Flooding in Townsville, North Queensland, Australia, in February 2019 and Its Effects on Mosquito-Borne Diseases
Abstract
1. Introduction
2. Materials and Methods
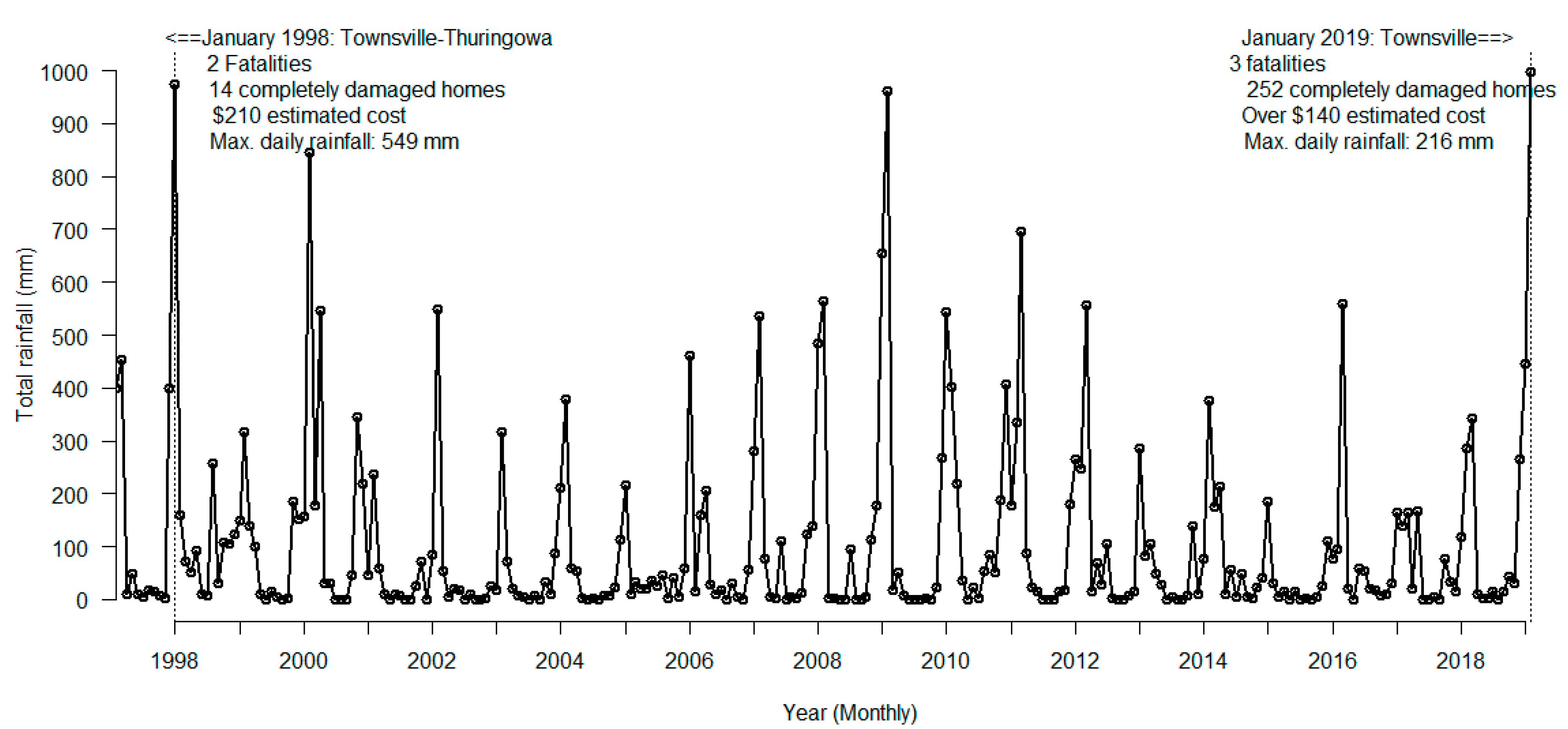
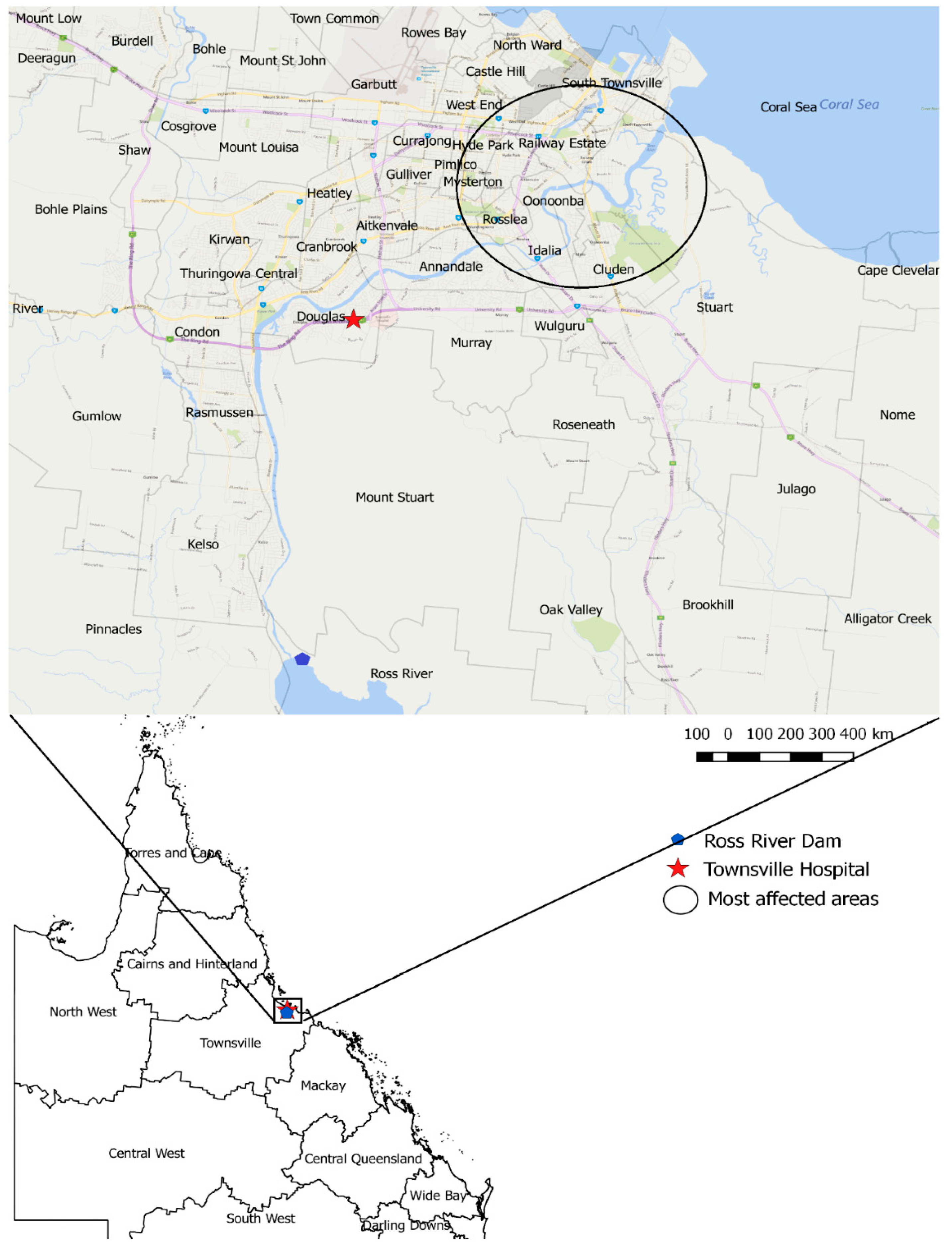
