Effects of Different Components of PM2.5 on the Expression Levels of NF-κB Family Gene mRNA and Inflammatory Molecules in Human Macrophage
Abstract
1. Introduction
2. Materials and Methods
2.1. PM2.5 Sampling and Constituent Preparation
2.1.1. PM2.5 Sampling
2.1.2. PM2.5 Component Extraction and Solution Preparation
2.2. Cell Culture and Exposure to PM2.5
2.3. MTT for the Cell Survival Rate
2.4. ELISA for Inflammatory Molecules and Cytokines in the Supernatant
2.5. Real-Time Quantitative PCR for the Expression Levels of NF-κB Family Genes
2.6. Statistical Analyses
3. Results
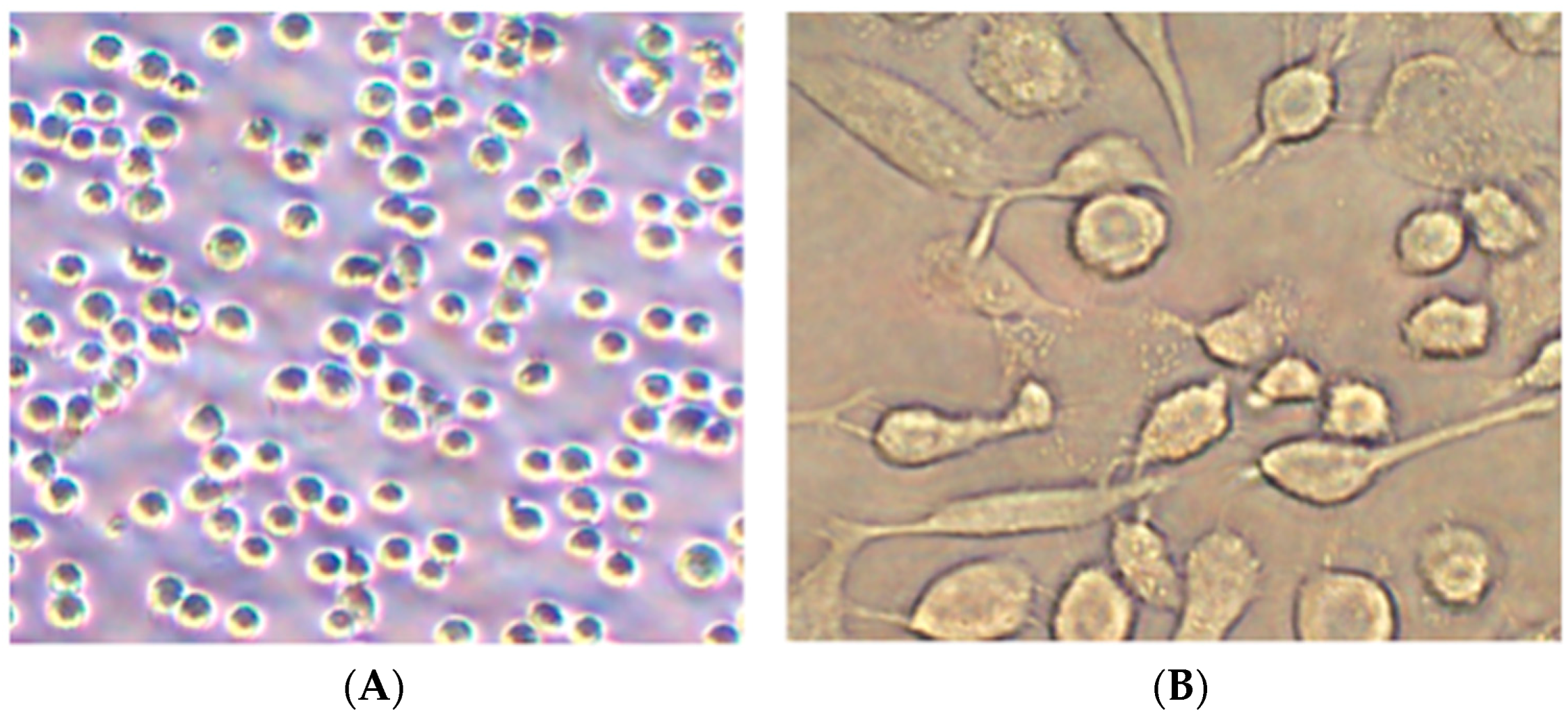
3.1. Differentiation of Monocytes
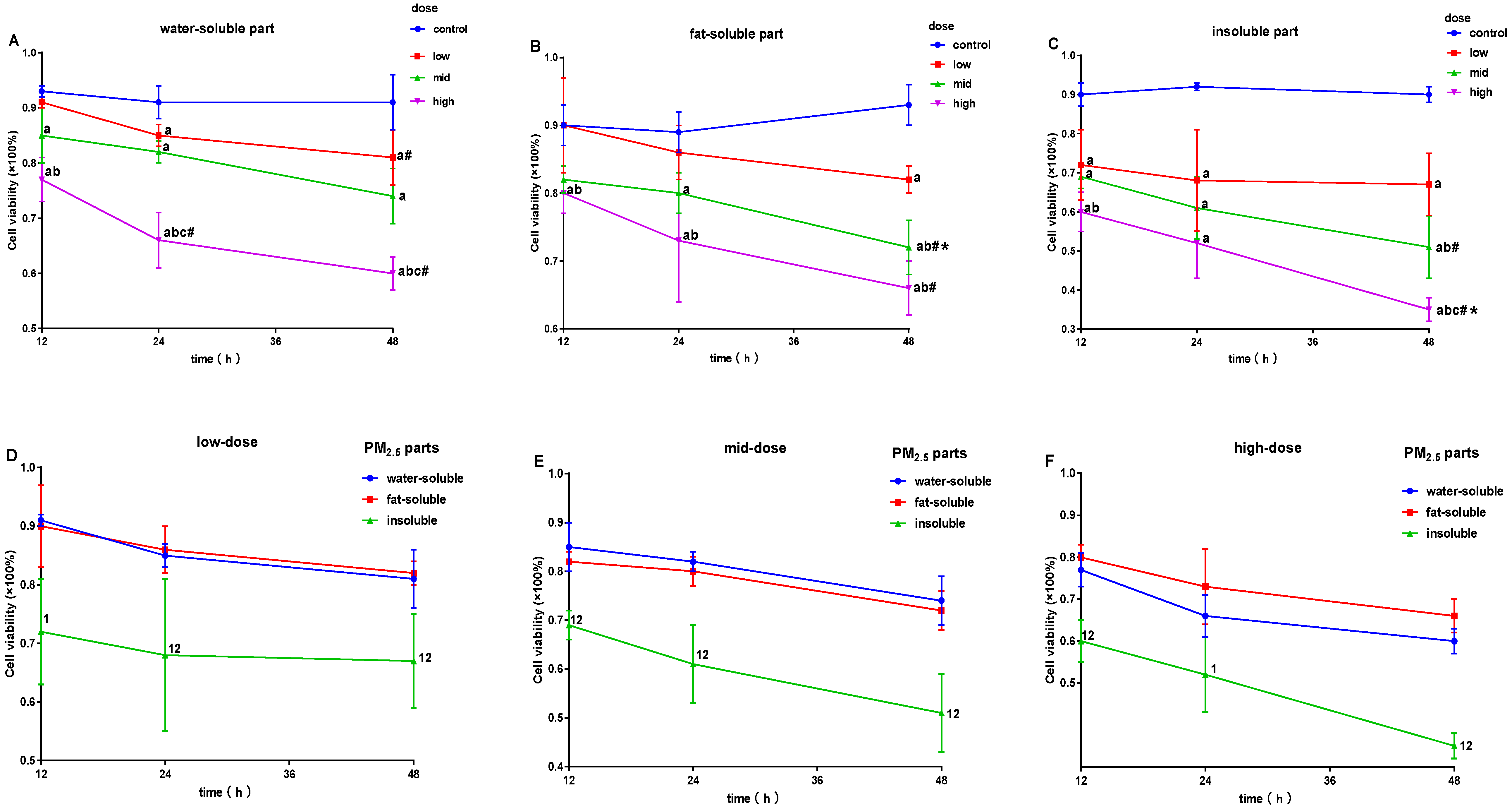
3.2. Effects of PM2.5 on Cell Survival Rate
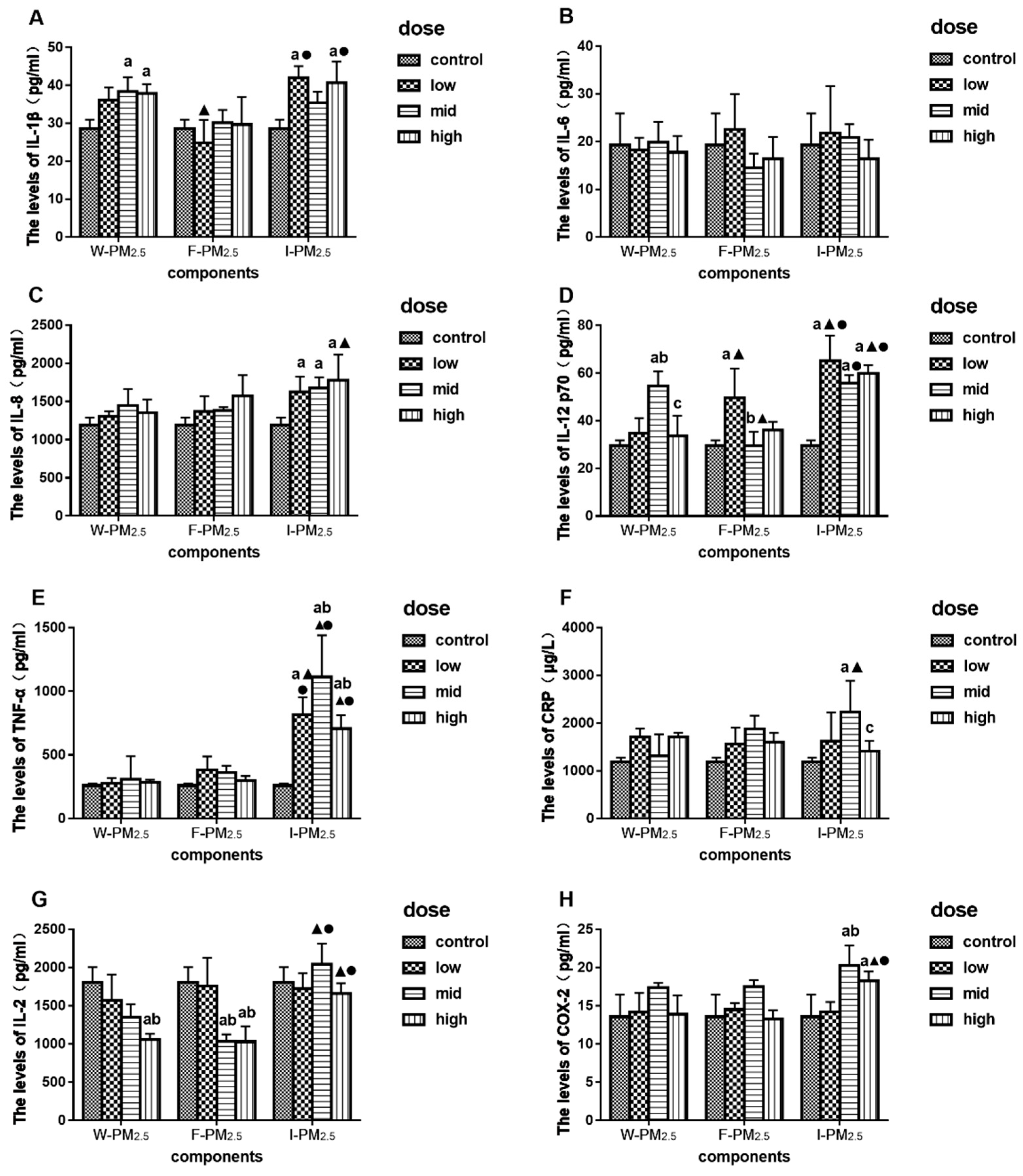
3.3. Effects of PM2.5 on the Levels of IL-1β, IL-6, IL-8, IL-12 p70, TNF-α, CRP, IL-2, and COX-2
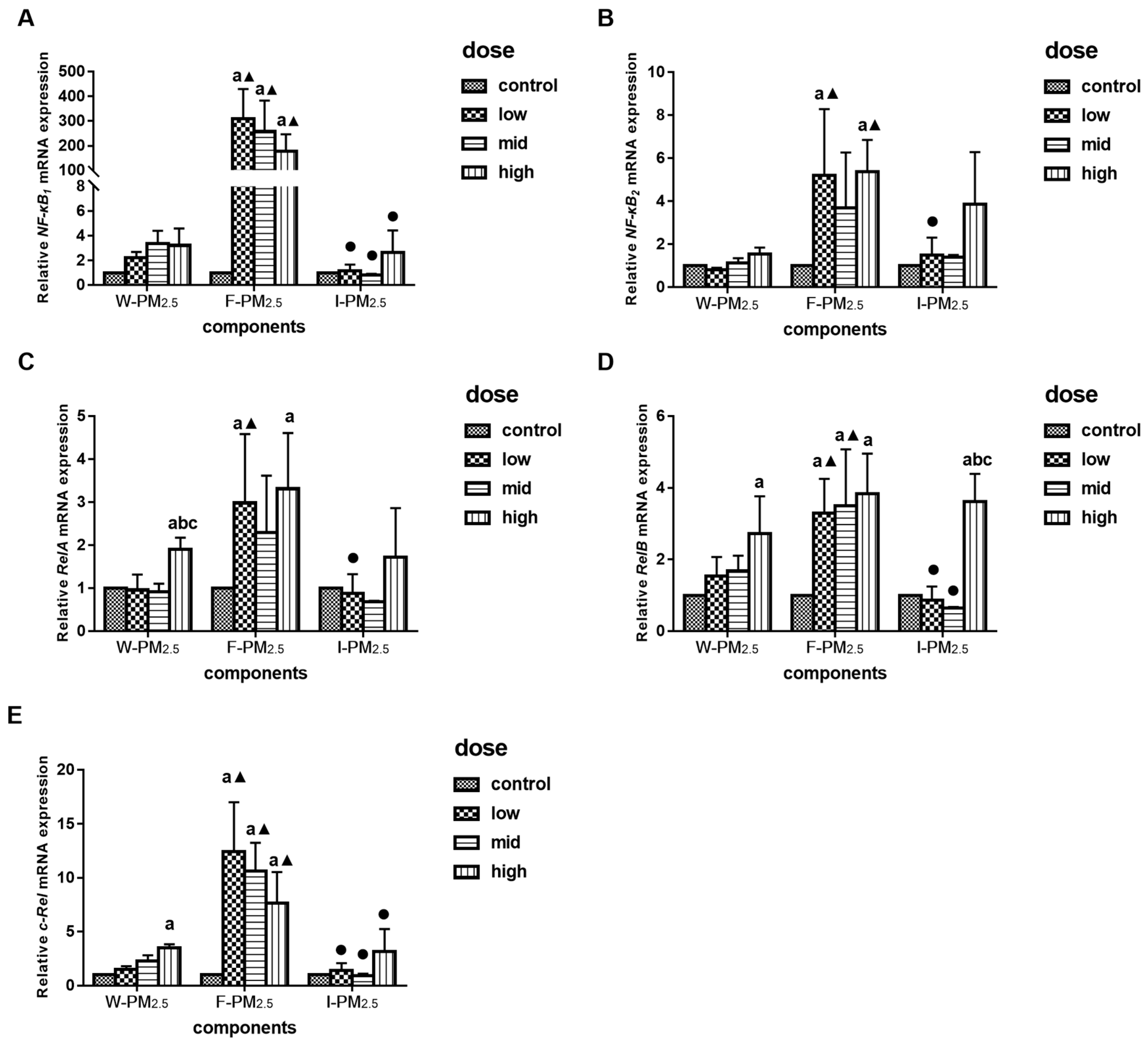
3.4. Effects of PM2.5 on the Relative mRNA Expression Levels of NF-KB Family Gene
3.5. The Connection between the Expression of NF-κB mRNA and Inflammatory Molecules
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pruss-Ustun, A.; Neira, M. Preventing Disease through Healthy Environments: A Global Assessment of the Environmental Burden of Disease Abstract. Available online: https://apps.who.int/iris/bitstream/handle/10665/204585/9789241565196_eng.pdf (accessed on 5 April 2019).
- Lo, W.C.; Shie, R.H.; Chan, C.C.; Lin, H.H. Burden of disease attributable to ambient fine particulate matter exposure in Taiwan. J. Formos Med. Assoc. 2017, 116, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, S.; Rochereau, T.; Maesano, C.N.; Com-Ruelle, L.; Annesi-Maesano, I. Long-Term Effect of Outdoor Air Pollution on Mortality and Morbidity: A 12-Year Follow-Up Study for Metropolitan France. Int. J. Environ. Res. Public Health 2018, 15, 2487. [Google Scholar] [CrossRef] [PubMed]
- EEA. Ambient (Outdoor) Air Quality and Health. Available online: http://www.who.int/mediacentre/factsheets/fs313/en/ (accessed on 5 April 2019).
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [PubMed]
