-Value of 2-Sample Chi-Square Test for Equality of Proportions |
|---|---|---|---|
| 2000–2006 | 2014–2016 | ||
| Nyabessan | |||
| Nb. pnc. | 53 | 56 | |
| A. gambiae s.l. | 190 (5.1) | 358 (15.0) | <0.0001 |
| A. moucheti | 1455 (38.9) | 817 (34.2) | 0.0002 |
| A. ovengensis | 1992 (53.3) | 651 (27.2) | <0.0001 |
| A. paludis | 4 (0.1) | 524 (21.9) | <0.0001 |
| A. ziemanni | 0 | 11 (0.5) | 0.0001 |
| A. marshallii | 97 (2.6) | 14 (0.6) | <0.0001 |
| A. coustani | 0 | 13 (0.5) | <0.0001 |
| A. obscurus | 0 | 1 (0.04) | 0.8214 |
| Total | 3738 (100) | 2389 (100) | |
| Olama | |||
| Nb. pnc | 70 | 36 | |
| A. gambiae s.l. | 45 (0.9) | 10 (1.1) | 0.6503 |
| A. moucheti | 5037 (97.9) | 814 (89.0) | <0.0001 |
| A. nili | 10 (0.2) | 1 (0.1) | 0.8925 |
| A. paludis | 5 (0.1) | 4 (0.4) | 0.0460 |
| A. ziemanni | 41 (0.8) | 83 (9.1) | <0.0001 |
| A. marshallii | 9 (0.2) | 3 (0.3) | 0.5783 |
| Total | 5147 (100) | 915 (100) |
| Variable | Sum Sq | Df | F Value | Pr (>F) |
|---|---|---|---|---|
| Period | 807.14 | 1 | 65.99 | <0.0000 * |
| Location | 29.12 | 1 | 2.38 | 0.1254 |
| Species | 551.60 | 3 | 15.03 | <0.0000 * |
| Position | 5.96 | 1 | 0.49 | 0.4864 |
| Hour | 182.87 | 4 | 3.74 | 0.0066 * |
| Period:Location | 11.02 | 1 | 0.90 | 0.3444 |
| Period:Species | 306.99 | 2 | 12.55 | <0.0000 * |
| Period:Position | 77.74 | 1 | 6.36 | 0.0130 * |
| Location:Position | 18.66 | 1 | 1.53 | 0.2191 |
| Species:Position | 103.11 | 3 | 2.81 | 0.0422 * |
| Period:Hour | 36.34 | 4 | 0.74 | 0.5645 |
| Location:Hour | 121.40 | 4 | 2.48 | 0.0472 * |
| Species:Hour | 197.81 | 12 | 1.35 | 0.2003 |
| Position:Hour | 57.92 | 4 | 1.18 | 0.3212 |
| Period:Location:Position | 47.27 | 1 | 3.86 | 0.0515 |
| Period:Species:Position | 12.06 | 2 | 0.49 | 0.6119 |
| Period:Location:Hour | 101.69 | 4 | 2.08 | 0.0875 |
| Period:Species:Hour | 107.66 | 8 | 1.10 | 0.3675 |
| Period:Position:Hour | 54.23 | 4 | 1.11 | 0.3556 |
| Location:Position:Hour | 20.33 | 4 | 0.42 | 0.7972 |
| Species:Position:Hour | 97.86 | 12 | 0.67 | 0.7803 |
| Period:Location:Position:Hour | 12.25 | 4 | 0.25 | 0.9090 |
| Period:Species:Position:Hour | 42.31 | 8 | 0.43 | 0.8997 |
| Factor | Estimate | Std. Error | t Value | p-Value |
|---|---|---|---|---|
| Intercept | 2.5602 | 0.9717 | 2.635 | 0.0091 * |
| Hour (ref = 07–09 p.m.) | ||||
| 09–11 p.m. | 1.5566 | 0.9024 | 1.725 | 0.0860. |
| 11 p.m.–01 a.m. | 2.7241 | 0.9024 | 3.019 | 0.0029 * |
| 01–03 a.m. | 2.2428 | 0.9024 | 2.485 | 0.0137 * |
| 03–05 a.m. | 1.4813 | 0.9024 | 1.642 | 0.1022 |
| Species (ref = A. gambae s.l.) | ||||
| A. moucheti | 5.4189 | 0.7578 | 7.151 | <0.0001 * |
