Anti-Blastocystis Activity In Vitro of Egyptian Herbal Extracts (Family: Asteraceae) with Emphasis on Artemisia judaica
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Faecal Samples
2.2. Cultivation of Blastocystis spp. In Vitro
2.3. Molecular Identification of Blastocystis spp. Subtypes
2.4. Collection of Plant Materials
2.5. Plants Extraction
2.6. Use of Metronidazole as a Reference Anti-Protozoal Drug
2.7. In Vitro Exposure of Blastocystis spp. to Egyptian Herbal Extracts
2.8. Counting the Treated Blastocystis in Suspensions to Examine the Efficacy of the Herbal Extracts Tested
2.9. Fractionation of A. judaica Extract
2.10. Statistics
3. Results
3.1. Blastocystis spp. Isolates and Its Molecular Identification
3.2. Anti-Blastocystis Activity
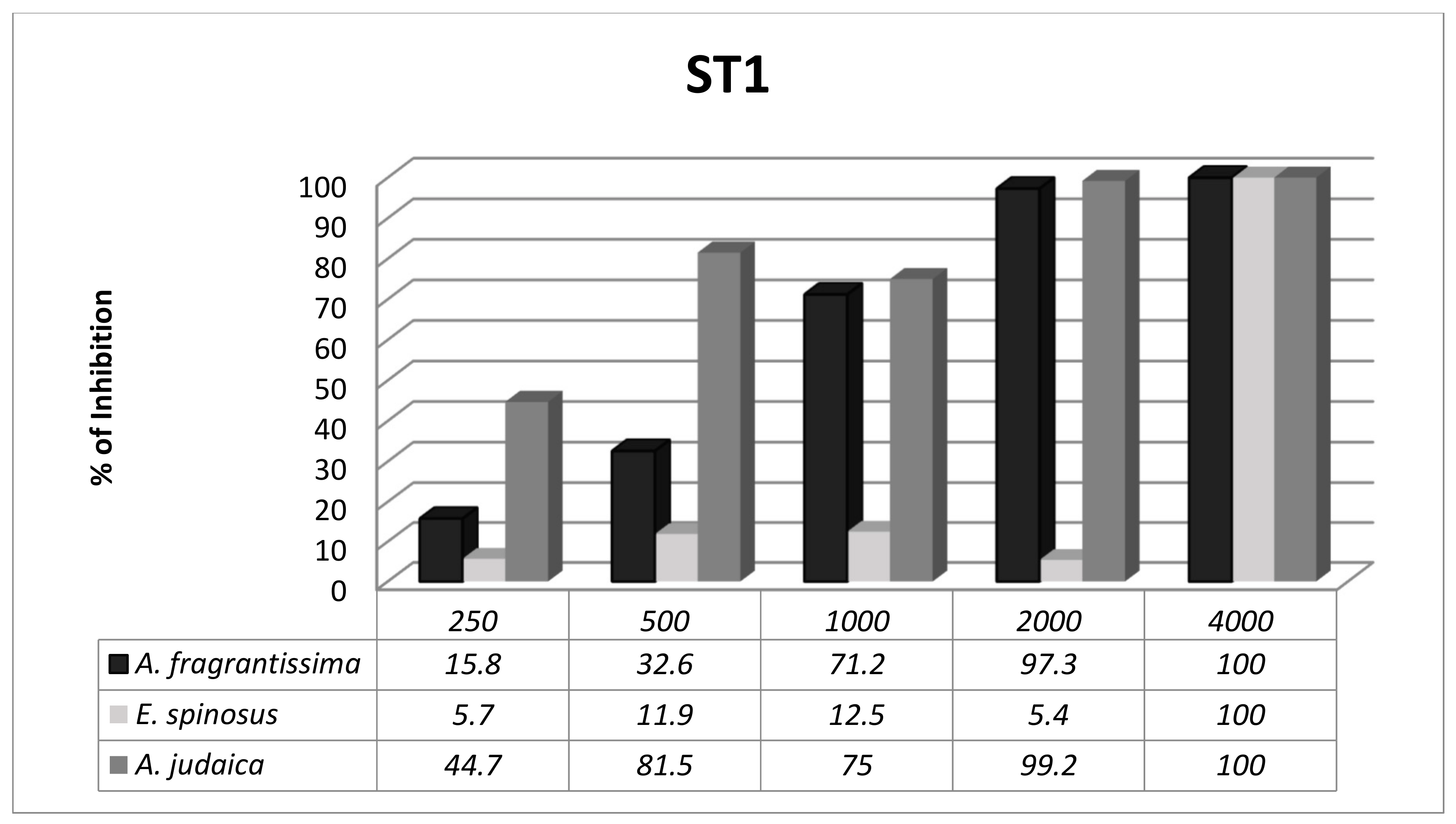
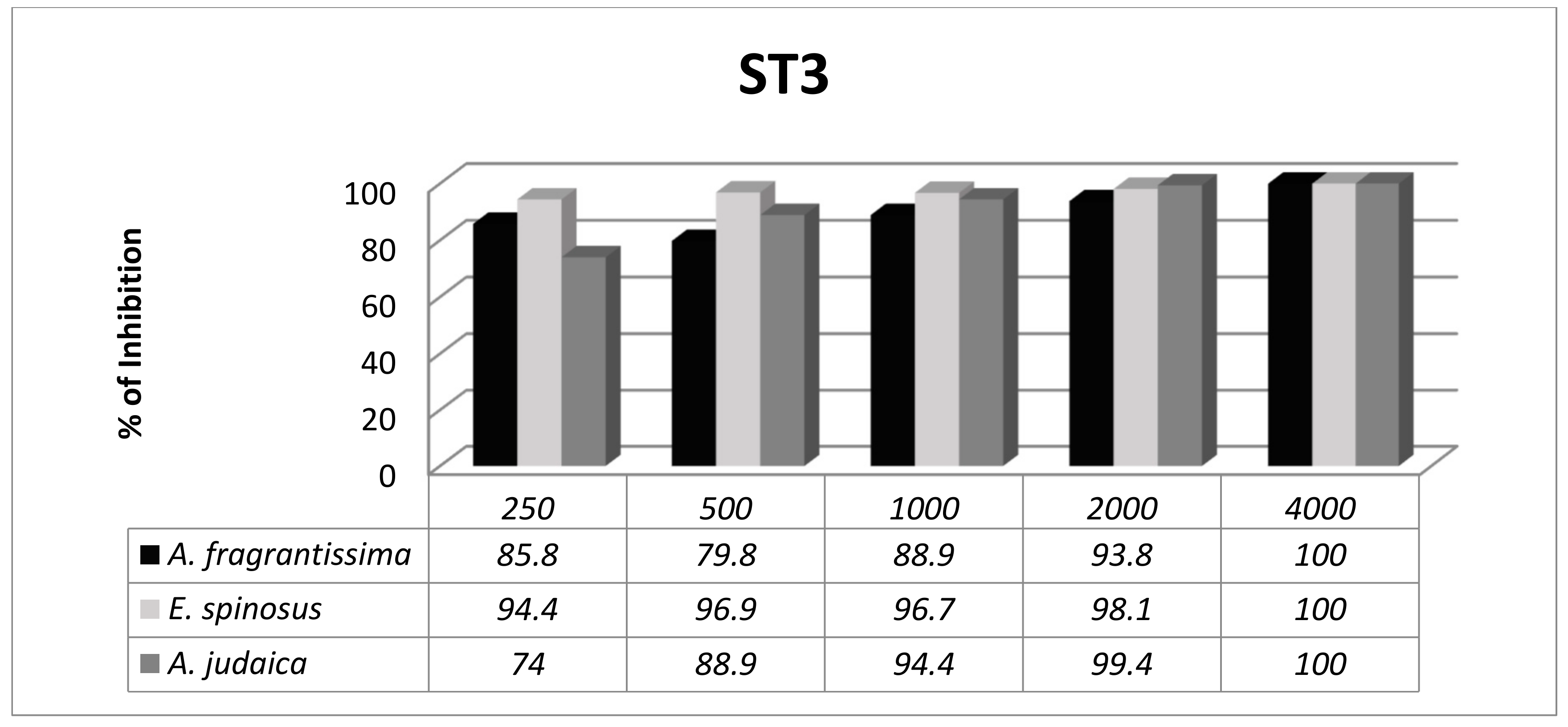
3.3. Subtype-Dependent Variations of Blastocystis
3.4. Identification of Active Fractions in A. judaica
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoshikawa, H.; Koyama, Y.; Tsuchiya, E.; Takami, K. Blastocystis phylogeny among various isolates from humans to insects. Parasitol. Int. 2016, 65, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.W. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008, 21, 639–665. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Karanis, P. Blastocystis spp., Ubiquitous Parasite of Human, animals and Environment. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–6. [Google Scholar]
- Lee, I.L.; Tan, T.C.; Govind, S.K. Establishing a protocol for water sample processing for the detection of Blastocystis sp. Exp. Parasitol. 2019, 198, 105–110. [Google Scholar] [CrossRef]
- Seyer, A.; Karasartova, D.; Ruh, E.; Güreser, A.S.; Turgal, E.; Imir, T.; Taylan-Ozkan, A. Epidemiology and prevalence of Blastocystis spp. in North Cyprus. Am. J. Trop. Med. Hyg. 2017, 96, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- El Safadi, D.; Gaayeb, L.; Meloni, D.; Cian, A.; Poirier, P.; Wawrzyniak, I.; Delbac, F.; Dabboussi, F.; Delhaes, L.; Seck, M.; et al. Children of Senegal River Basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect. Dis. 2014, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Lepczyńska, M.; Białkowska, J.; Dzika, E.; Piskorz-Ogórek, K.; Korycińska, J. Blastocystis: How do specific diets and human gut microbiota affect its development and pathogenicity? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1531–1540. [Google Scholar] [CrossRef]