- Park, E.J.; Roh, J.; Kim, Y.; Park, K.; Kim, D.S.; Yu, S.D. PM2.5 collected in a residential area induced Th1-type inflammatory responses with oxidative stress in mice. Environ. Res. 2011, 111, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Huang, D.Y.; Zhang, H.J.; Wang, S.; Chen, X.F. Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int. Immunopharmacol. 2017, 50, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.; Birmili, W.; Albrecht, C.; Hellack, B.; Jermann, E.; Wick, G.; Harrison, R.M.; Schins, R.P.F. Oxidant Generation and Toxicity of Size-Fractionated Ambient Particles in Human Lung Epithelial Cells. Environ. Sci. Technol. 2010, 44, 3539–3545. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Liu, Y.H.; Duan, F.K.; Qin, M.N.; Wu, F.C.; Sheng, W.; Yang, L.X.; Liu, J.G.; He, K.B. Transcriptomic Analyses of the Biological Effects of Airborne PM2.5 Exposure on Human Bronchial Epithelial Cells. PLoS ONE 2015, 10, e0138267. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Kou, X.J.; Geng, H.; Xie, J.F.; Yang, Z.H.; Zhang, Y.X.; Cai, Z.W.; Dong, C. Effect of Ambient PM2.5 on Lung Mitochondrial Damage and Fusion/Fission Gene Expression in Rats. Chem. Res. Toxicol. 2015, 28, 408–418. [Google Scholar] [CrossRef]
- Zhao, Q.J.; Chen, H.; Yang, T.; Rui, W.; Liu, F.; Zhang, F.; Zhao, Y.; Ding, W.J. Direct effects of airborne PM2.5 exposure on macrophage polarizations. Biochim. Biophys. Acta. Gen. Subj. 2016, 1860, 2835–2843. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-kappaB signaling pathway. J. Thorac. Dis. 2017, 9, 4398–4412. [Google Scholar] [CrossRef] [PubMed]
- Rumelhard, M.; Ramgolam, K.; Hamel, R.; Marano, F.; Baeza-Squiban, A. Expression and role of EGFR ligands induced in airway cells by PM2.5 and its components. Eur. Respir. J. 2007, 30, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.X.; Qin, Y.T.; Lou, D.S.; Li, Q.; Li, Y.Y.; Wang, Z.M.; Yang, W.W.; Wang, M.; Liu, N.; Wang, Z.; et al. iRhom2 deficiency relieves TNF-alpha associated hepatic dyslipidemia in long-term PM2.5-exposed mice. Biochem. Biophys. Res. Commun. 2017, 493, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ichinose, T.; Yoshida, S.; Ito, T.; He, C.Y.; Yoshida, Y.; Arashidani, K.; Takano, H.; Sun, G.F.; Shibamoto, T. PM2.5-induced lung inflammation in mice: Differences of inflammatory response in macrophages and type II alveolar cells. J. Appl. Toxicol. 2017, 37, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhao, X.H.; Bi, T.T.; Yuan, X.Y.; Guo, J.B.; Peng, S.Q. PM2.5 obtained from urban areas in Beijing induces apoptosis by activating nuclear factor-kappa B. Mil. Med. Res. