Exposure to Atmospheric Ultrafine Particles Induces Severe Lung Inflammatory Response and Tissue Remodeling in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. PM Sampling
2.2. Particle Size Distribution
2.3. Trace Elements and PAH Analysis in FF and UFP
2.4. Animal Housing
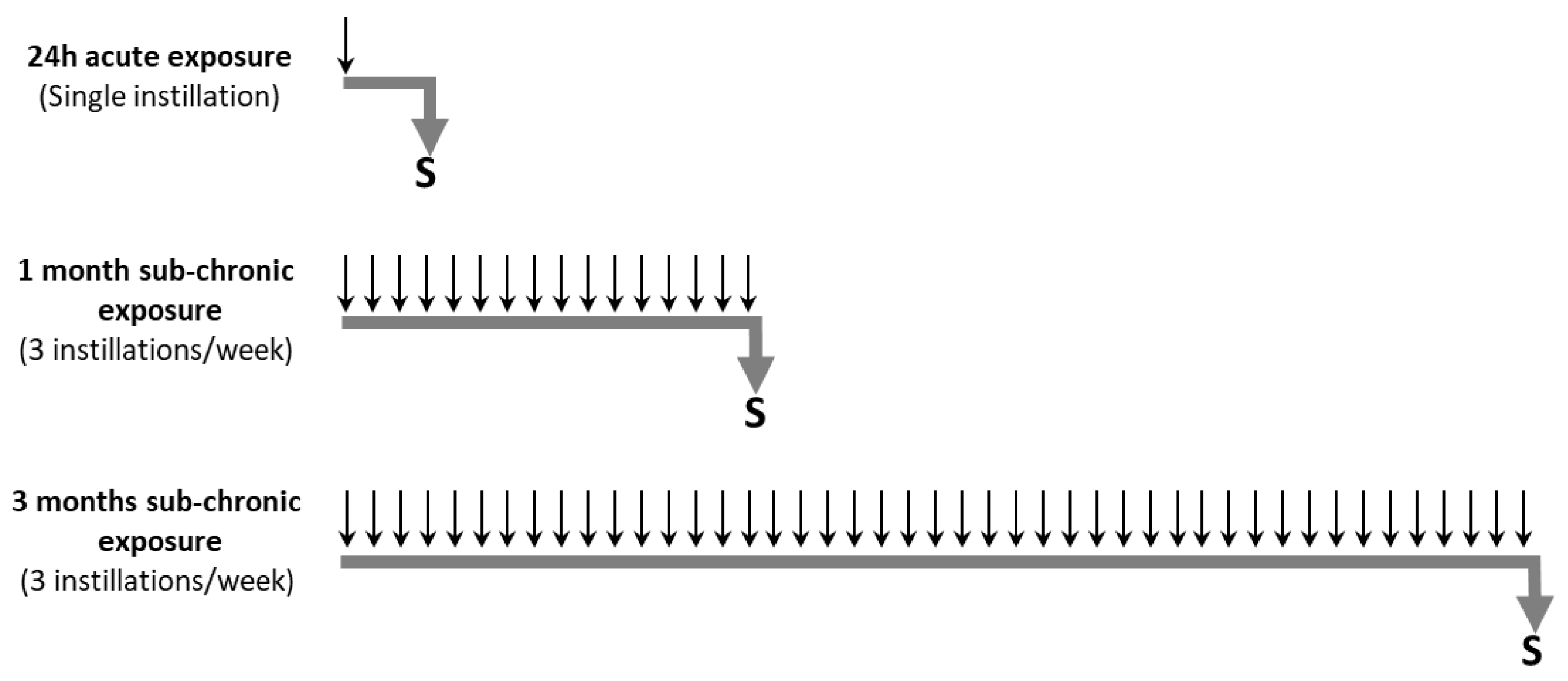
2.5. Animal Exposure to Particles
2.6. Biological Sample Collection
2.7. Numeration of BAL Cells
2.8. Lung Histology and Lung Injury Scoring
2.9. RNA Extraction and Real-Time PCR
2.10. Statistical Analysis
3. Results
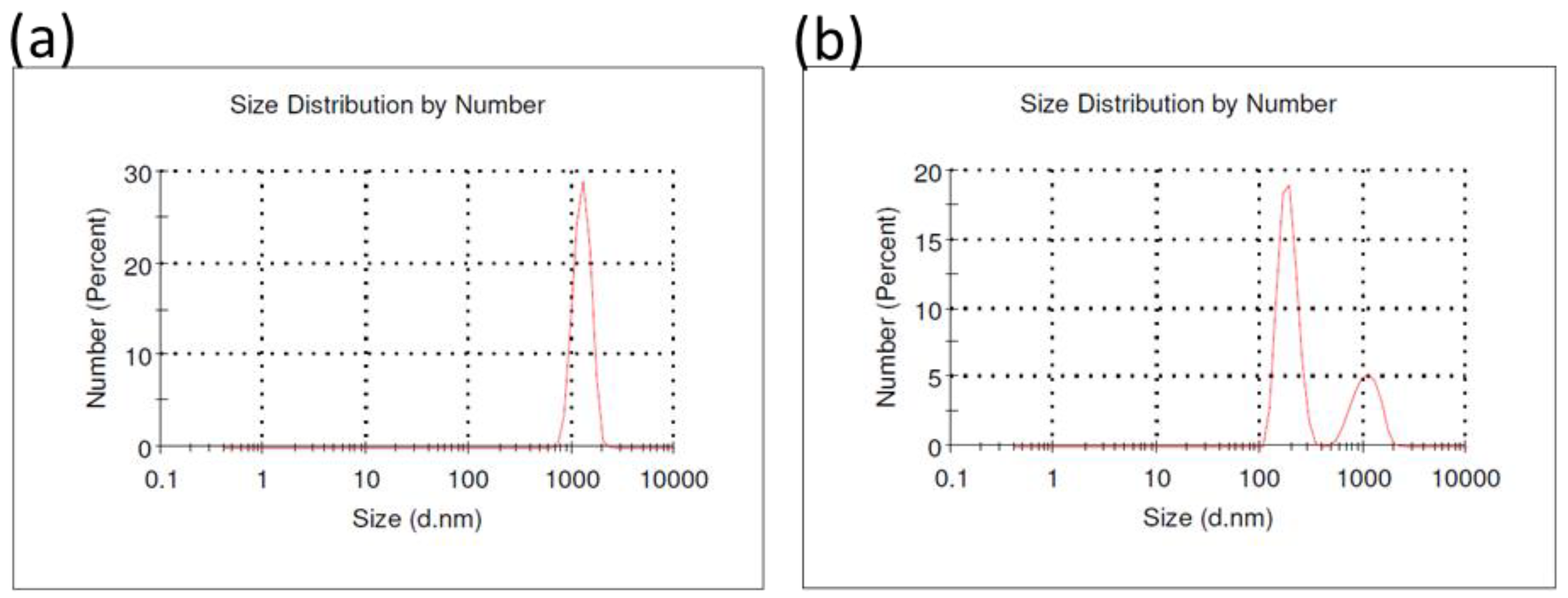
3.1. Physicochemical Characterization of Particles
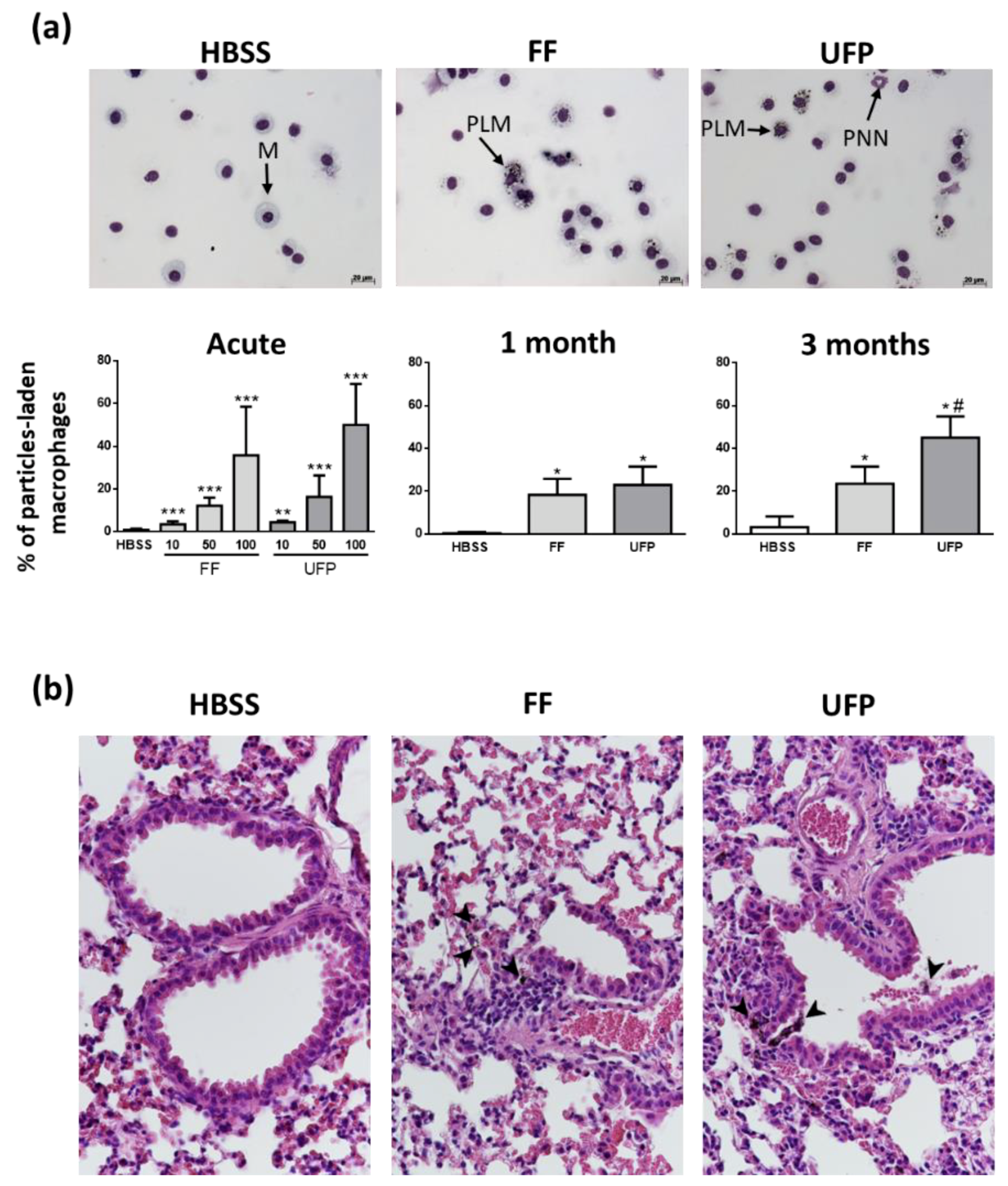
3.2. Internalization of Particles in Lungs
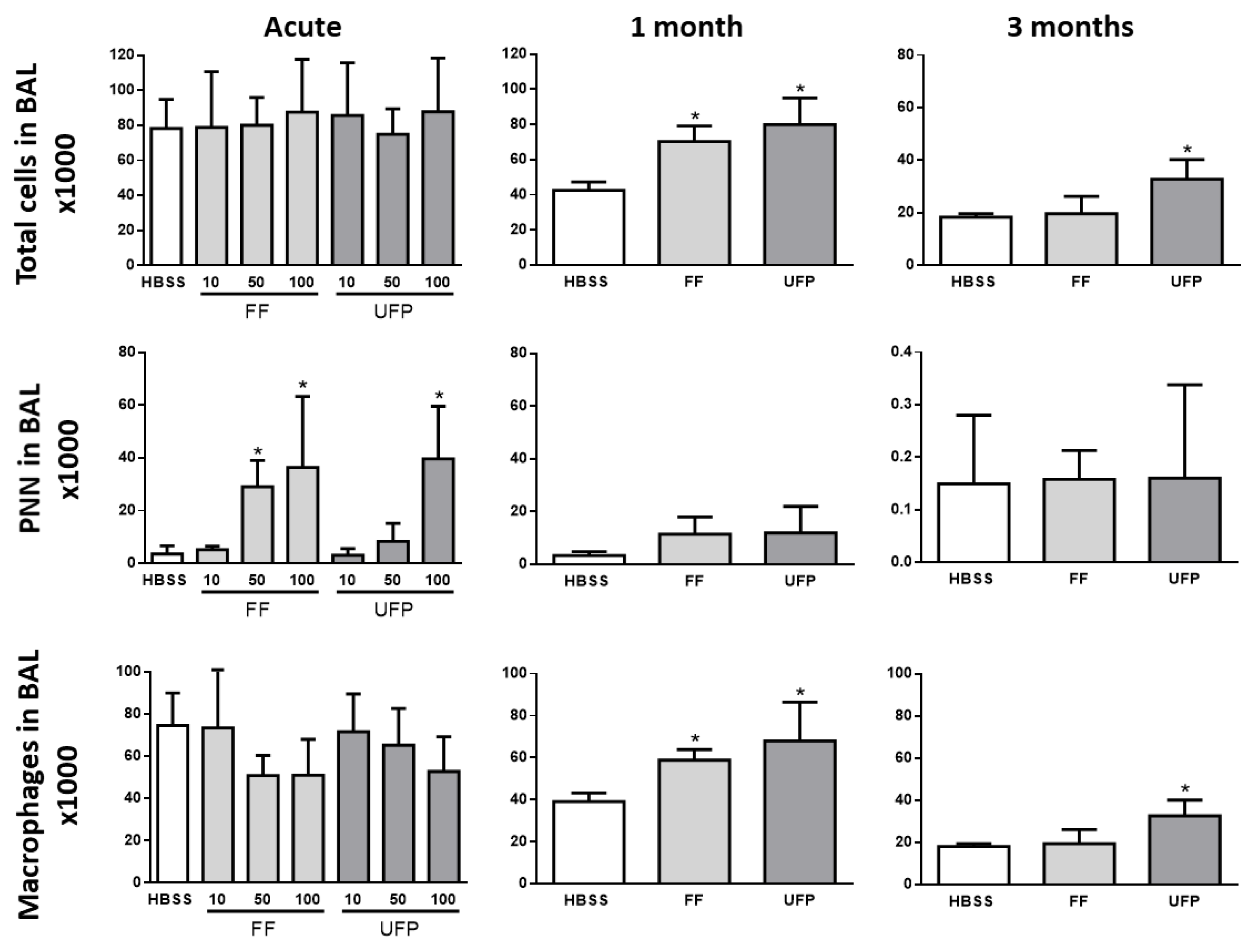
3.3. Inflammatory Cell Analysis in BAL
3.4. Differential Expression of Inflammation-Related Genes in the Lung of Mice Exposed to FF or UFP
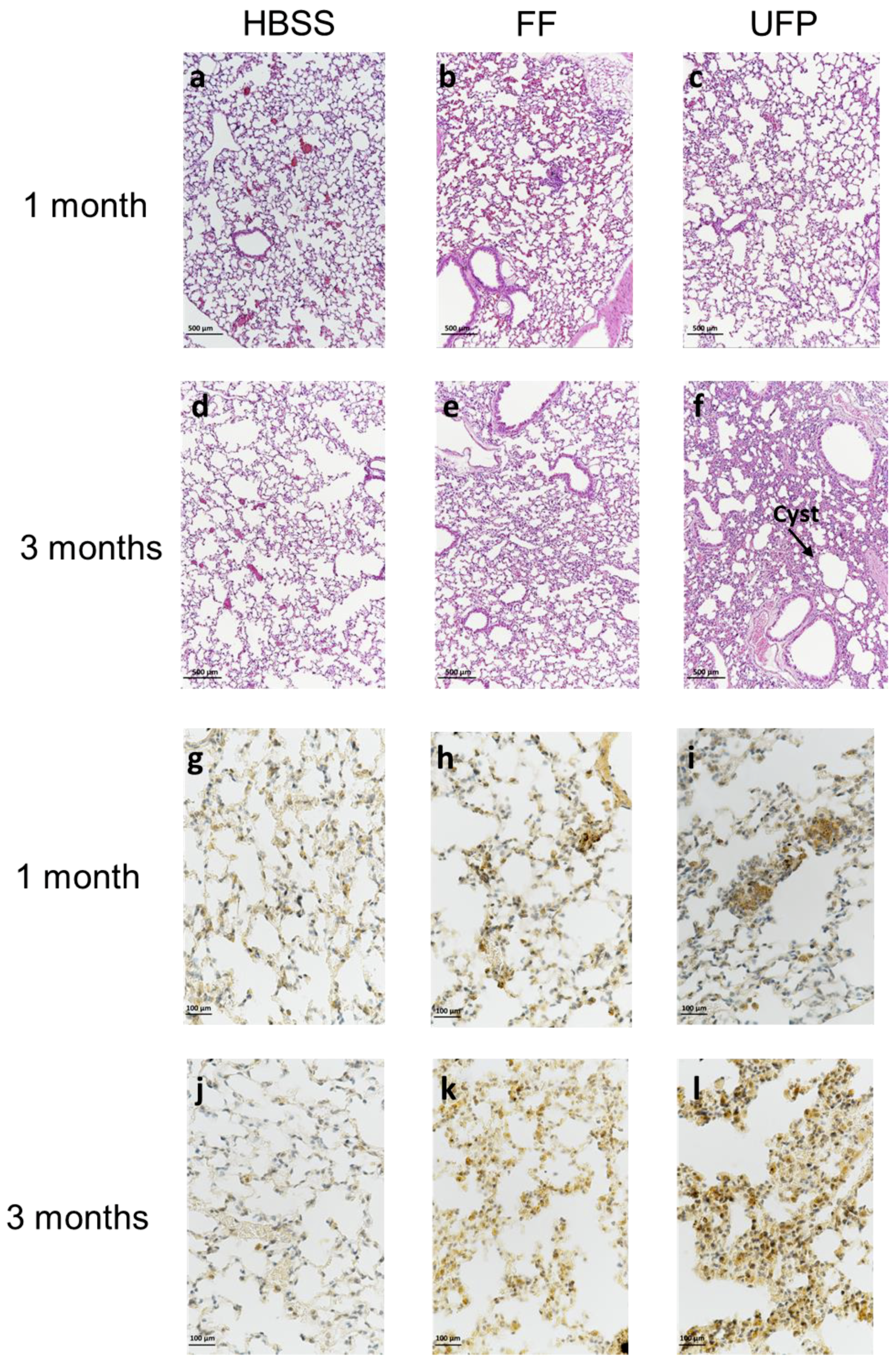
3.5. Lung Tissue Remodeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lelieveld, J.; Klingmüller, K.; Pozzer, A.; Pöschl, U.; Fnais, M.; Daiber, A.; Münzel, T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, composition, and lung diseases. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.Q.; FitzGerald, J.M.; Carlsten, C.; Sadatsafavi, M.; Brauer, M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am. J. Respir. Crit. Care. Med. 2013, 187, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Song, H.; Chen, L.; Hu, B.; Bai, R.; Xu, D.; Liu, Y.; Zhao, Y.; Chen, C. Early-life exposure to three size-fractionated ultrafine and fine atmospheric particulates in Beijing exacerbates asthma development in mature mice. Part. Fibre Toxicol. 2018, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Huang, Y.; Han, J.; Song, F.; Chen, K. Ambient particulate matter and lung cancer incidence and mortality: A meta-analysis of prospective studies. Eur. J. Public Health 2015, 25, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Lippmann, M. Effects of metals within ambient air particulate matter (PM) on human health. Inhal. Toxicol. 2009, 21, 1–31. [Google Scholar] [CrossRef]
- Tyler, C.R.; Zychowski, K.E.; Sanchez, B.N.; Rivero, V.; Lucas, S.; Herbert, G.; Liu, J.; Irshad, H.; McDonald, J.D.; Bleske, B.E. Surface area-dependence of gas-particle interactions influences pulmonary and neuroinflammatory outcomes. Part. Fibre Toxicol. 2016, 13, 64. [Google Scholar] [CrossRef]
