The Relationship between Body Fat Percentage and Some Anthropometric and Physical Fitness Characteristics in Pre- and Peripubertal Boys
Abstract
1. Introduction
2. Materials and Methods
- Standing long jump (SLJ) for the estimation of explosive strength of the lower limbs and leg-trunk-arm coordination. Reading accuracy was 1 cm. The subjects had three trials, and the best score was entered into the statistical analysis.
- Fistball throw (FBT) for the estimation of explosive strength of the shoulder and arm muscles and arm-trunk-leg coordination. Reading accuracy was 0.1 m. The subjects had three trials, and the best score was entered into the statistical analysis (a fistball is a 120g, 8cm diameter ball).
- 30 m dash for the estimation of running speed. Reading accuracy was 0.01 s.
- 1200 m run for the estimation of running endurance (outdoor). Reading accuracy was 1 s.
Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation. Obesity—Preventing and Managing the Global Epidemic; Report of a WHO Consultation; WHO, Technical Report Service: Geneva, Switzerland, 2000; pp. 1–253. [Google Scholar]
- Lobstein, T.; Frelut, M.L. Prevalence of overweight among children in Europe. Obes. Rev. 2003, 4, 195–200. [Google Scholar] [CrossRef]
- McDowell, M.A.; Fryar, C.D.; Ogden, C.L.; Flegal, K.M. Anthropometric Reference Data for Children and Adults: United States, 2003–2006; National Health Statistics Report No. 10; National Center for Health Statistics: Hewittsville, MD, USA, 2008.
- Ji, C.Y.; Cheng, T.J. Epidemic increase in overweight and obesity in Chinese children from 1985 to 2005. Int. J. Cardiol. 2009, 132, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kipping, R.R.; Howe, L.D.; Jago, R.; Campbell, R.; Wells, S.; Chittleborough, C.R.; Mytton, J.; Noble, S.M.; Peters, T.J.; Lawlor, D.A. Effect of intervention aimed at increasing physical activity, reducing sedentary behaviour, and increasing fruit and vegetable consumption in children: active for Life Year 5 (AFLY5) school based cluster randomised controlled trial. BMJ 2014, 27, 348:g3256. [Google Scholar] [CrossRef] [PubMed]
- Bodzsár, É.B.; Zsákai, A. Present State of Secular Trend in Hungary. In New Perspectives and Problems in Anthropology; Bodzsár, É.B., Zsákai, A., Eds.; Cambridge Scholars Publishing: Newcastle, UK, 2007. [Google Scholar]
- Bodzsár, É.B.; Zsákai, A. Recent trends in childhood obesity and overweight in the transition countries of Eastern and Central Europe. Ann. Hum. Biol. 2014, 41, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W.S.; Campell, C.C.; Frongillo, E.A.; Haas, J.D.; Melnik, T.A. Overweight schoolchildren in New York State: Prevalence and charateristics. Am. J. Public Health 1994, 84, 807–813. [Google Scholar] [CrossRef]
- Llorente-Cantarero, F.J.; Perez-Navero, J.L.; Benitez-Sillero, D.; Munoz-Villanueva, M.C.; Gil-Campos, M. Evaluation of metabolic risk in prepubertal girls versus boys in relation to fitness and physical activity. Gend. Med. 2012, 9, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Troiano, R.P.; Flegal, K.M. Overweight children and adolescents: Description, epidemiology and demographics. Pediatrics 1998, 101, 497–504. [Google Scholar] [PubMed]
- Wang, Y.; Wang, J.Q. A comparison of international references for the assessment of child and adolescent overweight and obesity in different populations. Eur. J. Clin. Nutr. 2002, 56, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, R.; Ceciliani, A.; Garulli, A.; Masotti, A.; Poletti, G.; Beltrami, P.; Leoni, E. Physical fitness of primary school children in relation to overweight prevalence and physical activity habits. J. Sports Sci. 2012, 30, 633–640. [Google Scholar] [CrossRef]
- Bodzsár, É.B.; Zsákai, A. Physical Developmental Status of Hungarian Children and Adolescents. Hungarian National Growth Study 2003–2006; Plantin Kiadó: Budapest, Hungary, 2012. [Google Scholar]
- Shang, X.; Liu, A.; Li, Y.; Hu, X.; Du, L.; Ma, J.; Xu, G.; Li, Y.; Guo, H.; Ma, G. The association of weight status with physical fitness among Chinese children. Int. J. Pediatr. 2010, 2010, 515414. [Google Scholar] [CrossRef]