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.; Li, B.; Gao, R.; Zhang, Y.; Yan, W.; Ji, X.; Li, G.; Sang, N. NF-kappaB-regulated microRNA-574-5p underlies synaptic and cognitive impairment in response to atmospheric PM2.5 aspiration. Part. Fibre Toxicol. 2017, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhao, L.; Tong, J.; Yan, Y.; Xu, C. Fine Particulate Matter and Sulfur Dioxide Coexposures Induce Rat Lung Pathological Injury and Inflammatory Responses Via TLR4/p38/NF-kappaB Pathway. Int. J. Toxicol. 2017, 36, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Dagher, Z.; Garcon, G.; Billet, S.; Verdin, A.; Ledoux, F.; Courcot, D.; Aboukais, A.; Shirali, P. Role of nuclear factor-kappa B activation in the adverse effects induced by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. J. Appl. Toxicol. 2007, 27, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.; Jamison, S.; Yue, Y.; Durose, W.; Schmidt-Ullrich, R.; Lin, W. NF-κB Activation Protects Oligodendrocytes against Inflammation. J. Neurosci. 2017, 37, 9332–9344. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, K.B.; Du, Z.Y.; Zheng, M.; Duan, F.K.; Ma, Y.L. Humidity plays an important role in the PM2.5 pollution in Beijing. Environ. Pollut. 2015, 197, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.B.; Pakbin, P.; Shafer, M.M.; Antkiewicz, D.; Schauer, J.J.; Sioutas, C. Macrophage reactive oxygen species activity of water-soluble and water-insoluble fractions of ambient coarse, PM2.5 and ultrafine particulate matter (PM) in Los Angeles. Atmos. Environ. 2013, 77, 301–310. [Google Scholar] [CrossRef]
- Zou, Y.J.; Jin, C.Y.; Su, Y.; Li, J.R.; Zhu, B.S. Water soluble and insoluble components of urban PM2.5 and their cytotoxic effects on epithelial cells (A549) in vitro. Environ. Pollut. 2016, 212, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.; West, J.J.; Zhang, Y.Q.; Anenberg, S.C.; Lamarque, J.F.; Shindell, D.T.; Collins, W.J.; Dalsoren, S.; Faluvegi, G.; Folberth, G.; et al. Global premature mortality due to anthropogenic outdoor air pollution and the contribution of past climate change. Environ. Res. Lett. 2013, 8, 031002. [Google Scholar] [CrossRef]