- Ebersviller, S.; Lichtveld, K.; Sexton, K.G.; Zavala, J.; Lin, Y.-H.; Jaspers, I.; Jeffries, H.E. Gaseous VOCs rapidly modify particulate matter and its biological effects—Part 1: Simple VOCs and model PM. Atmos. Chem. Phys. 2012, 12, 5065–5105. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Peters, J.I. Williams, R.O. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Utell, M.J. Ultrafine particles in the urban air: To the respiratory tract—And beyond? Environ. Health Perspect. 2002, 110, 440–441. [Google Scholar] [CrossRef]
- Hussein, T.; Puustinen, A.; Aalto, P.P.; Mäkelä, J.M.; Hämeri, K.; Kulmala, M. Urban aerosol number size distributions. Atmos. Chem. Phys. 2004, 4, 391–411. [Google Scholar] [CrossRef]
- Shi, J.P.; Evans, D.E.; Khan, A.A.; Harrison, R.M. Sources and concentration of nanoparticles (<10 nm diameter) in the urban atmosphere. Atmos. Environ. 2001, 35, 1193–1202. [Google Scholar] [CrossRef]
- Baldauf, R.W.; Devlin, R.B.; Gehr, P.; Giannelli, R.; Hassett-Sipple, B.; Jung, H.; Martini, G.; McDonald, J.; Sacks, J.; Walker, K. Ultrafine particle metrics and research considerations: Review of the 2015 UFP workshop. Int. J. Environ. Res. Public Health 2016, 13, 1054. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, A.; Hsu, J.; Yip, F. Respiratory health effects of ultrafine particles in children: A literature review. Water Air Soil Pollut. 2016, 227. [Google Scholar] [CrossRef] [PubMed]
- Riffault, V.; Arndt, J.; Marris, H.; Mbengue, S.; Setyan, A.; Alleman, L.Y.; Deboudt, K.; Flament, P.; Augustin, P.; Delbarre, H.; et al. Fine and ultrafine particles in the vicinity of industrial activities: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2305–2356. [Google Scholar] [CrossRef]
- Li, N.; Georas, S.; Alexis, N.; Fritz, P.; Xia, T.; Williams, M.A.; Horner, E.; Nel, A. A work group report on ultrafine particles (AAAAI) why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in humans. J. Allergy Clin. Immunol. 2016, 138, 386–396. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler-Behnke, M.; Möller, W. Ultrafine particle-lung interactions: Does size matter? J. Aerosol Med. 2006, 19, 74–83. [Google Scholar] [CrossRef]
- Hullmann, M.; Albrecht, C.; van Berlo, D.; Gerlofs-Nijland, M.E.; Wahle, T.; Boots, A.W.; Krutmann, J.; Cassee, F.R.; Bayer, T.A.; Schins, R.P.F. Diesel engine exhaust accelerates plaque formation in a mouse model of Alzheimer’s disease. Part. Fibre Toxicol. 2017, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Renwick, L.C.; Donaldson, K.; Clouter, A. Impairment of alveolar macrophage phagocytosis by ultrafine particles. Toxicol. Appl. Pharmacol. 