Molecular Study of Thyroid Cancer in World Trade Center Responders
Abstract
1. Introduction
2. Methods
2.1. Selection and Enrollment of Study Participants
2.2. Assessment of Clinical Characteristics
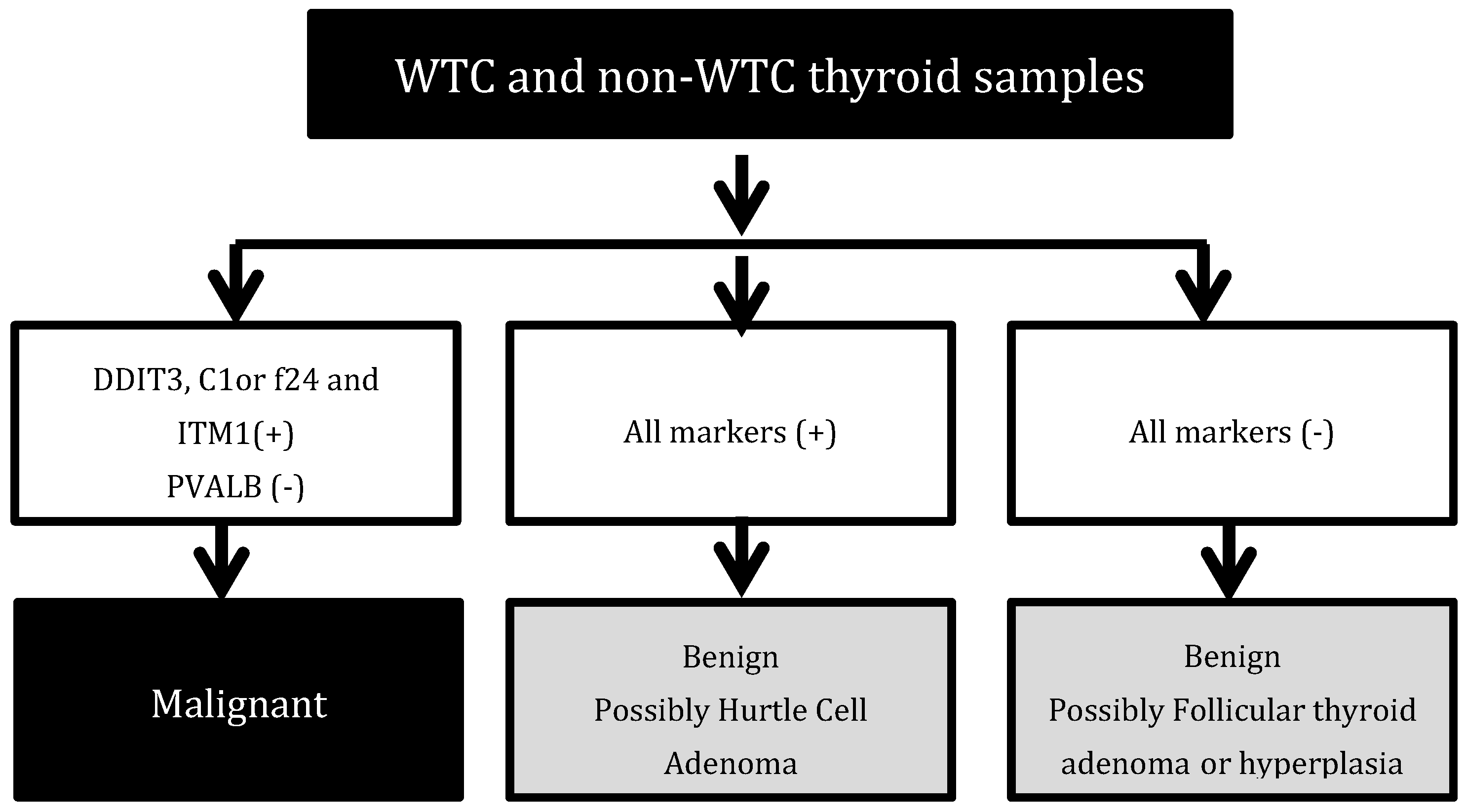
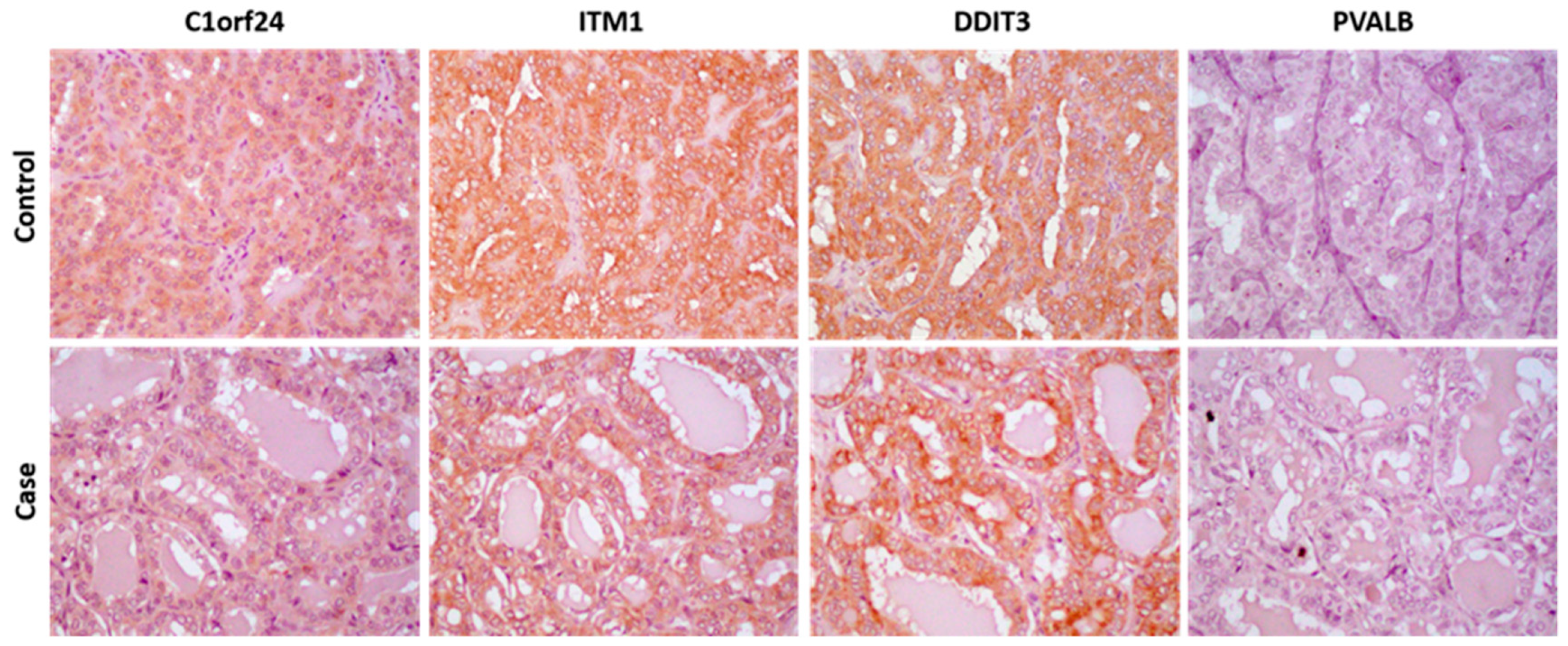
2.3. Immunohistochemistry Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solan, S.; Wallenstein, S.; Shapiro, M.; Teitelbaum, S.L.; Stevenson, L.; Kochman, A.; Kaplan, J.; Dellenbaugh, C.; Kahn, A.; Biro, F.N.; et al. Cancer Incidence in World Trade Center Rescue and Recovery Workers, 2001–2008. Environ. Health Perspect. 2013, 121, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Zeig-Owens, R.; Webber, M.P.; Hall, C.B.; Schwartz, T.; Jaber, N.; Weakley, J.; Rohan, T.E.; Cohen, H.W.; Derman, O.; Aldrich, T.K.; et al. Early assessment of cancer outcomes in New York City firefighters after the 9/11 attacks: An observational cohort study. Lancet 2011, 378, 898–905. [Google Scholar] [CrossRef]
- Jordan, H.T.; Brackbill, R.M.; Cone, J.E.; Debchoudhury, I.; Farfel, M.R.; Greene, C.M.; Hadler, J.L.; Kennedy, J.; Li, J.; Liff, J.; et al. Mortality among survivors of the Sept 11, 2001, World Trade Center disaster: Results from the World Trade Center Health Registry cohort. Lancet 2011, 378, 879–887. [Google Scholar] [CrossRef]
- Lioy, P.J.; Georgopoulos, P. The Anatomy of the Exposures That Occurred around the World Trade Center Site. Ann. N. Y. Acad. Sci. 2006, 1076, 54–79. [Google Scholar] [CrossRef] [PubMed]
- Siemiatycki, J.; Richardson, L.; Straif, K.; Latreille, B.; Lakhani, R.; Campbell, S.; Rousseau, M.-C.; Boffetta, P. Listing Occupational Carcinogens. Environ. Health Perspect. 2004, 112, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Welch, H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Brawley, O.W. Some Thoughts on Exposure to the World Trade Center Wreckage and Cancer. JAMA Oncol. 2018, 4, 775–776. [Google Scholar] [CrossRef]