- Zeng, Q.; Ni, Y.; Jiang, G.H.; Li, G.X.; Pan, X.C. The short term burden of ambient particulate matters on non-accidental mortality and years of life lost: A ten-year multi-district study in Tianjin, China. Environ. Pollut. 2017, 220, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Krewski, D.; Pope, C.A.; Chen, Y.; Gapstur, S.M.; Thun, M.J. Long-term Ambient Fine Particulate Matter Air Pollution and Lung Cancer in a Large Cohort of Never-Smokers. Am. J. Respir. Crit. Care 2011, 184, 1374–1381. [Google Scholar] [CrossRef]
- Ye, X.Q.; Hong, W.; Hao, B.W.; Peng, G.Y.; Huang, L.M.; Zhao, Z.X.; Zhou, Y.M.; Zheng, M.N.; Li, C.L.; Liang, C.X.; et al. PM2.5 promotes human bronchial smooth muscle cell migration via the sonic hedgehog signaling pathway. Respir. Res. 2018, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhu, T.; Lenz, A.G.; Frankenberger, B.; Tian, F.; Chen, C.Y.; Stoeger, T. Reduced in vitro toxicity of fine particulate matter collected during the 2008 summer Olympic Games in Beijing: The roles of chemical and biological components. Toxicol. In Vitro 2013, 27, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Wang, S.Y.; Zhu, J.; Li, C.Y.; Zhang, T.R.; Liu, H.B.; Xu, Q.; Ye, X.F.; Zhou, L.T.; Ye, L. Effect of Atmospheric PM2.5 on Expression Levels of NF-kappa B Genes and Inflammatory Cytokines Regulated by NF-kappa B in Human Macrophage. Inflammation 2018, 41, 784–794. [Google Scholar] [CrossRef]
- Chen, S.; Wu, X.; Hu, J.; Dai, G.; Rong, A.; Guo, G. PM2.5 exposure decreases viability, migration and angiogenesis in human umbilical vein endothelial cells and human microvascular endothelial cells. Mol. Med. Rep. 2017, 16, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Pavagadhi, S.; Betha, R.; Venkatesan, S.; Balasubramanian, R.; Hande, M.P. Physicochemical and toxicological characteristics of urban aerosols during a recent Indonesian biomass burning episode. Environ. Sci. Pollut. Res. 2013, 20, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Steenhof, M.; Gosens, I.; Strak, M.; Godri, K.J.; Hoek, G.; Cassee, F.R.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Lebret, E.; et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential—The RAPTES project. Part. Fibre Toxicol. 2011, 8, 26. [Google Scholar] [CrossRef]
- Cavanagh, J.A.E.; Trought, K.; Brown, L.; Duggan, S. Exploratory investigation of the chemical characteristics and relative toxicity of ambient air particulates from two New Zealand cities. Sci. Total Environ. 2009, 407, 5007–5018. