Age Differences in the Relationship between Secondhand Smoke Exposure and Risk of Metabolic Syndrome: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Study Characteristics and Quality Assessment
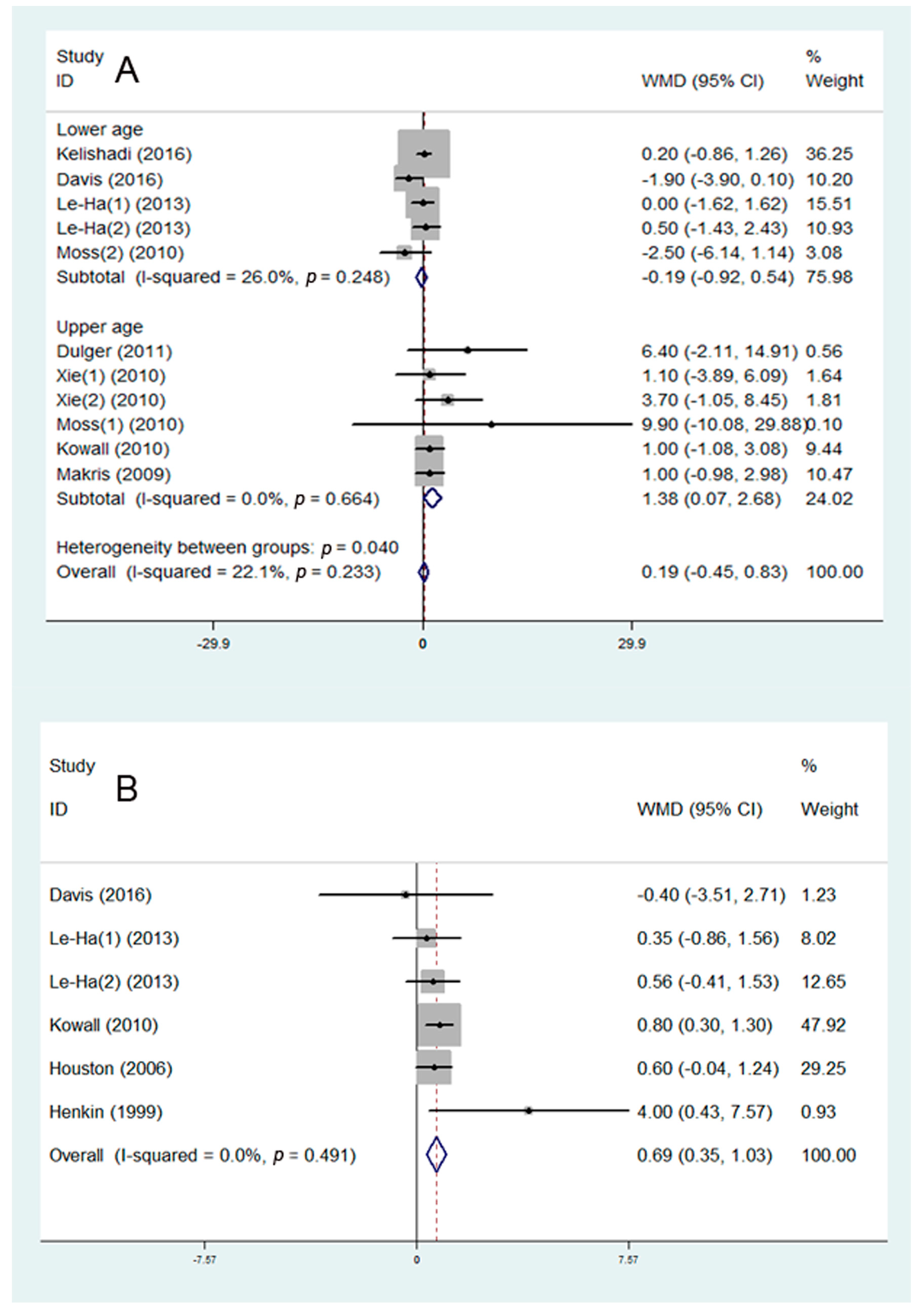
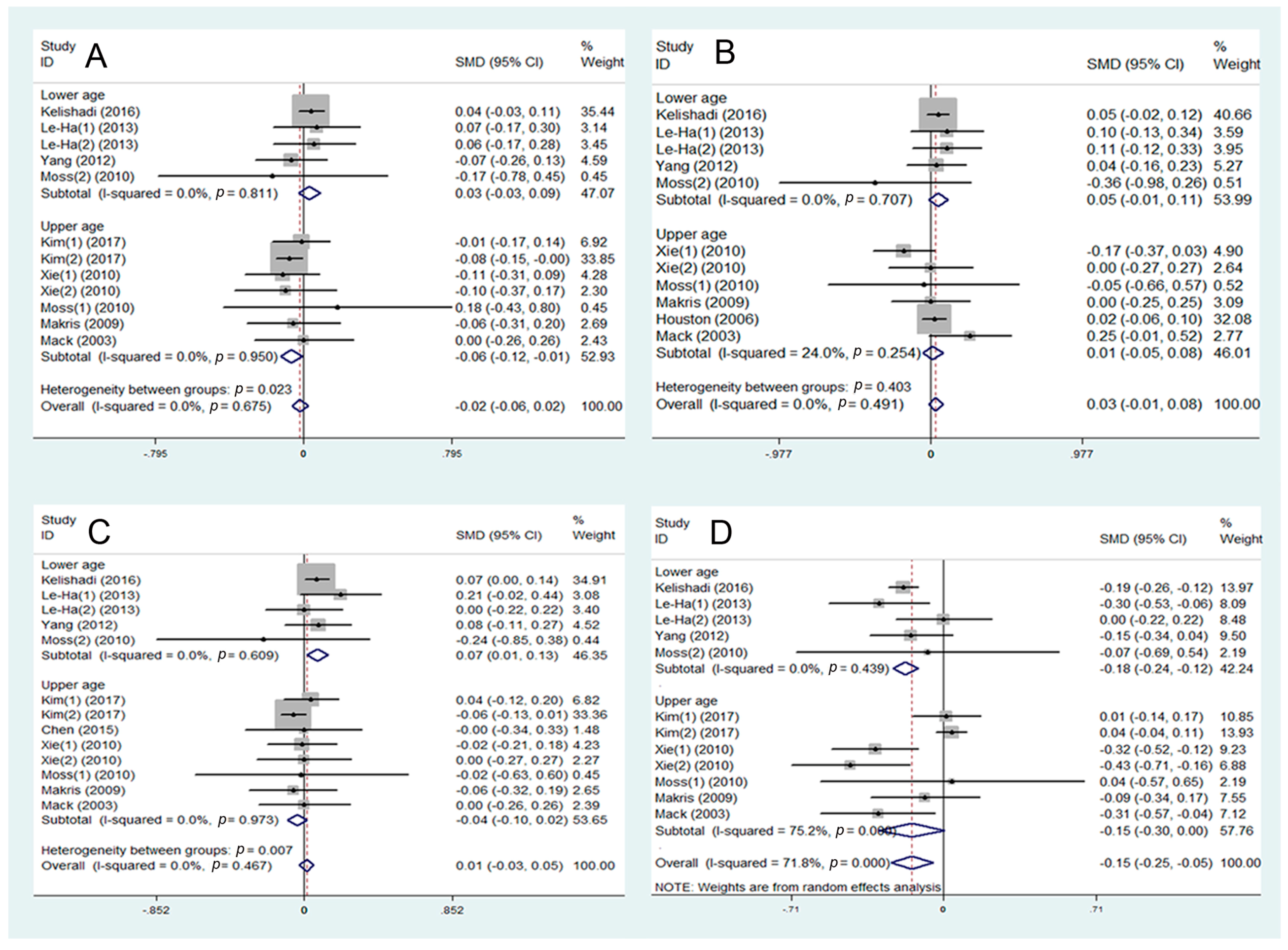
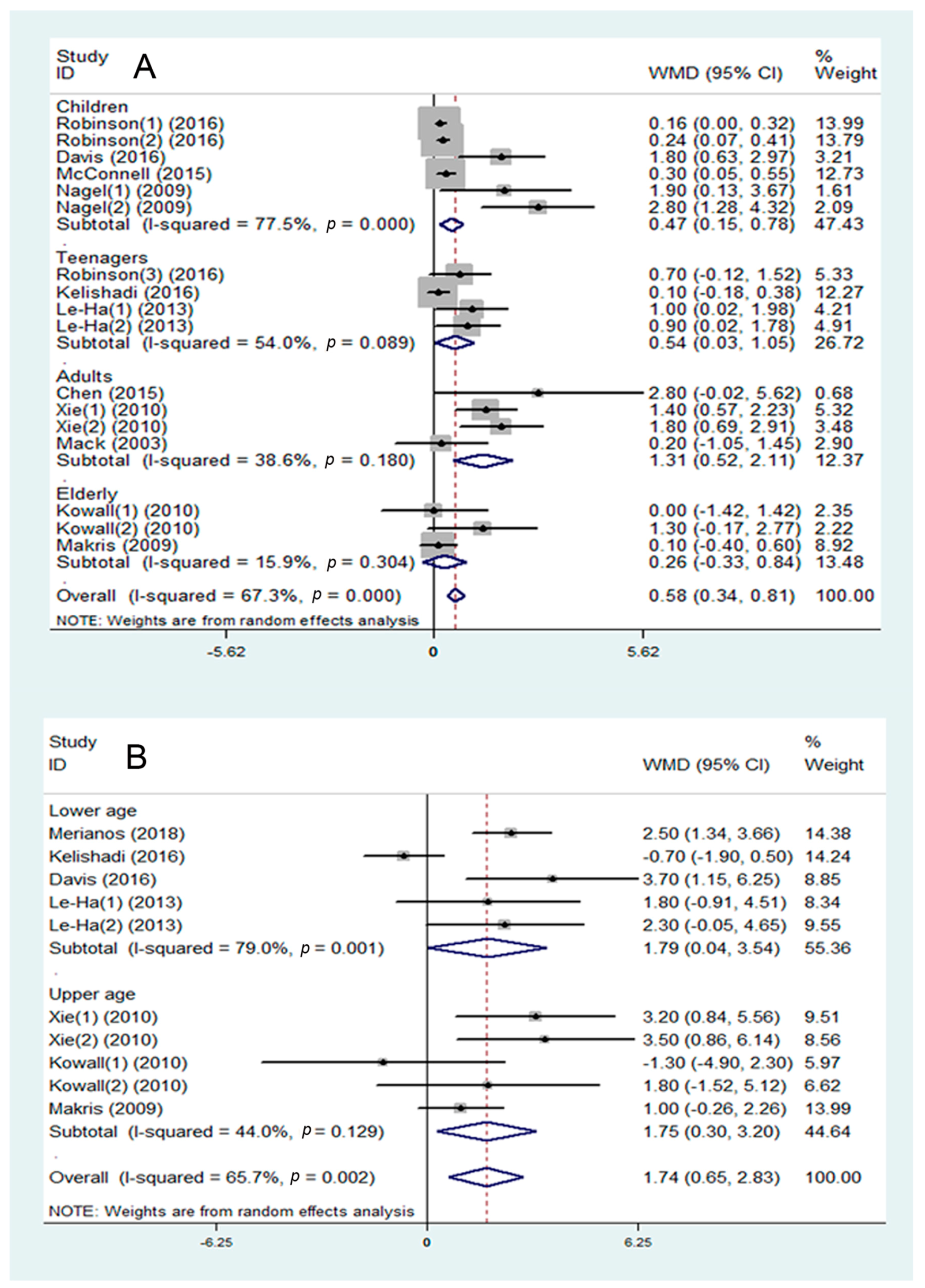
3.3. SHS and Disorder of Glucose Metabolism
3.4. SHS and Disorder of Lipid Metabolism
3.5. SHS and Risk of Abdominal Obesity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Pruss-Ustun, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef]
- Law, M.R.; Morris, J.K.; Wald, N.J. Environmental tobacco smoke exposure and ischaemic heart disease: An evaluation of the evidence. BMJ 1997, 315, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Hollenbach, J.P.; Schifano, E.D.; Hammel, C.; Cloutier, M.M. Exposure to secondhand smoke and asthma severity among children in Connecticut. PLoS ONE 2017, 12, e0174541. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, A.K.; Law, M.R.; Wald, N.J. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ 1997, 315, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Barnoya, J.; Glantz, S.A. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation 2005, 111, 2684–2698. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Choy, Y.S.; Park, E.C. Association between secondhand smoke and glycemic control in adult diabetes patients. Prev. Med. 2017, 94, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Oba, S.; Suzuki, E.; Yamamoto, M.; Horikawa, Y.; Nagata, C.; Takeda, J.; The Gifu Diabetes Study Group. Active and passive exposure to tobacco smoke in relation to insulin sensitivity and pancreatic beta-cell function in Japanese subjects. Diabetes Metab. 2015, 41, 160–167. [Google Scholar] [CrossRef]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Li, C.; Zhao, G.; Pearson, W.S.; Mokdad, A.H. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes care 2008, 31, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Sherling, D.H.; Perumareddi, P.; Hennekens, C.H. Metabolic Syndrome. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 365–367. [Google Scholar] [CrossRef]