2.1. Study Area and Data Sources
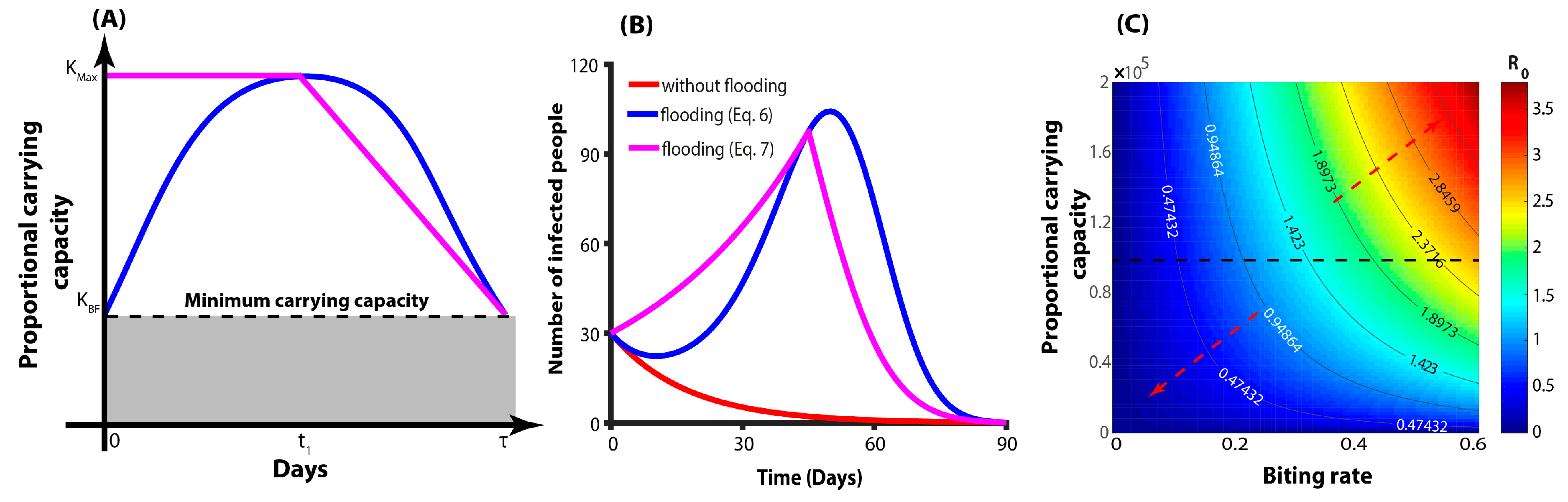
2.2. Mathematical Models
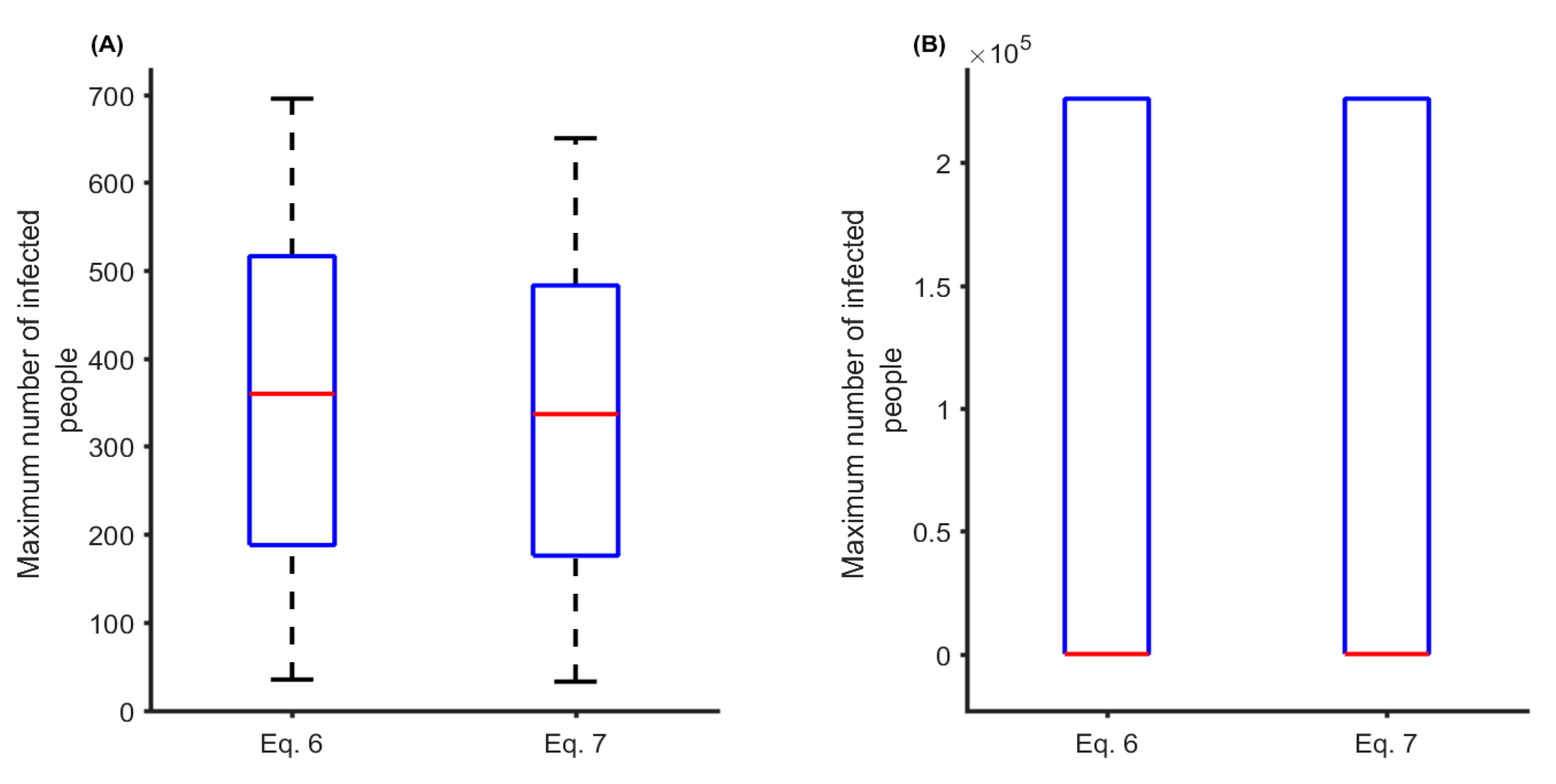
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armstrong, C. Flood crisis brings out best in community as thousands impacted. Townsville Bullettin. 5 February 2019. Available online: https://www.townsvillebulletin.com.au/news/flood-crisis-brings-out-best-in-community-as-thousands-impacted/news-story/00e6758bce1f7c31ad0691f7e8cefd09 (accessed on 8 February 2019).
- City of Townsville. Mosquito Borne Diseases. Available online: https://www.townsville.qld.gov.au/community-support/community-safety/mosquitoes/mosquito-borne-diseases (accessed on 8 February 2019).