2001, 172, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lanzinger, S.; Schneider, A.; Breitner, S.; Stafoggia, M.; Erzen, I.; Dostal, M.; Pastorkova, A.; Bastian, S.; Cyrys, J.; Zscheppang, A.; et al. Associations between ultrafine and fine particles and mortality in five central European cities—Results from the UFIREG study. Environ. Int. 2016, 88, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Frampton, M.W.; Rich, D.Q. Does particle size matter? Ultrafine particles and hospital visits in eastern Europe. Am. J. Respir. Crit. Care Med. 2016, 194, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.A.; Halterman, J.S.; Hopke, P.K.; Fagnano, M.; Rich, D.Q. Increased ultrafine particles and carbon monoxide concentrations are associated with asthma exacerbation among urban children. Environ. Res. 2014, 129, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Samoli, E.; Andersen, Z.J.; Katsouyanni, K.; Hennig, F.; Kuhlbusch, T.A.J.; Bellander, T.; Cattani, G.; Cyrys, J.; Forastiere, F.; Jacquemin, B.; et al. Exposure to ultrafine particles and respiratory hospitalisations in five European cities. Eur. Respir. J. 2016, 48, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, J.; Lanki, T.; Tiittanen, P.; Aalto, P.P.; Koskentalo, T.; Kulmala, M.; Salomaa, V.; Pekkanen, J. Associations of fine and ultrafine particulate air pollution with stroke mortality in an area of low air pollution levels. Stroke 2007, 38, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A. Are Ultrafine particles a risk factor for cardiovascular diseases? Rev. Esp. Cardiol. 2011, 64, 642–645. [Google Scholar] [CrossRef]
- Sun, Y.; Song, X.; Han, Y.; Ji, Y.; Gao, S.; Shang, Y.; Lu, S.E.; Zhu, T.; Huang, W. Size-fractioned ultrafine particles and black carbon associated with autonomic dysfunction in subjects with diabetes or impaired glucose tolerance in Shanghai, China. Part. Fibre Toxicol. 2015, 12. [Google Scholar] [CrossRef]
- Misra, C.; Kim, S.; Shen, S.; Sioutas, C. A high flow rate, very low pressure drop impactor for inertial separation of ultrafine from accumulation mode particles. J. Aerosol Sci. 2002, 33, 735–752. [Google Scholar] [CrossRef]
- Mbengue, S.; Alleman, L.Y.; Flament, P. Erratum to “Metal-bearing fine particle sources in a coastal industrialized environment” [Atmos. Res. 183 (2017) 202–211]. Atmos. Res. 2017, 189, 162. [Google Scholar] [CrossRef]
- Canepari, S.; Padella, F.; Astolfi, M.L.; Marconi, E.; Perrino, C. Elemental concentration in atmospheric particulate matter: Estimation of nanoparticle contribution. Aerosol Air Qual. Res. 2013, 13, 1619–1629. [Google Scholar] [CrossRef]
- Alleman, L.Y.; Lamaison, L.; Perdrix, E.; Robache, A.; Galloo, J.-C. PM10 metal concentrations and source identification using positive matrix factorization and wind sectoring in a French industrial zone. Atmos. Res. 2010, 96, 612–625. [Google Scholar] [CrossRef]