- Hanson, R.L.; Imperatore, G.; Bennett, P.H.; Knowler, W.C. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes 2002, 51, 3120–3127. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yadav, D.; Ahn, S.V.; Koh, S.B.; Park, J.T.; Yoon, J.; Yoo, B.S.; Lee, S.H. A prospective study of total sleep duration and incident metabolic syndrome: The ARIRANG study. Sleep Med. 2015, 16, 1511–1515. [Google Scholar] [CrossRef]
- Wennberg, M.; Gustafsson, P.E.; Wennberg, P.; Hammarstrom, A. Irregular eating of meals in adolescence and the metabolic syndrome in adulthood: Results from a 27-year prospective cohort. Public Health Nutr. 2016, 19, 667–673. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef]
- Hu, J.; Dong, Y.; Chen, X.; Liu, Y.; Ma, D.; Liu, X.; Zheng, R.; Mao, X.; Chen, T.; He, W. Prevalence of suicide attempts among Chinese adolescents: A meta-analysis of cross-sectional studies. Compr. Psychiatry 2015, 61, 78–89. [Google Scholar] [CrossRef]
- Zhong, G.C.; Liu, Y.; Chen, N.; Hao, F.B.; Wang, K.; Cheng, J.H.; Gong, J.P.; Ding, X. Reproductive factors, menopausal hormone therapies and primary liver cancer risk: A systematic review and dose-response meta-analysis of observational studies. Hum. Reprod. Update 2016, 23, 126–138. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Merianos, A.L.; Jandarov, R.A.; Khoury, J.C.; Mahabee-Gittens, E.M. Tobacco Smoke Exposure Association with Lipid Profiles and Adiposity Among U.S. Adolescents. J. Adolesc. Health 2018, 62, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, S.H.; Han, D.; Kim, S.H.; Kim, H.J.; Park, J.J.; Cho, Y.; Yoon, Y.E.; Han, K.D.; Oh, I.Y.; et al. Relation between secondhand smoke exposure and cardiovascular risk factors in never smokers. J. Hypertens. 2017, 35, 1976–1982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robinson, O.; Martinez, D.; Aurrekoetxea, J.J.; Estarlich, M.; Somoano, A.F.; Iniguez, C.; Santa-Marina, L.; Tardon, A.; Torrent, M.; Sunyer, J.; et al. The association between passive and active tobacco smoke exposure and child weight status among Spanish children. Obesity 2016, 24, 1767–1777. [Google Scholar] [CrossRef]