- Gonzalez-Arenas, N.R.; Villalobos, G.; Vargas-Sanchez, G.B.; Avalos-Galarza, C.A.; Marquez-Valdelamar, L.M.; Ramirez-Miranda, M.E.; Olivo-Diaz, A.; Romero-Valdovinos, M.; Martinez-Hernandez, F.; Maravilla, P. Is the genetic variability of Cathepsin B important in the pathogenesis of Blastocystis spp.? Parasitol. Res. 2018, 117, 3935–3943. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C. Blastocystis: Genetic diversity and molecular methods for diagnosis and epidemiology. Trop. Parasitol. 2013, 3, 26–34. [Google Scholar] [CrossRef]
- Alfellani, M.A.; Taner-Mulla, D.; Jacob, A.S.; Imeede, C.A.; Yoshikawa, H.; Stensvold, C.R.; Clark, C.G. Genetic diversity of Blastocystis in livestock and zoo animals. Protist 2013, 164, 497–509. [Google Scholar] [CrossRef]
- Cifre, S.; Gozalbo, M.; Ortiz, V.; Soriano, J.M.; Merino, J.F.; Trelis, M. Blastocystis subtypes and their association with irritable bowel syndrome. Med. Hypotheses 2018, 116, 4–9. [Google Scholar] [CrossRef]
- Dogruman-Al, F.; Kustimur, S.; Yoshikawa, H.; Tuncer, C.; Simsek, Z.; Tanyuksel, M.; Araz, E.; Boorom, K. Blastocystis subtypes in irritable bowel syndrome and inflammatory bowel disease in Ankara, Turkey. Mem. Inst. Oswaldo Cruz 2009, 104, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Kök, M.; Çekin, Y.; Çekin, A.; Uyar, S.; Harmandar, F.; Şahintürk, Y. The role of Blastocystis hominis in the activation of ulcerative colitis. Turkish J. Gastroenterol. 2018, 30, 40–46. [Google Scholar]
- Lepczyńska, M.; Chen, W.-C.; Dzika, E. Mysterious chronic urticaria caused by Blastocystis spp.? Int. J. Dermatol. 2016, 55, 259–266. [Google Scholar] [CrossRef]
- Micheloud, D.; Jensen, J.; Fernandez-Cruz, E.; Carbone, J. Chronic angioedema and Blastocystis hominis infection. Rev. Gastroenterol. Peru 2007, 27, 191–193. [Google Scholar] [PubMed]
- El Deeb, H.K.; Khodeer, S. Blastocystis spp.: Frequency and subtype distribution in iron deficiency anemic versus non-anemic subjects from Egypt. J. Parasitol. 2013, 99, 599–602. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, L.B.E.D.; El-Faramawy, A.A.M.; El-Sayed, N.M.; Ismail, K.A.; Fouad, S.M. Intestinal parasitic infection among Egyptian children with chronic liver diseases. J. Parasit. Dis. 2015, 39, 7–12. [Google Scholar] [CrossRef]
- Tejera, B.; Grados, D.; Martinez-Morillo, M.; Roure, S. Artritis reactiva por Blastocistys hominis. Reumatol. Clínica 2012, 8, 50–51. [Google Scholar] [CrossRef]