- Webb, C.; Doggett, S.; Russell, R. A Guide to Mosquitoes of Australia; CSIRO Publishing: Clayton Victoria, Australia, 2016. [Google Scholar]
- Kearney, M.; Porter, W.P.; Williams, C.; Ritchie, S.; Hoffmann, A.A. Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: The dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 2009, 23, 528–538. [Google Scholar] [CrossRef]
- Vasconcelos, P. Flooding in Europe: A brief review of the health risks. Euro Surveill. 2006, 11, 2947. [Google Scholar] [CrossRef]
- Schmid, D.; Lederer, I.; Much, P.; Pichler, A.-M.; Allerberger, F. Outbreak of norovirus infection associated with contaminated flood water, Salzburg. Euro Surveill. 2005, 10, 2727. [Google Scholar]
- Saulnier, D.D.; Hanson, C.; Ir, P.; Mölsted, A.H.; von Schreeb, J. The Effect of Seasonal Floods on Health: Analysis of Six Years of National Health Data and Flood Maps. Int. J. Environ. Res. Public Health 2018, 15, 665. [Google Scholar] [CrossRef] [PubMed]
- Duchet, C.; Moraru, G.M.; Segev, O.; Spencer, M.; Hayoon, A.G.; Blaustein, L. Effects of flash flooding on mosquito and community dynamics in experimental pools. J. Vector Ecol. 2017, 42, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Tall, J.A.; Gatton, M.L.; Tong, S. Ross River Virus Disease Activity Associated with Naturally Occurring Nontidal Flood Events in Australia: A Systematic Review. J. Med. Entomol. 2014, 51, 1097–1108. [Google Scholar] [CrossRef]
- Hashizume, M.; Dewan, A.M.; Sunahara, T.; Rahman, M.Z.; Yamamoto, T. Hydroclimatological variability and dengue transmission in Dhaka, Bangladesh: A time-series study. BMC Infect. Dis. 2012, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Webb, C. After the Floods Come the Mosquitoes—But the Disease Risk is More Difficult to Predict: The Conversation. 2019. Available online: https://theconversation.com/after-the-floods-come-the-mosquitoes-but-the-disease-risk-is-more-difficult-to-predict-111173 (accessed on 8 February 2019).
- Warfe, D.M.; Pettit, N.E.; Davies, P.M.; Pusey, B.J.; Hamilton, S.; Kennard, M.J.; Townsend, S.A.; Bayliss, P.; Ward, D.P.; Douglas, M.M.; et al. The ‘wet–dry’in the wet–dry tropics drives river ecosystem structure and processes in northern Australia. Freshw. Biol. 2011, 56, 2169–2195. [Google Scholar] [CrossRef]
- Petheram, C.; McMahon, T.A.; Peel, M.C. Flow characteristics of rivers in northern Australia: Implications for development. J. Hydrol. 2008, 357, 93–111. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. 2016 Census QuickStats. Available online: http://quickstats.censusdata.abs.gov.au/census_services/getproduct/census/2016/quickstat/318?opendocument. (accessed on 12 February 2019).
- Queensland Health. Notifiable Conditions Weekly Totals 2019. Available online: https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/diseases-infection/surveillance/reports/notifiable/weekly (accessed on 7 February 2019).