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Guo, X.; Liu, H.; Fang, X.; Yang, M.; Chen, W. Effects of dust storm PM2.5 on cell proliferation and cell cycle in human lung fibroblasts. Toxicol. In Vitro 2007, 21, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Jalava, P.I.; Hirvonen, M.R.; Sillanpaa, M.; Pennanen, A.S.; Happo, M.S.; Hillamo, R.; Cassee, F.R.; Gerlofs-Nijland, M.; Borm, P.J.A.; Schins, R.P.F.; et al. Associations of urban air particulate composition with inflammatory and cytotoxic responses in RAW 246.7 cell line. Inhal. Toxicol. 2009, 21, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Palleschi, S.; Rossi, B.; Armiento, G.; Montereali, M.R.; Nardi, E.; Tagliani, S.M.; Inglessis, M.; Gianfagna, A.; Silvestroni, L. Toxicity of the readily leachable fraction of urban PM2.5 to human lung epithelial cells: Role of soluble metals. Chemosphere 2018, 196, 35–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Shi, Z.; Wu, J.; Yang, X.; Feng, L.; Ren, L.; Duan, J.; Sun, Z. Metabolic impact induced by total, water soluble and insoluble components of PM2.5 acute exposure in mice. Chemosphere 2018, 207, 337–346. [Google Scholar] [CrossRef]
- Loxham, M.; Morgan-Walsh, R.J.; Cooper, M.J.; Blume, C.; Swindle, E.J.; Dennison, P.W.; Howarth, P.H.; Cassee, F.R.; Teagle, D.A.H.; Palmer, M.R.; et al. The Effects on Bronchial Epithelial Mucociliary Cultures of Coarse, Fine, and Ultrafine Particulate Matter from an Underground Railway Station. Toxicol. Sci. 2015, 145, 98–107. [Google Scholar] [CrossRef]
- Klinke, D.J. The ratio of P40 monomer to dimer is an important determinant of IL-12 bioactivity. J. Theor. Biol. 2006, 240, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.L.; Yang, M.H.; Wu, H.; Ma, T.; He, C.J.; Chai, M.L.; Zhang, X.S.; Zhang, J.L.; Ding, F.R.; Wang, S.T.; et al. Effects of AANAT overexpression on the inflammatory responses and autophagy activity in the cellular and transgenic animal levels. Autophagy 2018, 14, 1850–1869. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Mattei, E.; Rivera, E.; Gioda, A.; Sanchez-Rivera, D.; Roman-Velazquez, F.R.; Jimenez-Velez, B.D. Use of human bronchial epithelial cells (BEAS-2B) to study immunological markers resulting from exposure to PM2.5 organic extract from Puerto Rico. Toxicol. Appl. Pharm. 2010, 243, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Stonehuerner, J.; Dailey, L.A.; Carter, J.D. Metals associated with both the water-soluble and insoluble fractions of an ambient air pollution particle catalyze an oxidative stress. Inhal. Toxicol. 1999, 11, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.L.; Stenstrom, M.K. Metals and PAHs adsorbed to street particles. Water Res. 2005, 39, 4083–4092. [Google Scholar] [CrossRef]