- Mbengue, S.; Alleman, L.Y.; Flament, P. Size-distributed metallic elements in submicronic and ultrafine atmospheric particles from urban and industrial areas in northern France. Atmos. Res. 2014, 135–136, 35–47. [Google Scholar] [CrossRef]
- Crenn, V.; Chakraborty, A.; Fronval, I.; Petitprez, D.; Riffault, V. Fine particles sampled at an urban background site and an industrialized coastal site in Northern France—Part 2: Comparison of offline and online analyses for carbonaceous aerosols. Aerosol Sci. Technol. 2018, 52, 287–299. [Google Scholar] [CrossRef]
- Meuwissen, R.; Berns, A. Mouse models for human lung cancer. Genes Dev. 2005, 19, 643–664. [Google Scholar] [CrossRef]
- Dergham, M.; Lepers, C.; Verdin, A.; Billet, S.; Cazier, F.; Courcot, D.; Shirali, P.; Garçon, G. Prooxidant and proinflammatory potency of air pollution particulate matter (PM2.5–0.3) produced in rural, urban, or industrial surroundings in human bronchial epithelial cells (BEAS-2B). Chem. Res. Toxicol. 2012, 25, 904–919. [Google Scholar] [CrossRef]
- Dergham, M.; Lepers, C.; Verdin, A.; Cazier, F.; Billet, S.; Courcot, D.; Shirali, P.; Garçon, G. Temporal–spatial variations of the physicochemical characteristics of air pollution Particulate matter (PM2.5–0.3) and toxicological effects in human bronchial epithelial cells (BEAS-2B). Environ. Res. 2015, 137, 256–267. [Google Scholar] [CrossRef]
- Saarnio, K.; Sillanpää, M.; Hillamo, R.; Sandell, E.; Pennanen, A.S.; Salonen, R.O. Polycyclic aromatic hydrocarbons in size-segregated particulate matter from six urban sites in Europe. Atmos. Environ. 2008, 42, 9087–9097. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, W.; Meng, Q.; Lin, Q.; Fang, C.; Huang, X.; Li, C.; Huang, Y.; Tan, J. Ambient PM2.5 exposure exacerbates severity of allergic asthma in previously sensitized mice. J. Asthma 2015, 52, 785–794. [Google Scholar] [CrossRef]
- Churg, A.; Brauer, M.; del Carmen Avila-Casado, M.; Fortoul, T.I.; Wright, J.L. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ. Health Perspect. 2003, 111, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Grunig, G.; Marsh, L.M.; Esmaeil, N.; Jackson, K.; Gordon, T.; Reibman, J.; Kwapiszewska, G.; Park, S.H. Perspective: Ambient air pollution: Inflammatory response and effects on the lung’s vasculature. Pulm. Circ. 2014, 4, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Ferin, J.; Lehnert, B.E. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994, 102, 173–179. [Google Scholar] [PubMed]