- Martcheva, M. An Introduction to Mathematical Epidemiology; Springer: New York, NY, USA, 2015; Volume 61. [Google Scholar]
- Flaxman, J.P.; Smith, D.W.; Mackenzie, J.S.; Fraser, J.R.E.; Bass, S.P.; Hueston, L.; Lindsay, M.D.A.; Cunningham, A.L. A comparison of the diseases caused by Ross River virus and Barmah Forest virus. Med. J. Aust. 1998, 169, 159–163. [Google Scholar] [CrossRef]
- Warrilow, D.; Northill, J.A.; Pyke, A.T. Sources of dengue viruses imported into Queensland, Australia, 2002–2010. Emerg. Infect. Dis. 2012, 18, 1850. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.L.; Ryan, P.A.; Turley, A.P.; Wilson, G.; Retzki, K.; Iturbe-Ormaetxe, I.; Dong, Y.; Kenny, N.; Paton, C.J.; Ritchie, S.A.; et al. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res. 2018, 2, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.M.; Kien, D.T.; Clapham, H.; Aguas, R.; Trung, V.T.; Chau, T.N.; Popovici, J.; Ryan, P.A.; O’Neill, S.L.; McGraw, E.A.; et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci. Transl. Med. 2015, 279, 279ra37. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Pyke, A.T.; Hall-Mendelin, S.; Day, A.; Mores, C.N.; Christofferson, R.C.; Gubler, D.J.; Bennett, S.N.; van den Hurk, A.F. An explosive epidemic of DENV-3 in Cairns, Australia. PLoS ONE 2013, 8, e68137. [Google Scholar] [CrossRef]
- Polwiang, S. Estimation of dengue infection for travelers in Thailand. Travel Med. Infect. Dis. 2016, 14, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Comiskey, C.; Lindsay, M.D.A.; Cross, J.A.; Anderson, M. Modelling the transmission dynamics of Ross River virus in southwestern Australia. Math. Med. Biol. A J. IMA 2002, 19, 61–74. [Google Scholar] [CrossRef]
- Glass, K. Ecological mechanisms that promote arbovirus survival: A mathematical model of Ross River virus transmission. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Romeo-Aznar, V.; Paul, R.; Telle, O.; Pascual, M. Mosquito-borne transmission in urban landscapes: The missing link between vector abundance and human density. Proc. Biol. Sci. 2018, 285, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Deaths in Australia 2018. Available online: https://www.aihw.gov.au/reports/life-expectancy-death/deaths-in-australia/contents/life-expectancy. (accessed on 12 February 2019).
- Li, J.; Blakeley, D. The failure of 𝑅0. Comput. Math. Methods Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Garba, S.M.; Gumel, A.B.; Abu Bakar, M.R. Backward bifurcations in dengue transmission dynamics. Math. Biosci. 2008, 215, 11–25. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Description | Value (Range) | Unit | References |
|---|---|---|---|---|
| Transmission probability | 0.25–0.75 | Dimensionless | [21,22,23] | |
| Biting rate | 0.172–0.375 | Person per mosquito per day | [21,24] | |
| Mosquito birth rate | 0.09 | Per day | [25] | |
| Proportional carrying capacity | 100,000 | Mosquitoes | Estimated | |
| Maximum proportional carrying capacity due to flooding | 200,000 | Mosquitoes | Assumed | |
| Total population | 226,031 | People | [16] | |
| Time until carrying capacity normalizes | 90 | Day | Assumed | |
| Recovery rate | 0.143–0.22 | Per day | [21,24] | |
| Mortality rate | 0.000034 | Per day | [26] | |
| Mosquito death rate | 0.026–0.036 | Per day | [21] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adekunle, A.I.; Adegboye, O.A.; Rahman, K.M. Flooding in Townsville, North Queensland, Australia, in February 2019 and Its Effects on Mosquito-Borne Diseases. Int. J. Environ. Res. Public Health 2019, 16, 1393. https://doi.org/10.3390/ijerph16081393
Adekunle AI, Adegboye OA, Rahman KM. Flooding in Townsville, North Queensland, Australia, in February 2019 and Its Effects on Mosquito-Borne Diseases. International Journal of Environmental Research and Public Health. 2019; 16(8):1393. https://doi.org/10.3390/ijerph16081393
Chicago/Turabian StyleAdekunle, Adeshina I., Oyelola A. Adegboye, and Kazi Mizanur Rahman. 2019. "Flooding in Townsville, North Queensland, Australia, in February 2019 and Its Effects on Mosquito-Borne Diseases" International Journal of Environmental Research and Public Health 16, no. 8: 1393. https://doi.org/10.3390/ijerph16081393
APA StyleAdekunle, A. I., Adegboye, O. A., & Rahman, K. M. (2019). Flooding in Townsville, North Queensland, Australia, in February 2019 and Its Effects on Mosquito-Borne Diseases. International Journal of Environmental Research and Public Health, 16(8), 1393. https://doi.org/10.3390/ijerph16081393