- Kelishadi, R.; Noori, A.; Qorbani, M.; Rahimzadeh, S.; Djalalinia, S.; Shafiee, G.; Motlagh, M.E.; Ardalan, G.; Ansari, H.; Asayesh, H.; et al. Are active and passive smoking associated with cardiometabolic risk factors in adolescents? The CASPIAN-III Study. Paediatr. Int. Child. Health 2016, 36, 181–188. [Google Scholar] [CrossRef]
- Davis, C.L.; Tingen, M.S.; Jia, J.; Sherman, F.; Williams, C.F.; Bhavsar, K.; Wood, N.; Kobleur, J.; Waller, J.L. Passive Smoke Exposure and Its Effects on Cognition, Sleep, and Health Outcomes in Overweight and Obese Children. Child. Obes. 2016, 12, 119–125. [Google Scholar] [CrossRef]
- McConnell, R.; Shen, E.; Gilliland, F.D.; Jerrett, M.; Wolch, J.; Chang, C.C.; Lurmann, F.; Berhane, K. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: The Southern California Children’s Health Study. Environ. Health Perspect. 2015, 123, 360–366. [Google Scholar] [CrossRef]
- Chen, W.; Yun, M.; Fernandez, C.; Li, S.; Sun, D.; Lai, C.C.; Hua, Y.; Wang, F.; Zhang, T.; Srinivasan, S.R.; et al. Secondhand smoke exposure is associated with increased carotid artery intima-media thickness: The Bogalusa Heart Study. Atherosclerosis 2015, 240, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Le-Ha, C.; Beilin, L.J.; Burrows, S.; Huang, R.C.; Oddy, W.H.; Hands, B.; Mori, T.A. Gender difference in the relationship between passive smoking exposure and HDL-cholesterol levels in late adolescence. J. Clin. Endocrinol. Metab. 2013, 98, 2126–2135. [Google Scholar] [CrossRef]
- Yang, B.; Li, M.; Chen, B.; Xu, Y.; Li, T.D. Deterioration of endothelial function and carotid intima-media thickness in Tibetan male adolescents exposed to second-hand smoke. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Dulger, H.; Donder, A.; Sekeroglu, M.R.; Erkoc, R.; Ozbay, B. Investigation of the relationship between serum levels of cotinine and the renal function in active and passive smokers. Ren. Fail. 2011, 33, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Palmer, P.H.; Pang, Z.; Sun, P.; Duan, H.; Johnson, C.A. Environmental tobacco use and indicators of metabolic syndrome in Chinese adults. Nicotine Tob. Res. 2010, 12, 198–206. [Google Scholar] [CrossRef]