- Swensen, S.J.; Jett, J.R.; Hartman, T.E.; Midthun, D.E.; Sloan, J.A.; Sykes, A.-M.; Aughenbaugh, G.L.; Clemens, M.A. Lung Cancer Screening with CT: Mayo Clinic Experience. Radiology 2003, 226, 756–761. [Google Scholar] [CrossRef]
- Wisnivesky, J.P.; Teitelbaum, S.L.; Todd, A.C.; Boffetta, P.; Crane, M.; Crowley, L.; de la Hoz, R.E.; Dellenbaugh, C.; Harrison, D.; Herbert, R.; et al. Persistence of multiple illnesses in World Trade Center rescue and recovery workers: A cohort study. Lancet 2011, 378, 888–897. [Google Scholar] [CrossRef]
- Saposnik, G.; Redelmeier, D.; Ruff, C.C.; Tobler, P.N. Cognitive biases associated with medical decisions: A systematic review. BMC Med. Inform. Decis. Mak. 2016, 16, 138. [Google Scholar] [CrossRef]
- Herbert, R.; Moline, J.; Skloot, G.; Metzger, K.; Baron, S.; Luft, B.; Markowitz, S.; Udasin, I.; Harrison, D.; Stein, D.; et al. The World Trade Center disaster and the health of workers: Five-year assessment of a unique medical screening program. Environ. Health Perspect. 2006, 114, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Lieberman-Cribbin, W.; Tuminello, S.; Gillezeau, C.; van Gerwen, M.; Brody, R.; Donovan, M.; Taioli, E. The development of a Biobank of cancer tissue samples from World Trade Center responders. J. Transl. Med. 2018, 16, 280. [Google Scholar] [CrossRef] [PubMed]
- Topliss, D. Thyroid incidentaloma: The ignorant in pursuit of the impalpable. Clin. Endocrinol. 2004, 60, 18–20. [Google Scholar] [CrossRef]
- Cerutti, J.M.; Delcelo, R.; Amadei, M.J.; Nakabashi, C.; Maciel, R.M.B.; Peterson, B.; Shoemaker, J.; Riggins, G.J. A preoperative diagnostic test that distinguishes benign from malignant thyroid carcinoma based on gene expression. J. Clin. Investig. 