- Tamaoki, J.; Isono, K.; Takeyama, K.; Tagaya, E.; Nakata, J.; Nagai, A. Ultrafine carbon black particles stimulate proliferation of human airway epithelium via EGF receptor-mediated signaling pathway. Am. J. Physiol. Lung. Cell Mol. Physiol. 2004, 287, L1127–L1133. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, B.; Platel, A.; Antherieu, S.; Alleman, L.Y.; Hardy, E.M.; Perdrix, E.; Grova, N.; Riffault, V.; Appenzeller, B.M.; Happillon, M.; et al. Genetic and epigenetic alterations in normal and sensitive COPD-diseased human bronchial epithelial cells repeatedly exposed to air pollution-derived PM 2.5. Environ. Pollut. 2017, 230, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Dawidowski, L.; Mandalunis, P.; Cereceda-Balic, F.; Tasat, D.R. Characterization and biological effect of Buenos Aires urban air particles on mice lungs. Environ. Res. 2007, 105, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Southam, D.S.; Dolovich, M.; O’Byrne, P.M.; Inman, M.D. Distribution of intranasal instillations in mice: Effects of volume, time, body position, and anesthesia. Am. J. Physiol. Lung. Cell Mol. Physiol. 2002, 282, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Van Berlo, D.; Albrecht, C.; Knaapen, A.M.; Cassee, F.R.; Gerlofs-Nijlandm, M.E.; Kooter, I.M.; Palomero-Gallagher, N.; Bidmon, H.J.; van Schootenm, F.J.; Krutmann, J.; et al. Comparative evaluation of the effects of short-term inhalation exposure to diesel engine exhaust on rat lung and brain. Arch. Toxicol. 2010, 84, 553–562. [Google Scholar] [CrossRef]
- Van Winkle, L.S.; Bein, K.; Anderson, D.; Pinkerton, K.E.; Tablin, F.; Wilson, D.; Wexler, A.S. Biological dose response to PM2.5: Effect of particle extraction method on platelet and lung responses. Toxicol. Sci. 2015, 143, 349–359. [Google Scholar] [CrossRef]
- Kusaka, T.; Nakayama, M.; Nakamura, K.; Ishimiya, M.; Furusawa, E.; Ogasawara, K. Effect of silica particle size on macrophage inflammatory responses. PLoS ONE 2014, 9, e92634. [Google Scholar] [CrossRef]
- Levy, B.D.; Serhan, C.N. Resolution of acute inflammation in the lung. Annu. Rev. Physiol. 2014, 76, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, L.; Ju, W.; Wang, X.; Dong, L.; Zhang, Y.; Ya, P.; Yang, C.; Li, F. The acute airway inflammation induced by PM2.5 exposure and the treatment of essential oils in Balb/c mice. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.O.; Van Dyke, T.E. Natural resolution of inflammation. Periodontol 2013, 63, 149–164. [Google Scholar] [CrossRef]
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009, 2, 1. [Google Scholar] [PubMed]