- Moss, D.R.; Lucht, L.A.; Kip, K.E.; Reis, S.E. Acute physiologic effects of secondhand smoke exposure in children. Nicotine Tob. Res. 2010, 12, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Kowall, B.; Rathmann, W.; Strassburger, K.; Heier, M.; Holle, R.; Thorand, B.; Giani, G.; Peters, A.; Meisinger, C. Association of passive and active smoking with incident type 2 diabetes mellitus in the elderly population: The KORA S4/F4 cohort study. Eur. J. Epidemiol. 2010, 25, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Arnold, F.J.; Wilhelm, M.; Link, B.; Zoellner, I.; Koenig, W. Environmental tobacco smoke and cardiometabolic risk in young children: Results from a survey in south-west Germany. Eur. Heart J. 2009, 30, 1885–1893. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Makris, T.K.; Thomopoulos, C.; Papadopoulos, D.P.; Bratsas, A.; Papazachou, O.; Massias, S.; Michalopoulou, E.; Tsioufis, C.; Stefanadis, C. Association of passive smoking with masked hypertension in clinically normotensive nonsmokers. Am. J. Hypertens. 2009, 22, 853–859. [Google Scholar] [CrossRef]
- Houston, T.K.; Person, S.D.; Pletcher, M.J.; Liu, K.; Iribarren, C.; Kiefe, C.I. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ 2006, 332, 1064–1069. [Google Scholar] [CrossRef]
- Mack, W.J.; Islam, T.; Lee, Z.; Selzer, R.H.; Hodis, H.N. Environmental tobacco smoke and carotid arterial stiffness. Prev. Med. 2003, 37, 148–154. [Google Scholar] [CrossRef]
- Henkin, L.; Zaccaro, D.; Haffner, S.; Karter, A.; Rewers, M.; Sholinsky, P.; Wagenknecht, L. Cigarette smoking, environmental tobacco smoke exposure and insulin sensitivity: The Insulin Resistance Atherosclerosis Study. Ann. Epidemiol. 1999, 9, 290–296. [Google Scholar] [CrossRef]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef]
- Li, C.; Hsieh, M.C.; Chang, S.J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013, 25, 210–216. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Bacopoulou, F.; Efthymiou, V.; Landis, G.; Rentoumis, A.; Chrousos, G.P. Waist circumference, waist-to-hip ratio and waist-to-height ratio reference percentiles for abdominal obesity among Greek adolescents. BMC Pediatr. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Andres, R. Aging and diabetes. Med. Clin. N. Am. 1971, 55, 835–846. [Google Scholar] [CrossRef]