2004, 113, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, J.M.; Latini, F.R.M.; Nakabashi, C.; Delcelo, R.; Andrade, V.P.; Amadei, M.J.; Maciel, R.M.B.; Hojaij, F.C.; Hollis, D.; Shoemaker, J.; et al. Diagnosis of suspicious thyroid nodules using four protein biomarkers. Clin. Cancer Res. 2006, 12, 3311–3318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cerutti, J.M.; Oler, G.; Delcelo, R.; Gerardt, R.; Michaluart, P.; de Souza, S.J.; Galante, P.A.F.; Huang, P.; Riggins, G.J. PVALB, a new Hürthle adenoma diagnostic marker identified through gene expression. J. Clin. Endocrinol. Metab. 2011, 96, E151–E160. [Google Scholar] [CrossRef] [PubMed]
- Tuminello, S.; van Gerwen, M.A.G.; Genden, E.; Crane, M.; Lieberman-Cribbin, W.; Taioli, E. Increased incidence of thyroid cancer among World Trade Center first responders: A descriptive epidemiological assessment. Int. J. Environ. Res. Public Health 2019, 16, 1258. [Google Scholar] [CrossRef] [PubMed]
- Cardis, E.; Kesminiene, A.; Ivanov, V.; Malakhova, I.; Shibata, Y.; Khrouch, V.; Drozdovitch, V.; Maceika, E.; Zvonova, I.; Vlassov, O.; et al. Risk of thyroid cancer after exposure to 131I in childhood. J. Natl. Cancer Inst. 2005, 18, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bishop, J.; Shan, Y.; Pai, S.; Liu, D.; Murugan, A.K.; Sun, H.; El-Naggar, A.K.; Xing, M. Highly prevalent TERT promoter mutations in aggressive thyroid tumors. Endocr. Relat. Cancer 2013, 20, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, R.; Xing, M. A six-type genetic prognostic model for papillary thyroid cancer. Endocr. Relat. Cancer 2017, 24, 41–52. [Google Scholar] [CrossRef] [PubMed]
| Clinical–Pathological Features | WTC Thyroid Cancer Cases | Non-WTC Controls | |||
|---|---|---|---|---|---|
| Eligible (n = 43) | Included (n = 30) | p Value a | Included (n = 30) | p Value b | |
| Age at Diagnosis (years) | 48.5 (SD 7.7) | 49.3 (SD 8.6) | 0.65 | 47.8 (SD 11.3) | 0.5652 |