- de Haar, C.; Hassing, I.; Bol, M.; Bleumink, R.; Pieters, R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin. Exp. Allergy 2006, 36, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Cox, G. IL-10 enhances resolution of pulmonary inflammation in vivo by promoting apoptosis of neutrophils. Am. J. Physiol. 1996, 271, 566–571. [Google Scholar] [CrossRef]
- Brown, D.M.; Dickson, C.; Duncan, P.; Al-Attili, F.; Stone, V. Interaction between nanoparticles and cytokine proteins: Impact on protein and particle functionality. Nanotechnology 2010, 21, 215104. [Google Scholar] [CrossRef] [PubMed]
- Veranth, J.M.; Kaser, E.G.; Veranth, M.M.; Koch, M.; Yost, G.S. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part. Fibre Toxicol. 2007, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Caramori, G.; Casolari, P.; Barczyk, A.; Durham, A.L.; Di Stefano, A.; Adcock, I. COPD immunopathology. Semin. Immunopathol. 2016, 38, 497–515. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.; Sood, R.G.; Sarkar, M.; Thakur, V. Quantitative computed tomography (CT) assessment of emphysema in patients with severe chronic obstructive pulmonary disease (COPD) and its correlation with age, sex, pulmonary function tests, BMI, smoking, and biomass exposure. Pol. J. Radiol. 2017, 82, 760–766. [Google Scholar] [CrossRef]
- Kariisa, M.; Foraker, R.; Pennell, M.; Buckley, T.; Diaz, P.; Criner, G.J.; Wilkins, J.R. Short-and long-term effects of ambient ozone and fine particulate matter on the respiratory health of chronic obstructive pulmonary disease subjects. Arch. Environ. Occup. Health 2015, 70, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Adar, S.D.; Kaufman, J.D.; Diez-Roux, A.V.; Hoffman, E.A.; D’Souza, J.; Stukovsky, K.H.; Rich, S.S.; Rotter, J.I.; Guo, X.; Raffel, L.J.; et al. Air pollution and percent emphysema identified by computed tomography in the Multi-Ethnic study of Atherosclerosis. Environ. Health Perspect. 2015, 123, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Tsai, C.-J.; Huang, C.-Y.; Chen, H.-D.; Chen, S.-J.; Lin, C.-C.; Tsai, J.H.; Chou, C.; Lung, C.; Huang, W.R.; et al. Chemical mass closure and chemical characteristics of ambient ultrafine particles and other PM Fractions. Aerosol Sci. Technol. 2010, 44, 713–723. [Google Scholar] [CrossRef]
- Xia, T.; Zhu, Y.; Mu, L.; Zhang, Z.-F.; Liu, S. Pulmonary diseases induced by ambient ultrafine and engineered nanoparticles in twenty-first century. Natl. Sci. Rev. 2016, 3, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, A.; Kanno, S.; Kobayashi, T.; Hirano, S. Extrapulmonary translocation of intratracheally instilled fine and ultrafine particles via direct and alveolar macrophage-associated routes. Arch. Toxicol. 