- Esteghamati, A.; Khanaliha, K.; Bokharaei-Salim, F.; Sayyahfar, S.; Ghaderipour, M. Prevalence of intestinal parasitic infection in cancer, organ transplant and primary immunodeficiency patients in Tehran, Iran. Asian Pacific J. Cancer Prev. 2019, 20, 495–501. [Google Scholar] [CrossRef]
- Bednarska, M.; Jankowska, I.; Pawelas, A.; Piwczyńska, K.; Bajer, A.; Wolska-Kuśnierz, B.; Wielopolska, M.; Welc-Falęciak, R. Prevalence of Cryptosporidium, Blastocystis, and other opportunistic infections in patients with primary and acquired immunodeficiency. Parasitol. Res. 2018, 117, 2869–2879. [Google Scholar] [CrossRef]
- Rajamanikam, A.; Hooi, H.S.; Kudva, M.; Samudi, C.; Kumar, S. Resistance towards metronidazole in Blastocystis sp.: A pathogenic consequence. PLoS ONE 2019, 14, e0212542. [Google Scholar] [CrossRef]
- Rajamanikam, A.; Kumar, S.; Samudi, C.; Kudva, M. Exacerbated symptoms in Blastocystis sp.-infected patients treated with metronidazole: Two case studies. Parasitol. Res. 2018, 117, 2585–2590. [Google Scholar] [CrossRef]
- Panda, S.K.; Luyten, W. Antiparasitic activity in Asteraceae with special attention to ethnobotanical use by the tribes of Odisha, India. Parasite 2018, 25, 10. [Google Scholar] [CrossRef]
- Hussain, A.; Hayat, M.Q.; Sahreen, S.; Ul Ain, Q.; Bokhari, S.A.I. Pharmacological promises of genus Artemisia (Asteraceae): A Review. B Life Environ. Sci. 2017, 54, 265–287. [Google Scholar]
- Abd El-Ghani, M.M.; Abdel-Khalik, K.N. Floristic diversity and phytogeography of the Gebel Elba National Park, south-east Egypt. Turk. J. Botany 2006, 30, 121–136. [Google Scholar]
- Lin, Y.-L.; Chang, C.-C.; Lee, I.-J. Review on phytochemical study of Asteraceae in Taiwan. J. Chin. Med. 2008, 19, 135–149. [Google Scholar]
- Adekenov, S.M. Sesquiterpene lactones with unusual structure. Their biogenesis and biological activity. Fitoterapia 2017, 121, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Picman, A.K. Biological activities of sesquiterpene lactones. Biochem. Syst. Ecol. 1986, 14, 255–281. [Google Scholar] [CrossRef]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene lactones from Artemisia genus: Biological activities and methods of analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef]
- Yahyaoui, A.; Khedher, O.; Rigane, G.; Salem, R.B.; Moussaoui, Y. Chemical analysis of essential oil from Echinops spinosus L. roots: Antimicrobial and antioxidant activities. Rev. Roum. Chim. 2018, 63, 199–204. [Google Scholar]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Ruedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef]