| A. ovengensis | 2.9990 | 0.8821 | 3.400 | 0.0008 * |
| A. paludis | 3.0243 | 1.0094 | 2.996 | 0.0031 * |
| A. marshallii | −2.682 | 7.699 | −0.348 | 0.7289 |
| Variable | Sum Sq | Df | F Value | Pr (>F) |
|---|---|---|---|---|
| Period | 17.48 | 1 | 1.9789 | 0.16785 |
| Species | 114.85 | 6 | 2.1667 | 0.06856 |
| Location | 2.46 | 1 | 0.2787 | 0.60073 |
| Period:Species | 37.20 | 6 | 0.7017 | 0.64996 |
| Period:Location | 1.04 | 1 | 0.1177 | 0.73350 |
| Species:Location | 4.66 | 4 | 0.1317 | 0.96978 |
| Period:Species:Location | 12.05 | 3 | 0.4545 | 0.71569 |
| Variable | Sum Sq | Df | F Value | Pr (>F) |
|---|---|---|---|---|
| Dam_Construction | 0.856 | 1 | 4.056 | 0.0636 |
| Species | 2.484 | 5 | 2.355 | 0.0948 |
| Location | 0.864 | 1 | 4.095 | 0.0625 |
| Dam_Construction:Species | 0.079 | 2 | 0.188 | 0.8311 |
| Dam_Construction:Location | 0.652 | 1 | 3.092 | 0.1005 |
| Species:Location | 1.301 | 2 | 3.083 | 0.0777 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbakop, L.R.; Awono-Ambene, P.H.; Mandeng, S.E.; Ekoko, W.E.; Fesuh, B.N.; Antonio-Nkondjio, C.; Toto, J.-C.; Nwane, P.; Fomena, A.; Etang, J. Malaria Transmission around the Memve’ele Hydroelectric Dam in South Cameroon: A Combined Retrospective and Prospective Study, 2000–2016. Int. J. Environ. Res. Public Health 2019, 16, 1618. https://doi.org/10.3390/ijerph16091618
Mbakop LR, Awono-Ambene PH, Mandeng SE, Ekoko WE, Fesuh BN, Antonio-Nkondjio C, Toto J-C, Nwane P, Fomena A, Etang J. Malaria Transmission around the Memve’ele Hydroelectric Dam in South Cameroon: A Combined Retrospective and Prospective Study, 2000–2016. International Journal of Environmental Research and Public Health. 2019; 16(9):1618. https://doi.org/10.3390/ijerph16091618
Chicago/Turabian StyleMbakop, Lili R., Parfait H. Awono-Ambene, Stanislas E. Mandeng, Wolfgang E. Ekoko, Betrand N. Fesuh, Christophe Antonio-Nkondjio, Jean-Claude Toto, Philippe Nwane, Abraham Fomena, and Josiane Etang. 2019. "Malaria Transmission around the Memve’ele Hydroelectric Dam in South Cameroon: A Combined Retrospective and Prospective Study, 2000–2016" International Journal of Environmental Research and Public Health 16, no. 9: 1618. https://doi.org/10.3390/ijerph16091618
APA StyleMbakop, L. R., Awono-Ambene, P. H., Mandeng, S. E., Ekoko, W. E., Fesuh, B. N., Antonio-Nkondjio, C., Toto, J.-C., Nwane, P., Fomena, A., & Etang, J. (2019). Malaria Transmission around the Memve’ele Hydroelectric Dam in South Cameroon: A Combined Retrospective and Prospective Study, 2000–2016. International Journal of Environmental Research and Public Health, 16(9), 1618. https://doi.org/10.3390/ijerph16091618