2009, 83, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Ferin, J.; Oberdörster, G.; Penney, D.P. Pulmonary retention of ultrafine and fine particles in rats. Am. J. Respir. Cell Mol. Biol. 1992, 6, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Cheng, W.-Y.; Samet, J.M.; Gilmour, M.I.; Devlin, R.B. Differential cardiopulmonary effects of size-fractionated ambient particulate matter in mice. Cardiovasc. Toxicol. 2010, 10, 259–267. [Google Scholar] [CrossRef]
| Element (µg/g) | FF | UFP |
|---|---|---|
| As | 23.0 | 64.8 |
| Ba | 140.3 | 64.4 |
| Be | 0.4 | 0.3 |
| Cd | 16.9 | 19.3 |
| Ce | 8.5 | 6.3 |
| Co | 5.4 | 9.3 |
| Cs | 4.4 | 8.9 |
| Cu | 367.0 | 425.2 |
| La | 8.4 | 3.1 |
| Mn | 1350.4 | 582.2 |
| Mo | 25.6 | 36.7 |
| Ni | 98.7 | 199.7 |
| Pb | 399.4 | 541.9 |
| Rb | 26.3 | 44.1 |
| Sb | 47.1 | 49.3 |
| Sn | 49.6 | 111.1 |
| Sr | 91.8 | 49.3 |
| Ti | 3.0 | 5.7 |
| Zn | 4000.2 | 2460.7 |
| Cr | 97.3 | 120.7 |
| V | 75.0 | 196.3 |
| Al | 5252.2 | 3621.1 |
| Ca | 17,904.5 | 11,857.4 |
| Fe | 16,406.1 | 10,267.0 |
| K | 1240.3 | 30,368 * |
| Mg | 6591.3 | 6344.8 * |
| Na | 43,510.8 | 283,147 * |
| Si | 9383.5 | 7568.1 |
| PAH (ng/mg) | FF | UFP |
|---|---|---|
| Fluoranthene (FLA) | 5.1 | 4.3 |
| Pyrene (PYR) | 4.2 | 3.7 |
| Benzo(c)phenanthrene (BcPHE) | 0.4 | 0.2 |
| Benzo(a)anthracene (BaA) | 6.9 | 4.2 |
| Chrysene (CHR) | 6.8 | 2.8 |
| 5-Methylchrysene (5MCHR) | 1.1 | 3.4 |
| Benzo(e)pyrene (BeP) | 9.9 | 0.8 |
| Benzo(b)fluoranthene (BbF) | 7.6 | 8.0 |
| Benzo(j)fluoranthene (BjF) | 4.7 | 9.9 |
| Benzo(k)fluoranthene (BkF) | 7.4 | 4.6 |
| Benzo(a)pyrene (BaP) | 3.8 | 5.3 |
| Dibenzo(a,l)pyrene (DalP) | 0.8 | 0.4 |
| Dibenzo(a,h)anthracene (DahA) | 2.6 | 1.5 |
| Benzo(g,h,i)perylene (BghiP) | 12.0 | 12.5 |
| Indeno(1,2,3-c,d)pyrene (IP) | 14.0 | 12.1 |
| Dibenzo(a,e)pyrene (DaeP) | 3.6 | 2.7 |
| Anthanthrene (ANTH) | 0.1 | 3.3 |
| Coronene (COR) | 2.9 | 4.5 |
| Total | 94.0 | 84.0 |
| Acute Exposure | ||||||
|---|---|---|---|---|---|---|
| FF 10 | FF 50 | FF 100 | UFP 10 | UFP 50 | UFP 100 | |
| Cyp1a1 | 0.8 ± 0.5 | 1.2 ± 0.6 | 1.7 ± 0.5 * | 0.8 ± 0.5 | 1.4 ± 0.4 | 1.7 ±0.5 * |
| Cyp1b1 | 1.3 ± 0.3 | 1.6 ± 0.5 | 2.6 ± 1.6 ** | 0.9 ± 0.4 | 0.9 ± 0.2 | 2.2 ±1.0 * |
| Il-6 | 2.3 ± 2.5 | 1.3 ± 1.1 | 1.7 ± 1.9 | 1.8 ± 0.9 | 2.7 ±1.9 | 6.1 ±4.1 * |
| Il-1b | 0.7 ± 0.3 | 1.0 ± 0.3 | 0.8 ± 0.1 | 1.3 ± 0.2 | 1.7 ± 0.8 | 2.7 ± 1.2 |
| Il-10 | 1.1 ± 0.6 | 1.6 ± 0.5 | 1.0 ± 0.4 | 1.0 ± 0.7 | 1.6 ± 0.6 | 1.7 ± 0.8 |
| 1 Month | 3 Months | |||||
| FF 10 | UFP 10 | FF 10 | UFP 10 | |||
| Cyp1a1 | 7.8 ± 4.2 ** | 3.5 ± 2.6 ** | 5.6 ± 2.0 ** | 1.8 ± 0.6 | ||
| Cyp1b1 | 1..9 ± 0.2 | 1.6 ± 1.1 | 2.7 ± 0.9 * | 3.9 ± 2.2 ** | ||