- Choucry, M.A. Chemical composition and anticancer activity of Achillea fragrantissima (Forssk.) Sch. Bip. (Asteraceae) essential oil from Egypt. J. Pharmacogn. Phyther. 2017, 9, 1–5. [Google Scholar]
- Abdel-Rahman, R.F.; Alqasoumi, S.I.; El-Desoky, A.H.; Soliman, G.A.; Paré, P.W.; Hegazy, M.-E.F. Evaluation of the anti-inflammatory, analgesic and anti-ulcerogenic potentials of Achillea fragrantissima (Forssk.). South African J. Bot. 2015, 98, 122–127. [Google Scholar] [CrossRef]
- Mustafa, E.H.; Abu Zarga, M.; Abdalla, S. Effects of cirsiliol, a flavone isolated from Achillea fragrantissima, on rat isolated ileum. Gen. Pharmacol. 1992, 23, 555–560. [Google Scholar] [CrossRef]
- Khedher, O.; Moussaou, Y.; Ben Salem, R. Solvent effects on phenolic contents and antioxidant activities of the Echinops spinosus and the Limoniastrum monopetalum. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 66–76. [Google Scholar]
- Ahmed, E.S.; Mabrouk, D.M.; Hassanane, M.M.; Khalil, W.K.B.; Hu, Y. Protective effect of Artemisia judaica against doxorubicin-induced toxicity in mice. Annu. Res. Rev. Biol. 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Nofal, S.; Mahmoud, S.; Ramadan, A.; Soliman, G.; Fawzy, R. Anti-diabetic effect of Artemisia judaica extracts. Res. J. Med. Med. Sci. 2009, 4, 42–48. [Google Scholar]
- Jones, W.R. The experimental infection of rats with Entamoeba histolytica; with a method for evaluating the anti-amoebic properties of new compounds. Ann. Trop. Med. Parasitol. 1946, 40, 130–140. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Wu, Z.; Kimata, I.; Iseki, M.; Ali, I.; Hossain, M.; Zaman, V.; Haque, R.; Takahashi, Y. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol. Res. 2004, 92, 22–29. [Google Scholar]
- Mokhtar, A.B.; El-Gayar, E.K.; Habib, E.S. In vitro anti-protozoal activity of propolis extract and cysteine proteases inhibitor (phenyl vinyl sulfone) on Blastocystis species. J. Egypt. Soc. Parasitol. 2016, 46, 261–272. [Google Scholar] [CrossRef]
- Dinleyici, E.C.; Eren, M.; Dogan, N.; Reyhanioglu, S.; Yargic, Z.A.; Vandenplas, Y. Clinical efficacy of Saccharomyces boulardii or metronidazole in symptomatic children with Blastocystis hominis infection. Parasitol. Res. 2011, 108, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.W.; Singh, M.; Yap, E.H. Recent advances in Blastocystis hominis research: Hot spots in terra incognita. Int. J. Parasitol. 2002, 32, 789–804. [Google Scholar] [CrossRef]