- Defronzo, R.A. Glucose intolerance and aging: Evidence for tissue insensitivity to insulin. Diabetes 1979, 28, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Crockford, P.M.; Harbeck, R.J.; Williams, R.H. Influence of age on intravenous glucose tolerance and serum immunoreactive insulin. Lancet 1966, 1, 465–467. [Google Scholar] [CrossRef]
- Murakata, Y.; Fujimaki, T.; Yamada, Y. Age-related changes in clinical parameters and their associations with common complex diseases. Biomed. Rep. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Hedley, A.A.; Ogden, C.L.; Johnson, C.L.; Carroll, M.D.; Curtin, L.R.; Flegal, K.M. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 2004, 291, 2847–2850. [Google Scholar] [CrossRef]
- Nascimento, S.; Goethel, G.; Gauer, B.; Sauer, E.; Nardi, J.; Cestonaro, L.; Correia, D.; Peruzzi, C.; Mota, L.; Machry, R.V.; et al. Exposure to environment chemicals and its possible role in endocrine disruption of children from a rural area. Environ. Res. 2018, 167, 488–498. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Period | Study Design | SHS Assessment | Mean Age (Year) | Sample Size | Variables |
|---|---|---|---|---|---|---|
| Merianos et al. (2018) [19] | 1999–2012 | CS | Non-smoker, serum cotinine (0.05–2.99 ng/mL) | 12–19 | 11,550 | WC |
| Kim et al. (2017) [20] | 2008–2011 | CS | Self-report passive smoking | 45 | 7376 | TC, LDL-C, HDL-C |
| Robinson et al. (2016) [21] | 2003–2008 | C | Questionnaire: non-smoker, passive exposure at home/work | 1/4/14 | 3174 | BMI |
| Kelishadi et al. (2016) [22] | 2009–2010 | CS | Self-report: living with smokers | 10–18 | 5625 | BMI, WC, FPG, TC, TG, LDL-C, HDL-C |
| Davis et al. (2016) [23] | 2003–2006 | CS | Non-smoker, plasma cotinine ≥ 0.05 ng/mL | 7–11 | 222 | BMI, WC, INS, FPG |
| McConnell et al. (2015) [24] | NC | C | Questionnaire: living with smokers, quantity of smokers in the household | 10 | 3318 | BMI |
| Chen et al. (2015) [25] | 2004–2010 | C | Questionnaire: non-smoker, living together indoors with smokers, the number of exposed years | 26–48 | 415 | BMI, LDL-C |