| Gender | 0.16 | 1.00 | |||
| Male | 36 (83.7%) | 21 (70%) | 21 (70%) | ||
| Female | 7 (16.3%) | 9 (30%) | 9 (30%) | ||
| Histology | 0.74 | 1.00 | |||
| Papillary thyroid carcinoma | 27 (62.8%) | 21 (70%) | 21 (70%) | ||
| Papillary thyroid carcinoma, follicular variant | 12 (27.9%) | 6 (20%) | 6 (20%) | ||
| Other | 4 (9.3%) | 3 (10%) | 3 (10%) | ||
| Tumor Sizec (cm) | 1.38 (SD 1.16) | 1.46 (SD 0.93) | 0.78 | ||
| Microcarcinoma | |||||
| Yesc | 14 (51.85%) | 12 (40.0%) | 0.37 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Gerwen, M.A.G.; Tuminello, S.; Riggins, G.J.; Mendes, T.B.; Donovan, M.; Benn, E.K.T.; Genden, E.; Cerutti, J.M.; Taioli, E. Molecular Study of Thyroid Cancer in World Trade Center Responders. Int. J. Environ. Res. Public Health 2019, 16, 1600. https://doi.org/10.3390/ijerph16091600
van Gerwen MAG, Tuminello S, Riggins GJ, Mendes TB, Donovan M, Benn EKT, Genden E, Cerutti JM, Taioli E. Molecular Study of Thyroid Cancer in World Trade Center Responders. International Journal of Environmental Research and Public Health. 2019; 16(9):1600. https://doi.org/10.3390/ijerph16091600
Chicago/Turabian Stylevan Gerwen, Maaike A. G., Stephanie Tuminello, Gregory J. Riggins, Thais B. Mendes, Michael Donovan, Emma K.T. Benn, Eric Genden, Janete M. Cerutti, and Emanuela Taioli. 2019. "Molecular Study of Thyroid Cancer in World Trade Center Responders" International Journal of Environmental Research and Public Health 16, no. 9: 1600. https://doi.org/10.3390/ijerph16091600
APA Stylevan Gerwen, M. A. G., Tuminello, S., Riggins, G. J., Mendes, T. B., Donovan, M., Benn, E. K. T., Genden, E., Cerutti, J. M., & Taioli, E. (2019). Molecular Study of Thyroid Cancer in World Trade Center Responders. International Journal of Environmental Research and Public Health, 16(9), 1600. https://doi.org/10.3390/ijerph16091600