| Il-6 | 0.4 ± 0.2 ** | 0.4 ± 0.1 * | 2.5 ± 1.6 | 7.8 ± 2.9 * | ||
| Il-1b | 1.1 ± 0.6 | 0.9 ± 0.2 | 0.7 ± 0.1 | 1.0 ± 0.3 | ||
| Il-10 | 0.4 ± 0.2 | 0.5 ± 0.1 | 2.2 ± 1.9 | 3.9 ± 1.0 * | ||
| 1 Month | 3 Months | |||||
|---|---|---|---|---|---|---|
| HBSS | FF | UFP | HBSS | FF | UFP | |
| Peribronchiolar inflammation | 0 ± 0 | 0.7 ± 0.6 | 0.7 ± 0.6 | 0 ± 0 | 1 ± 0.0 | 1.7 ± 0.6 |
| Total macrophages/10 HPF | 45.3 ± 16.3 | 275 ± 81.8 | 342.7 ± 74.4 | 53 ± 12.0 | 211 ± 122.7 | 500 ± 213.7 |
| Cystic regions | 0 ± 0 | 1.3 ± 0.6 | 0.7 ± 0.6 | 0 ± 0 | 1.3 ± 0.6 | 1.7 ± 0.6 |
| Alveolar wall thickness | 0.7 ± 0.6 | 1.7 ± 0.6 | 1.7 ± 0.6 | 1.0 ± 0 | 1.7 ± 0.6 | 2.3 ± 0.6 |
| Lesioned parenchyma surface (mm2) | 0.6 ± 0.8 | 5.5 ± 1.0 | 9.8 ± 2.0 | 1.8 ± 0.8 | 21.9 ± 9.0 | 28.3 ± 3.8 |
| Overall lung surface (mm2) | 78.3 ± 4.3 | 66.3 ± 9.1 | 56.5 ± 8.4 | 64.8 ± 7.7 | 68.3 ± 14.0 | 61.7 ± 14.1 |
| Percentage of lesioned areas | 0.8 ± 0.9 | 8.3 ± 0.4 | 18 ± 6.6 | 2.7 ± 0.9 | 35 ± 21.2 | 47.3 ± 11.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, Y.; Antherieu, S.; Dusautoir, R.; Y. Alleman, L.; Sotty, J.; De Sousa, C.; Platel, A.; Perdrix, E.; Riffault, V.; Fronval, I.; et al. Exposure to Atmospheric Ultrafine Particles Induces Severe Lung Inflammatory Response and Tissue Remodeling in Mice. Int. J. Environ. Res. Public Health 2019, 16, 1210. https://doi.org/10.3390/ijerph16071210
Saleh Y, Antherieu S, Dusautoir R, Y. Alleman L, Sotty J, De Sousa C, Platel A, Perdrix E, Riffault V, Fronval I, et al. Exposure to Atmospheric Ultrafine Particles Induces Severe Lung Inflammatory Response and Tissue Remodeling in Mice. International Journal of Environmental Research and Public Health. 2019; 16(7):1210. https://doi.org/10.3390/ijerph16071210
Chicago/Turabian StyleSaleh, Yara, Sébastien Antherieu, Romain Dusautoir, Laurent Y. Alleman, Jules Sotty, Corentin De Sousa, Anne Platel, Esperanza Perdrix, Véronique Riffault, Isabelle Fronval, and et al. 2019. "Exposure to Atmospheric Ultrafine Particles Induces Severe Lung Inflammatory Response and Tissue Remodeling in Mice" International Journal of Environmental Research and Public Health 16, no. 7: 1210. https://doi.org/10.3390/ijerph16071210
APA StyleSaleh, Y., Antherieu, S., Dusautoir, R., Y. Alleman, L., Sotty, J., De Sousa, C., Platel, A., Perdrix, E., Riffault, V., Fronval, I., Nesslany, F., Canivet, L., Garçon, G., & Lo-Guidice, J.-M. (2019). Exposure to Atmospheric Ultrafine Particles Induces Severe Lung Inflammatory Response and Tissue Remodeling in Mice. International Journal of Environmental Research and Public Health, 16(7), 1210. https://doi.org/10.3390/ijerph16071210