| Le-Ha et al. (2013) [26] | 1989–2006 | C | Questionnaire: quantity of cigarettes smoked daily in the household | 17 | 1754 | BMI, WC, INS, FPG, TC, TG, LDL-C, HDL-C |
| Yang et al. (2012) [27] | NC | CS | Non-smoker, plasma cotinine ≥ 0.90 ng/mL | 16 | 624 | TC, TG, LDL-C, HDL-C |
| Dulger et al. (2011) [28] | NC | CS | Non-smoker, living with smokers for at least 5–6 h during the day | 27–33 | 40 | FPG |
| Xie et al. (2010) [29] | NC | CS | Questionnaire: number of days/one week in a room with smokers | 38 | 389 | BMI, WC, FPG, TC, TG, LDL-C, HDL-C |
| Moss et al. (2010) [30] | NC | CS | Self-report passive smoking | 30–50/10–12 | 41 | FPG, TC, TG, LDL-C, HDL-C |
| Kowall et al. (2010) [31] | 1999–2001 | C | Questionnaire: stay with smokers at home/work, quantity of other smokers | 55–74 | 1223 | BMI, WC, INS, FPG |
| Nagel et al. (2009) [32] | 1999–2008 | CS | Questionnaire: living with smokers, quantity of cigarettes smoked daily in the household | 10 | 450 | BMI |
| Makris et al. (2009) [33] | NC | CS | Non-smoker, at least 1 h daily domestic and/or workplace smoke exposure | 50–60 | 254 | BMI, WC, FPG, TC, TG, LDL-C, HDL-C |
| Houston et al. (2006) [34] | NC | C | Self-report and serum cotinine (1–15 ng/mL) | 18–30 | 4572 | INS, TG |
| Mack et al. (2003) [35] | NC | CS | Questionnaire: number of smokers and hours per day staying with smokers | 40 | 227 | BMI, TC, TG, LDL-C, HDL-C |
| Henkin et al. (1999) [36] | NC | CS | Structured interview: living with smokers | 40–69 | 1481 | INS |
| Cross-Sectional Study | Year | Research Elements | Quality Control | Data Integrity | Scores | Quality * | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||||
| Merianos | 2018 | ★ | ★ | ★ | ★ | - | ★ | ★ | ★ | - | ★ | - | 8 | High |
| Kim | 2017 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | - | - | - | 8 | High |
| Kelishadi | 2016 | ★ | - | ★ | - | - | ★ | - | ★ | - | ★ | - | 5 | Moderate |
| Davis | 2016 | ★ | ★ | ★ | - | ★ | ★ | - | ★ | - | - | ★ | 7 | Moderate |
| Yang | 2012 | ★ | ★ | - | - | ★ | ★ | ★ | ★ | - | - | - | 6 | Moderate |
| Dulger | 2011 | ★ | ★ | - | - | ★ | ★ | ★ | ★ | - | - | - | 6 | Moderate |
| Xie | 2010 | ★ | ★ | ★ | - | ★ | ★ | ★ | ★ | ★ | ★ | - | 9 | High |