- Naß, J.; Efferth, T. The activity of Artemisia spp. and their constituents against trypanosomiasis. Phytomed. 2018, 47, 184–191. [Google Scholar] [CrossRef]
- Girish, S.; Kumar, S.; Aminudin, N. Tongkat Ali (Eurycoma longifolia): A possible therapeutic candidate against Blastocystis sp. Parasit. Vectors 2015, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Abdelgaleil, S.A.M.; Abbassy, M.A.; Belal, A.-S.H.; Abdel Rasoul, M.A.A. Bioactivity of two major constituents isolated from the essential oil of Artemisia judaica L. Bioresour. Technol. 2008, 99, 5947–5950. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A.; BaAbbad, R.; Balash, A.; Al-Hemdan, N.A.; Softah, A. The potential anti Helicobacter pylori and antioxidant effects of Artemisia judaica. Funct. Foods Heal. Dis. 2013, 3, 332–340. [Google Scholar] [CrossRef][Green Version]
- Özbilgin, A.; Durmuşkahya, C.; Kilimcioğlu, A.A.; Kayalar, H.; Kurt, Ö.; Tabak, T.; Östan, İ. In vitro efficacy of Quercus infectoria oliv and Achillea millefolium L. extracts against Blastocystis spp. isolates. Kafkas Univ. Vet. Fak. Derg. 2013, 19, 511–516. [Google Scholar]
- Sawangjaroen, N.; Sawangjaroen, K. The effects of extracts from anti-diarrheic Thai medicinal plants on the in vitro growth of the intestinal protozoa parasite: Blastocystis hominis. J. Ethnopharmacol. 2005, 98, 67–72. [Google Scholar] [CrossRef]
- Kolören, O.; Kolören, Z.; Şekeroğlu, Z.A.; Çolayvaz, M.; Karanis, P. Amoebicidal and amoebistatic effects of Artemisia argyi methanolic extracts on Acanthamoeba castellanii trophozoites and cysts. Acta. Parasitol. 2019, 64, 63–70. [Google Scholar] [CrossRef]
- Loo, C.S.N.; Lam, N.S.K.; Yu, D.; Su, X.; Lu, F. Artemisinin and its derivatives in treating protozoan infections beyond malaria. Pharmacol. Res. 2017, 117, 192–217. [Google Scholar] [CrossRef]
- Emami, S.A.; Zamanai Taghizadeh Rabe, S.; Ahi, A.; Mahmoudi, M. Inhibitory activity of eleven Artemisia species from Iran against Leishmania major parasites. Iran. J. Basic Med. Sci. 2012, 15, 807–811. [Google Scholar]
- Yason, J.A.; Ajjampur, S.S.R.; Tan, K.S.W. Blastocystis isolate b exhibits multiple modes of resistance against antimicrobial peptide LL-37. Infect. Immun. 2016, 84, 2220–2232. [Google Scholar] [CrossRef]
- El-Sayed, S.H.; Amer, N.; Ismail, S.; Ali, I.; Rizk, E.; Magdy, M.; El-Badry, A.A.-M. In vitro and in vivo anti-Blastocystis efficacy of olive leaf extract and bee pollen compound. Res. J. Parasitol. 2017, 12, 33–44. [Google Scholar] [CrossRef]