- Feng, L.; Yang, X.Z.; Asweto, C.O.; Wu, J.; Zhang, Y.N.; Hu, H.J.; Shi, Y.F.; Duan, J.C.; Sun, Z.W. Low-dose combined exposure of nanoparticles and heavy metal compared with PM2.5 in human myocardial AC16 cells. Environ. Sci. Pollut. Res. 2017, 24, 27767–27777. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.L.; Shang, M.T.; Mu, K.; Jiang, N.; Wen, H.Y.; Wang, R.; Wu, H.; Li, W.Y. In vitro and in vivo toxic effects and inflammatory responses induced by carboxylated black carbon-lead complex exposure. Ecotoxicol. Environ. Saf. 2018, 165, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Plata, V.M.; Mullerova, H.; Toso, J.F.; Feudjo-Tepie, M.; Soriano, J.B.; Vessey, R.S.; Celli, B.R. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 2006, 61, 23–28. [Google Scholar] [CrossRef]
- Suwa, T.; Hogg, J.C.; Quinlan, K.B.; Ohgami, A.; Vincent, R.; van Eeden, S.F. Particulate air pollution induces progression of atherosclerosis. J. Am. Coll. Cardiol. 2002, 39, 935–942. [Google Scholar] [CrossRef]
- Bai, N.; Tranfield, E.M.; Kavanagh, T.J.; Kaufman, J.D.; Rosenfeld, M.E.; van Eeden, S.F. Exposure to diesel exhaust upregulates COX-2 expression in ApoE knockout mice. Inhal. Toxicol. 2012, 24, 518–527. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Gorshkova, I.; He, D.; Usatyuk, P.; Kalari, S.; Pendyala, S.; Garcia, J.G.; Berdyshev, E.; Natarajan, V. Sphingosine-1-Phosphate Lyase Regulates Lps-Induced Interleukin-6 Secretion in Pulmonary Microvascular Endothelial Cells and a Murine Model of Acute Lung Injury. J. Investig. Med. 2009, 57, 554. [Google Scholar]
- Vogel, C.F.A.; Sciullo, E.; Wong, P.; Kuzmicky, P.; Kado, N.; Matsumura, F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ. Health Perspect. 2005, 113, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.A.; Kado, S.Y.; Kobayashi, R.; Liu, X.X.; Wong, P.; Na, K.; Durbin, T.; Okamoto, R.A.; Kado, N.Y. Inflammatory marker and aryl hydrocarbon receptor-dependent responses in human macrophages exposed to emissions from biodiesel fuels. Chemosphere 2019, 220, 993–1002. [Google Scholar] [CrossRef]