- Wagner, I.V.; Sabin, M.A.; Pfäffle, R.W.; Hiemisch, A.; Sergeyev, E.; Körner, A.; Kiess, W. Effects of obesity on human sexual development. Nat. Rev. Endocrinol. 2012, 8, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Malina, R.M.; Bouchard, C.; Bar-Or, O. Growth, Maturation and Physical Activity; Human Kinetics: Champaign, IL, USA, 2005. [Google Scholar]
- Chumlea, W.C.; Siervogel, R.M.; Roche, A.F.; Mukherjee, D.; Webb, P. Changes in adipocyte cellularity in children 10 to 18 years of age. Int. J. Obes. 1981, 6, 383–389. [Google Scholar]
- Aguilera, C.M.; Gomez-Llorente, C.; Tofe, I.; Gil-Campos, M.; Cañete, R.; Gil, Á. Genome-wide expression in visceral adipose tissue from obese prepubertal children. Int. J. Mol. Sci. 2015, 16, 7723–7737. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Booth, N.J.; Laird, Y.; Sproule, J.; Reilly, J.J.; Saunder, H.D. Physical activity, diet and other behavioral interventions for improving cognition and school achievement in children and adolescents with obesity or overweight. Cochrane Database Syst. Rev. 2018, 3, CD009728. [Google Scholar] [CrossRef]
- Ekelund, U.; Andersen, L.B.; Froberg, K.; Sardinha, L.B.; Anderssen, S.A.; Brage, S. Independent associations between physical activity and aerobic fitness with metabolic risk factors in children: The European Youth Heart Study. Diabetologia 2007, 50, 1832–1840. [Google Scholar] [CrossRef]
- Ara, I.; Sanchez-Villegas, A.; Vicente-Rodriguez, G.; Moreno, L.A.; Leiva, M.T.; Martinez-Gonzalez, M.A.; Casajus, J.A. Physical fitness and obesity are associated in a dose-dependent manner in children. Ann. Nutr. Metab. 2010, 57, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ceschia, A.; Giacomini, S.; Santarossa, S.; Rugo, M.; Salvadego, D.; Da Ponte, A.; Driussi, C.; Mihaleje, M.; Poser, S.; Lazzer, S. Deleterious effects of obesity on physical fitness in pre-pubertal children. Eur. J. Sport Sci. 2016, 16, 271–278. [Google Scholar] [CrossRef]
- Brodney, S.; Blair, S.N.; Do Lee, C. Is it possible to be overweight or obese and fit and healthy. In Physical Activity and Obesity; Bouchard, C., Ed.; Human Kinetics: Champaign, IL, USA, 2000; pp. 355–371. [Google Scholar]
- Mészáros, J.; Király, T.; Zsidegh, M.; Prókai, A.; Szmodis, M. Body fat and endurance of prepubertal boys. Acta Kinesiologiae Universitatis Tartuensis. Abstr. 24th Pediatr. Work Physiol. Meet. 2007, 12, 139. [Google Scholar]
- Pampakas, P.; Mészáros, Z.; Király, T.; Szmodis, B.M.; Szakály, Z.; Zsidegh, M.; Mészáros, J. Longitudinal differences and trends in body fat and running endurance in Hungarian primary schoolboys. Anthrop. Anz. 2008, 66, 317–326. [Google Scholar]
- Szmodis, M.; Szmodis, I.; Farkas, A.; Mészáros, Z.; Mészáros, J. Relationships of relative fat content and some indices of body structure and motor performance in prepubertal boys. In Proceedings of the 2nd Joint Meeting of The North American Society for Pediatric Exercise Medicine and the European Group for Pediatric Work Physiology (NASPEM-PWP 2010), Niagara-on-the-Lake, ON, Canada, 22–26 September 2010; p. 69. [Google Scholar]
- Weiner, J.E.S.; Lourie, J.A. (Eds.) Human Biology. A Guide to Field Methods; IBP Handbook, No. 9; Blackwell: Oxford, UK, 1969. [Google Scholar]
- Drinkwater, D.T.; Ross, W.D. Anthropometric fractionation of body mass. In Kinanthropometry II; Ostyn, M., Beunen, G., Simons, J., Eds.; University Park Press: Baltimore, MD, USA, 1980. [Google Scholar]
- Conrad, K. Der Konstitutionstypus; Springer: Berlin, Germany, 1963. [Google Scholar]
- Szmodis, I. Physique and growth estimated by Conrad’s and Heath–Carter’s somatocharts in athletic children. In Growth and Development; Eiben, O.G., Ed.; Akadémiai Kiadó: Budapest, Hungary, 1977; pp. 407–415. [Google Scholar]
- Szabó, T. A Központi Sportiskola Kiválasztási Rendszere I. Az Általános Motorikus Próbák Tapasztalatai. (The Selection System of Central School of Sports I. Repert on the Physical Fitness Tests.); Utánpótlás-Nevelés. 1; Központi Sportiskola: Budapest, Hungary, 1977; pp. 3–54. [Google Scholar]
- EUROFIT Eurofit Tests of Physical Fitness, 2nd ed.; Council of Europe, Committee for the Development of Sport: Strasbourg, France, 1993.