- Acheuk, F.; Lakhdari, W.; Abdellaoui, K.; Belaid, M.; Allouane, R.; Halouane, F. Phytochemical study and bioinsecticidal effect of the crude ethonolic extract of the algerian plant Artemisia judaica L. (Asteraceae) against the black bean aphid, Aphis fabae scop. Agric. For. 2017, 63, 95–104. [Google Scholar] [CrossRef]
- Nibret, E.; Wink, M. Volatile components of four Ethiopian Artemisia species extracts and their in vitro antitrypanosomal and cytotoxic activities. Phytomed. 2010, 17, 369–374. [Google Scholar] [CrossRef]
- Abaza, S.; Rayan, H.; Soliman, R.; Nemr, N.; Mokhtar, A. Subtype analysis of Blastocystis spp. isolates from symptomatic and asymptomatic patients in Suez Canal University Hospitals, Ismailia, Egypt. Parasitol. United J. 2014, 7, 56–67. [Google Scholar] [CrossRef]
- Yakoob, J.; Abbas, Z.; Beg, M.A.; Naz, S.; Awan, S.; Hamid, S.; Jafri, W. In vitro sensitivity of Blastocystis hominis to garlic, ginger, white cumin, and black pepper used in diet. Parasitol. Res. 2011, 109, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Mirza, H.; Teo, J.D.W.; Upcroft, J.; Tan, K.S.W. A rapid, high-throughput viability assay for Blastocystis spp. reveals metronidazole resistance and extensive subtype-dependent variations in drug susceptibilities. Antimicrob. Agents Chemother. 2011, 55, 637–648. [Google Scholar] [CrossRef]
- Wu, Z.; Mirza, H.; Tan, K.S.W. Intra-subtype variation in enteroadhesion accounts for differences in epithelial barrier disruption and is associated with metronidazole resistance in Blastocystis subtype-7. PLoS Negl. Trop. Dis. 2014, 8, e2885. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Smith, H.V.; Nagel, R.; Olsen, K.E.P.; Traub, R.J. Eradication of Blastocystis carriage with antimicrobials: Reality or delusion? J. Clin. Gastroenterol. 2010, 44, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Bremer Christensen, C.; Soelberg, J.; Stensvold, C.R.; Jäger, A.K. Activity of medicinal plants from Ghana against the parasitic gut protist Blastocystis. J. Ethnopharmacol. 2015, 174, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Mojarrab, M.; Lagzian, M.-S.; Emami, S.A.; Asili, J.; Tayarani-Najaran, Z. In vitro anti-proliferative and apoptotic activity of different fractions of Artemisia armeniaca. Rev. Bras. Farmacogn. 2013, 23, 783–788. [Google Scholar] [CrossRef]
- Sainz, P.; Cruz-Estrada, A.; Díaz, C.; González-Coloma, A. The genus Artemisia: Distribution and phytochemistry in the Iberian Peninsula and the Canary and Balearic Islands. Phytochem. Rev. 2017, 16, 1–21. [Google Scholar] [CrossRef]