| Moss | 2010 | ★ | ★ | - | - | ★ | ★ | ★ | ★ | ★ | ★ | - | 8 | High |
| Nagel | 2009 | ★ | ★ | ★ | - | ★ | - | ★ | ★ | - | ★ | ★ | 8 | High |
| Makris | 2009 | ★ | ★ | - | ★ | ★ | ★ | ★ | ★ | - | ★ | ★ | 9 | High |
| Mack | 2003 | ★ | - | - | - | ★ | ★ | ★ | ★ | - | ★ | - | 6 | Moderate |
| Henkin | 1999 | ★ | ★ | - | - | ★ | ★ | ★ | ★ | - | ★ | ★ | 8 | High |
| Cohort Study | Year | Selection | Comparability | Result Evaluation | Scores | Quality * | ||||||||
| I | II | III | IV | V | VI | VII | VIII | |||||||
| Robinson | 2016 | ★ | ★ | - | ★ | ★★ | ★ | ★ | ★ | 8 | High | |||
| McConnell | 2015 | - | ★ | - | ★ | ★★ | ★ | ★ | ★ | 7 | High | |||
| Chen | 2015 | ★ | ★ | - | ★ | ★★ | ★ | ★ | ★ | 8 | High | |||
| Le-Ha | 2013 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | High | |||
| Kowall | 2010 | ★ | ★ | - | ★ | ★★ | - | ★ | ★ | 7 | High | |||
| Houston | 2006 | ★ | ★ | ★ | ★ | ★★ | - | ★ | ★ | 8 | High | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-J.; Li, G.-L.; Sun, A.; Peng, D.-S.; Zhang, W.-X.; Yan, Y.-E. Age Differences in the Relationship between Secondhand Smoke Exposure and Risk of Metabolic Syndrome: A Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 1409. https://doi.org/10.3390/ijerph16081409
Chen H-J, Li G-L, Sun A, Peng D-S, Zhang W-X, Yan Y-E. Age Differences in the Relationship between Secondhand Smoke Exposure and Risk of Metabolic Syndrome: A Meta-Analysis. International Journal of Environmental Research and Public Health. 2019; 16(8):1409. https://doi.org/10.3390/ijerph16081409
Chicago/Turabian StyleChen, Hui-Jian, Gai-Ling Li, Ao Sun, Dang-Sheng Peng, Wan-Xia Zhang, and You-E Yan. 2019. "Age Differences in the Relationship between Secondhand Smoke Exposure and Risk of Metabolic Syndrome: A Meta-Analysis" International Journal of Environmental Research and Public Health 16, no. 8: 1409. https://doi.org/10.3390/ijerph16081409
APA StyleChen, H.-J., Li, G.-L., Sun, A., Peng, D.-S., Zhang, W.-X., & Yan, Y.-E. (2019). Age Differences in the Relationship between Secondhand Smoke Exposure and Risk of Metabolic Syndrome: A Meta-Analysis. International Journal of Environmental Research and Public Health, 16(8), 1409. https://doi.org/10.3390/ijerph16081409