- Kemper, H.C.G. (Ed.) The Amsterdam Growth Study; HK Sport Science Monograph Series; Human Kinetics: Champaign, The Netherlands, 1995; Volume 6, p. 278. [Google Scholar]
- Lohman, T. Advances in Body Composition Assessment; Human Kinetics Publishers, Inc.: Champaign, IL, USA, 1992. [Google Scholar]
- Mészáros, J.; Othman, M.; Szabó, T. Anthropometry and motor performance scores in Hungarian schoolboys. A 25 years comparison. In The Exchange and Development of Sport Culture in East and West; Hank, J., Ed.; NTNU-AIESEP: Taipei, Taiwan, 2001; pp. 102–103. [Google Scholar]
- Othman, M.; Mészáros, J.; Szabó, T. Secular trend and motor performance in Hungarian schoolboys. Kinesiology 2002, 34, 127–133. [Google Scholar]
- Mohácsi, J.; Lee, C.P.; Zsidegh, M.; Tatár, A.; Mészáros, J. Physique and Motor Performances of 10-Year-Old Malaysian Boys. Coll. Antropol. 2002, 26, 142–143. [Google Scholar]
- Scheffler, C.; Obermüller, J. Development of fat distribution patterns in children and its association with the type of body shape assessed by the Metric-Index. Anthropol. Anz. 2012, 69, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Tatár, A.; Lee, C.P.; Zsidegh, M.; Mészáros, J.; Mohácsi, J. Growth type and running endurance in 13-year-old boys. Coll. Antropol. 2002, 26, 208–209. [Google Scholar]
- Mészáros, J.; Zsidegh, M.; Ihász, F.; Mészáros, Z.; Tatár, A.; Prókai, A.; Vajda, I.; Mohácsi, J. Physique body fat content and motor performance in twins. Revista Portuguesa de Ciencias do Desporto 2003, 3, 124–127. [Google Scholar]
- Ortega, F.B.; Ruiz, J.R.; Castillo, M.J. Physical activity, physical fitness, and overweight in children and adolescents: Evidence from epidemiologic studies. Endocrinol. Nutr. 2013, 60, 458–469. [Google Scholar] [CrossRef]
- Rauner, A.; Mess, F.; Woll, A. The relationship between physical activity, physical fitness and overweight in adolescents: A systematic review of studies published in or after 2000. BMC Pediatr. 2000, 13, 19. [Google Scholar]
- D’Hondt, E.; Deforche, B.; Gentier, I.; De Bourdeaudhuij, I.; Vaeyens, R.; Philippaerts, R.; Lenoir, M. A longitudinal analysis of gross motor coordination in overweight and obese children versus normal-weight peers. Int. J. Obes. 2013, 37, 61. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.M.; Bugge, A.; El-Naaman, B.; Eisenmann, J.C.; Froberg, K.; Pfeiffer, K.A.; Andersen, L.B. Inter-relationships among physical activity, body fat, and motor performance in 6- to 8-year-old Danish children. Pediatr. Exerc. Sci. 2012, 24, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chang, S.H.; Miyashita, M.; Stensel, D.; Chen, J.F.; Wen, L.T.; Nakamura, Y. Associations between health-related physical fitness and obesity in Taiwanese youth. J. Sports Sci. 2013, 31, 1797–1804. [Google Scholar] [CrossRef]
- Burns, R.; Hannon, J.C.; Brusseau, T.A.; Shultz, B.; Eisenman, P. Indices of abdominal adiposity and cardiorespiratory fitness test performance in middle-school students. J. Obes. 2013, 2013, 912460. [Google Scholar] [CrossRef]
- Malina, R.M.; Rogol, A.D. Sport training and the growth and pubertal maturation of young athletes. Pediatr. Endocrinol. Rev. 2011, 9, 441–455. [Google Scholar]
- Growth, Health and Fitness of Teenagers. Longitudinal Research in International Perspective. In Medicine and Sport Science; Kemper, H.C.G., Ed.; Karger: Basel, Switzerland; New York, NY, USA; Volume 20, p. 93.