- Esakky, P.; Hansen, D.A.; Drury, A.M.; Moley, K.H. Cigarette smoke-induced cell cycle arrest in spermatocytes [GC-2spd(ts)] is mediated through crosstalk between Ahr-Nrf2 pathway and MAPK signaling. J. Mol. Cell Biol. 2015, 7, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.X.; Song, X.L.; Li, S.S.; Lai, X.R.; Yang, Y.L.; Yang, G.; Li, Z.J.; Cui, Y.H.; Pan, H.W. Assessment of DNA Damage and Cell Senescence in Corneal Epithelial Cells Exposed to Airborne Particulate Matter (PM2.5) Collected in Guangzhou, China. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Y.; Chen, X.; Wang, S.; Liu, H.; Zhang, T.; Zhang, Y.; Xu, Q.; Han, X.; Zhao, Y.; et al. Over-expression of nuclear factor-kappaB family genes and inflammatory molecules is related to chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Natoli, G.; Chiocca, S. Nuclear ubiquitin ligases, NF-kappaB degradation, and the control of inflammation. Sci. Signal. 2008, 1. [Google Scholar] [CrossRef]
- Bourgeois, B.; Owens, J.W. The influence of Hurricanes Katrina and Rita on the inflammatory cytokine response and protein expression in A549 cells exposed to PM2.5 collected in the Baton Rouge-Port Allen industrial corridor of Southeastern Louisiana in 2005. Toxicol. Mech. Methods 2014, 24, 220–242. [Google Scholar] [CrossRef]
- Haddad, J.J. Redox and oxidant-mediated regulation of apoptosis signaling pathways: Immuno-pharmaco-redox conception of oxidative siege versus cell death commitment. Int. Immunopharmacol. 2004, 4, 475–493. [Google Scholar] [CrossRef]

| Doses | Concentration of PM2.5 (μg/mL) | |||
|---|---|---|---|---|
| W-PM2.5 | F-PM2.5 | I-PM2.5 | Total PM2.5 | |
| Low dose | 75 | 25 | 100 | 200 |
| Mid dose | 150 | 50 | 200 | 400 |
| High dose | 300 | 100 | 400 | 800 |
| Genes | Primer | Sequence |
|---|---|---|
| β-actin | Forward | 5′-CTGGAACGGTGAAGGTGACA-3′ |
| Reverse | 5′-CGGCCACATTGTGAACTTTG-3′ | |
| NF-κB1 | Forward | 5′-CACAAGGCAGCAAATAGACGAG-3′ |
| Reverse | 5′-TGGGGCATTTTGTTGAGAGTT-3′ | |
| NF-κB2 | Forward | 5′-GGCTGGTGCTGACATCCAT-3′ |
| Reverse | 5′-CTGCTTCGGGTGTCCTTCTC-3′ | |
| RelA | Forward | 5′-CCCCAGCCCTATCCCTTTAC-3′ |
| Reverse | 5′-TGCCCAGAAGGAAACACCA-3′ | |
| RelB | Forward | 5′-ATGAATGTGGTGAGGATCTGCTT-3′ |
| Reverse | 5′-CTCTGATGTGTTTGTGGATTTCTTG-3′ | |
| c-Rel | Forward | 5′-GACGACTGCTCTTCCTCCTGTT-3′ |
| Reverse | 5′-TCATCTCCTCCTCTGACACTTCC-3′ |
| Component | Cytokines | IL-1β | IL-6 | IL-8 | IL-12 p70 | CRP | TNF-α |
|---|---|---|---|---|---|---|---|