- Bakr, R. Microscopical and phytochemical investigation of Egyptian Artemisia judaica L. var. Sinaitica tackholm and its free radical scavenging activity. Int. J. Pharmacogn. Phytochem. Res. 2015, 6, 698–703. [Google Scholar]
- Khafagy, S.M.; El-Din, A.A.S.; Jakupovic, J.; Zdero, C.; Bohlmann, F. Glaucolide-like sesquiterpene lactones from Artemisia judaica. Phytochem. 1988, 27, 1125–1128. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A.; Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [PubMed]
- Calixto Júnior, J.T.; de Morais, S.M.; Gomez, C.V.; Molas, C.C.; Rolon, M.; Boligon, A.A.; Athayde, M.L.; de Morais Oliveira, C.D.; Tintino, S.R.; Henrique Douglas, M.C. Phenolic composition and antiparasitic activity of plants from the Brazilian Northeast “Cerrado”. Saudi J. Biol. Sci. 2016, 23, 434–440. [Google Scholar] [CrossRef]
| Isolate No. | Age of Patient | Sex | Symptoms | Since How Long | Stool Consistency | Microscopic Examination | Molecular Subtyping |
|---|---|---|---|---|---|---|---|
| Isolate 1 | 6 years | Male | Diarrhoea | 3.5 months | Semi-formed | 1–2 Blastocystis forms/field | ST1 |
| Isolate 2 | 8 years | Female | Abdominal pain | 7 months | Formed | 4–5 Blastocystis forms/field | ST3 |
| Subtypes | STs Primer Sets | Primers | Expected PCR Size | PCR Results of This Study’s Isolates |
|---|---|---|---|---|
| ST1 | SB83 | F 5′-GAAGGACTCTCTGACGATGA-3′ R 5′-GTCCAAATGAAAGGCAGC-3′ | 351 bp | ST1 |
| ST2 | SB340 | F 5′-TGTTCTTGTGTCTTCTCAGCTC-3′ R 5′-TTCTTTCACACTCCCGTCAT-3′ | 704 bp | - |
| ST3 | SB227 | F 5′-TAGGATTTGGTGTTTGGAGA-3′ R 5′-TTAGAAGTGAAGGAGATGGAAG-3′ | 526 bp | ST3 |
| ST4 | SB337 | F 5′-GTCTTTCCCTGTCTATTCTGCA-3′ R 5′-AATTCGGTCTGCTTCTTCTG-3′ | 487 bp | - |
| ST5 | SB336 | F 5′-GTGGGTAGAGGAAGGAAAACA-3′ R 5′-AGAACAAGTCGATGAAGTGAGAT-3′ | 317 bp | - |
| ST6 | SB332 | F 5′-GCATCCAGACTACTATCAACATT-3′ R 5′-CCATTTTCAGACAACCACTTA-3′ | 338 bp | - |
| ST7 | SB155 | F 5′-ATCAGCCTACAATCTCCTC-3′ R 5′-ATCGCCACTTCTCCAAT-3′ | 650 bp | - |
| Plant Species | MIC90 of Blastocystis in Culture † | Degree of Blastocystis elimination * | ||||||
|---|---|---|---|---|---|---|---|---|
| Conc. (µg/mL) | Blastocystis ×104 cells/mL after 24 h (% Inhibition) | Blastocystis ×104 cells/mL after 48 h (% Inhibition) | Blastocystis ×104 cells/mL after 72 h (% Inhibition) | |||||
| A. fragrantissima | 250 | 51.1 | (41.7) | 15 | (80.3) | 44.5 | (48.6) | ++ |
| 500 | 46.8 | (46.7) | 13.9 | (81.8) | 39.3 | (54.7) | ++ | |