| W-PM2.5 | NF-κB1 | 0.211 | −0.626 | −0.388 | 0.251 | 0.055 | −0.398 |
| NF-κB2 | 0.460 | −0.335 | −0.068 | −0.221 | −0.081 | −0.189 | |
| RelA | −0.020 | −0.330 | −0.369 | −0.468 | −0.289 | 0.287 | |
| RelB | 0.500 | −0.512 | −0.374 | −0.259 | −0.495 | −0.014 | |
| c-Rel | 0.479 | −0.519 | −0.278 | −0.085 | −0.305 | −0.030 | |
| F-PM2.5 | NF-κB1 | −0.370 | −0.178 | −0.584 | 0.580 | 0.100 | −0.117 |
| NF-κB2 | −0.440 | −0.212 | −0.048 | 0.641 * | −0.372 | −0.627 | |
| RelA | −0.428 | −0.325 | −0.164 | 0.445 | −0.466 | −0.325 | |
| RelB | −0.332 | −0.487 | −0.129 | 0.260 | −0.203 | −0.343 | |
| c-Rel | −0.424 | −0.263 | −0.696 * | 0.634 * | 0.047 | −0.156 | |
| I-PM2.5 | NF-κB1 | 0.674 * | −0.695 * | 0.605 | −0.173 | −0.131 | −0.424 |
| NF-κB2 | 0.636 * | −0.739 * | 0.641 * | −0.256 | −0.043 | −0.356 | |
| RelA | 0.681 * | −0.710 * | 0.577 | −0.167 | −0.148 | −0.405 | |
| RelB | 0.322 | −0.656 * | 0.212 | −0.093 | −0.175 | −0.444 | |
| c-Rel | 0.700 * | −0.681 * | 0.609 | −0.149 | −0.139 | −0.436 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhao, Y.; Gao, Y.; Li, C.; Zhou, L.; Qi, W.; Zhang, Y.; Ye, L. Effects of Different Components of PM2.5 on the Expression Levels of NF-κB Family Gene mRNA and Inflammatory Molecules in Human Macrophage. Int. J. Environ. Res. Public Health 2019, 16, 1408. https://doi.org/10.3390/ijerph16081408
Zhu J, Zhao Y, Gao Y, Li C, Zhou L, Qi W, Zhang Y, Ye L. Effects of Different Components of PM2.5 on the Expression Levels of NF-κB Family Gene mRNA and Inflammatory Molecules in Human Macrophage. International Journal of Environmental Research and Public Health. 2019; 16(8):1408. https://doi.org/10.3390/ijerph16081408
Chicago/Turabian StyleZhu, Jian, Yaming Zhao, Yizhen Gao, Chunyan Li, Liting Zhou, Wen Qi, Yuezhu Zhang, and Lin Ye. 2019. "Effects of Different Components of PM2.5 on the Expression Levels of NF-κB Family Gene mRNA and Inflammatory Molecules in Human Macrophage" International Journal of Environmental Research and Public Health 16, no. 8: 1408. https://doi.org/10.3390/ijerph16081408
APA StyleZhu, J., Zhao, Y., Gao, Y., Li, C., Zhou, L., Qi, W., Zhang, Y., & Ye, L. (2019). Effects of Different Components of PM2.5 on the Expression Levels of NF-κB Family Gene mRNA and Inflammatory Molecules in Human Macrophage. International Journal of Environmental Research and Public Health, 16(8), 1408. https://doi.org/10.3390/ijerph16081408