| 1000 | 22.9 | (73.9) | 13.5 | (82.3) | 17.75 | (79.4) | + | |
| 2000 | 5.9 | (93.3) | 3.8 | (95) | 3.75 | (95.6) | - | |
| 4000 | 0 | (100) | 0 | (100) | 0 | (100) | - | |
| E. spinosus | 250 | 30.9 | (64.8) | 19.3 | (74.7) | 45.6 | (47.3) | ++ |
| 500 | 33.8 | (61.5) | 18.5 | (75.8) | 41.9 | (54.7) | ++ | |
| 1000 | 23.5 | (73.2) | 8.9 | (88.2) | 41.5 | (52) | ++ | |
| 2000 | 12 | (86.3) | 29.3 | (61.6) | 44.5 | (48.6) | ++ | |
| 4000 | 0 | (100) | 0 | (100) | 0 | (100) | - | |
| A. judaica | 250 | 41 | (53.3) | 11.1 | (85.5) | 36 | (58.4) | ++ |
| 500 | 23.4 | (73.4) | 7.25 | (90.4) | 13 | (85) | + | |
| 1000 | 23.9 | (72.8) | 6 | (92.1) | 13.9 | (83.9) | + | |
| 2000 | 2.9 | (96.7) | 0.4 | (99.4) | 0.5 | (99.3) | - | |
| 4000 | 0 | (100) | 0 | (100) | 0 | (100) | - | |
| MTZ (Reference drug) | 5 | 14 | (84) | 12.5 | (83.6) | 9.5 | (89) | + |
| 10 | 2.5 | (97.2) | 7.6 | (90) | 14.75 | (82.8) | + | |
| 20 | 0.5 | (99.4) | 0 | (100) | 0 | (100) | - | |
| Fractions of A. judaica | Subtypes of Blastocystis | |||
|---|---|---|---|---|
| ST1 | ST3 | |||
| EtOAc | 100 | a | 100 | a |
| DCM | 100 | a | 100 | a |
| n-BuOH | 92.4 | c | 95 | b |
| n-hexane | 100 | a | 100 | a |
| Water-soluble fraction * | 95 | b | 94 | b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, A.B.; Ahmed, S.A.; Eltamany, E.E.; Karanis, P. Anti-Blastocystis Activity In Vitro of Egyptian Herbal Extracts (Family: Asteraceae) with Emphasis on Artemisia judaica. Int. J. Environ. Res. Public Health 2019, 16, 1555. https://doi.org/10.3390/ijerph16091555
Mokhtar AB, Ahmed SA, Eltamany EE, Karanis P. Anti-Blastocystis Activity In Vitro of Egyptian Herbal Extracts (Family: Asteraceae) with Emphasis on Artemisia judaica. International Journal of Environmental Research and Public Health. 2019; 16(9):1555. https://doi.org/10.3390/ijerph16091555
Chicago/Turabian StyleMokhtar, Amira B., Shahira A. Ahmed, Enas E. Eltamany, and Panagiotis Karanis. 2019. "Anti-Blastocystis Activity In Vitro of Egyptian Herbal Extracts (Family: Asteraceae) with Emphasis on Artemisia judaica" International Journal of Environmental Research and Public Health 16, no. 9: 1555. https://doi.org/10.3390/ijerph16091555
APA StyleMokhtar, A. B., Ahmed, S. A., Eltamany, E. E., & Karanis, P. (2019). Anti-Blastocystis Activity In Vitro of Egyptian Herbal Extracts (Family: Asteraceae) with Emphasis on Artemisia judaica. International Journal of Environmental Research and Public Health, 16(9), 1555. https://doi.org/10.3390/ijerph16091555