- Dwyer, T.; Blizzard, C. Defining obesity in children by biological endpoint rather than population distribution. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 472–480. [Google Scholar] [PubMed]
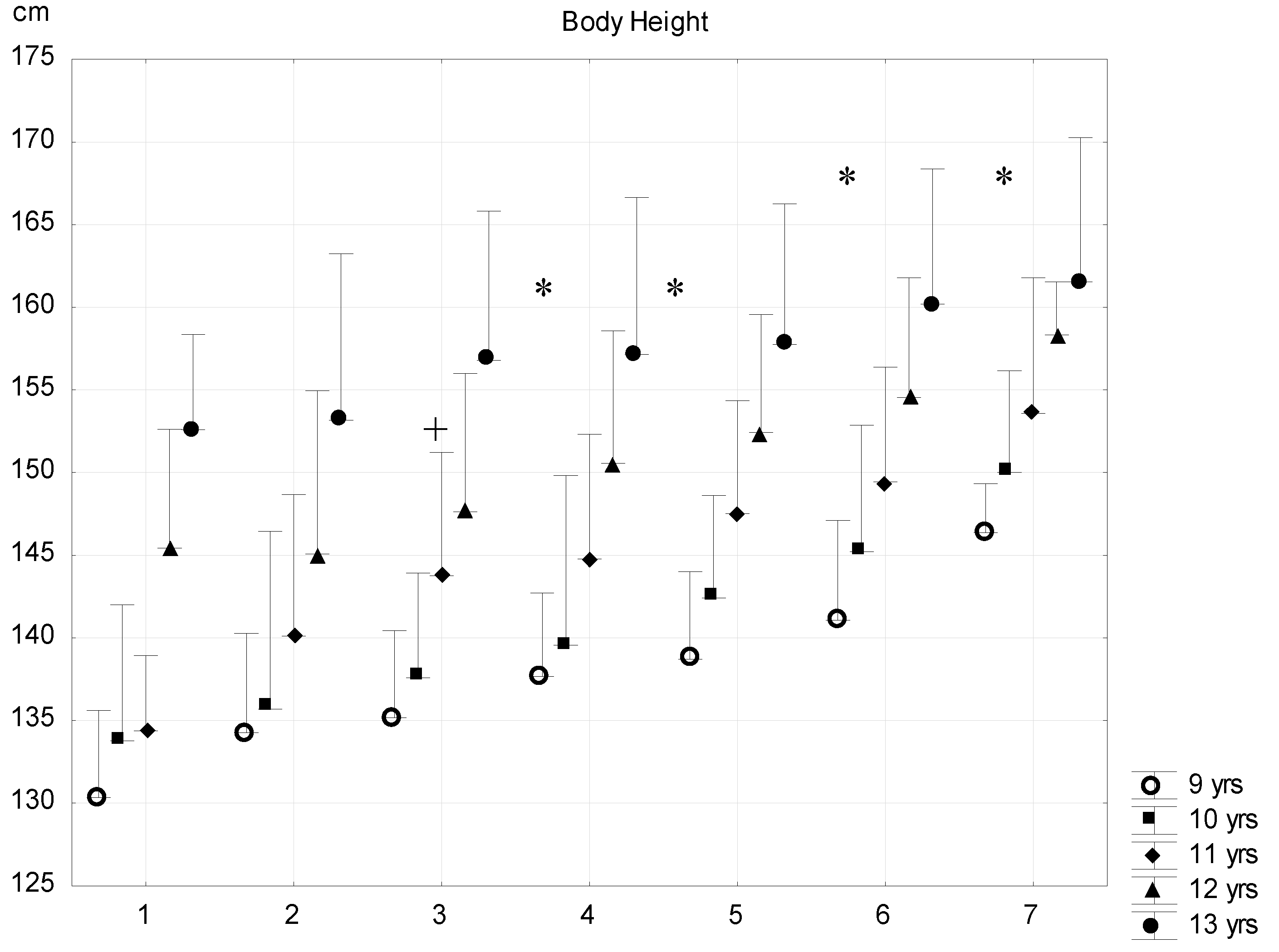
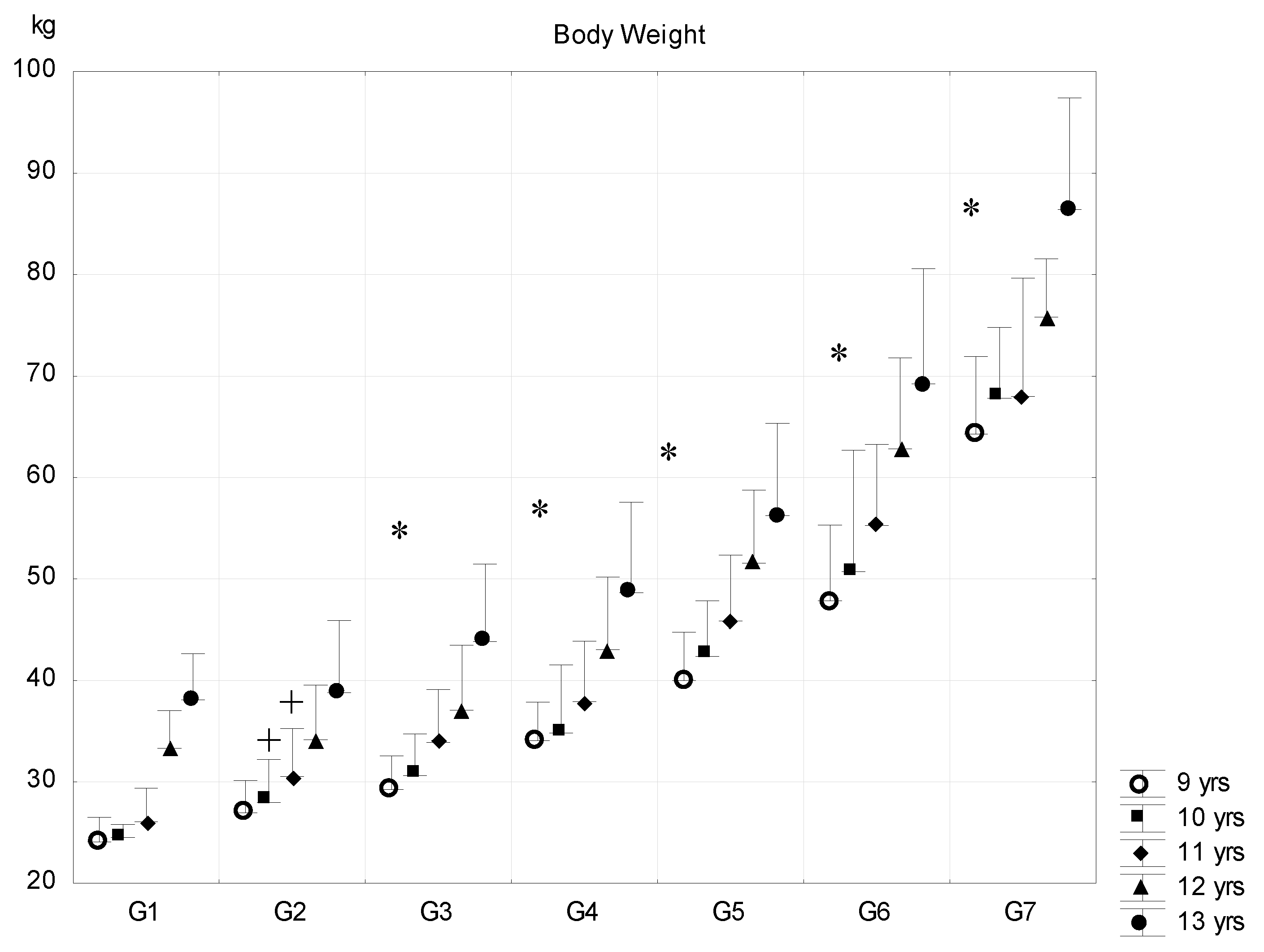
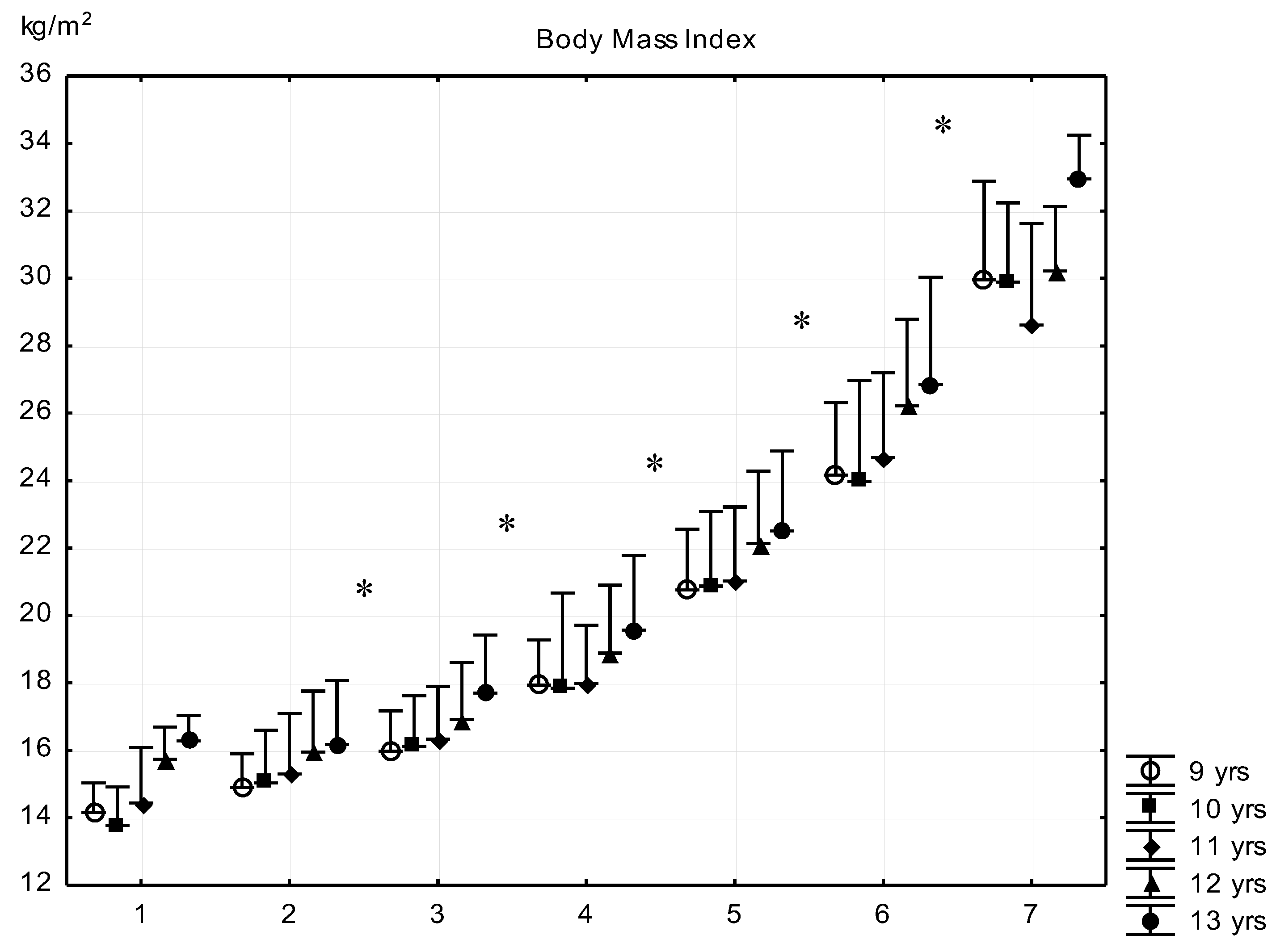
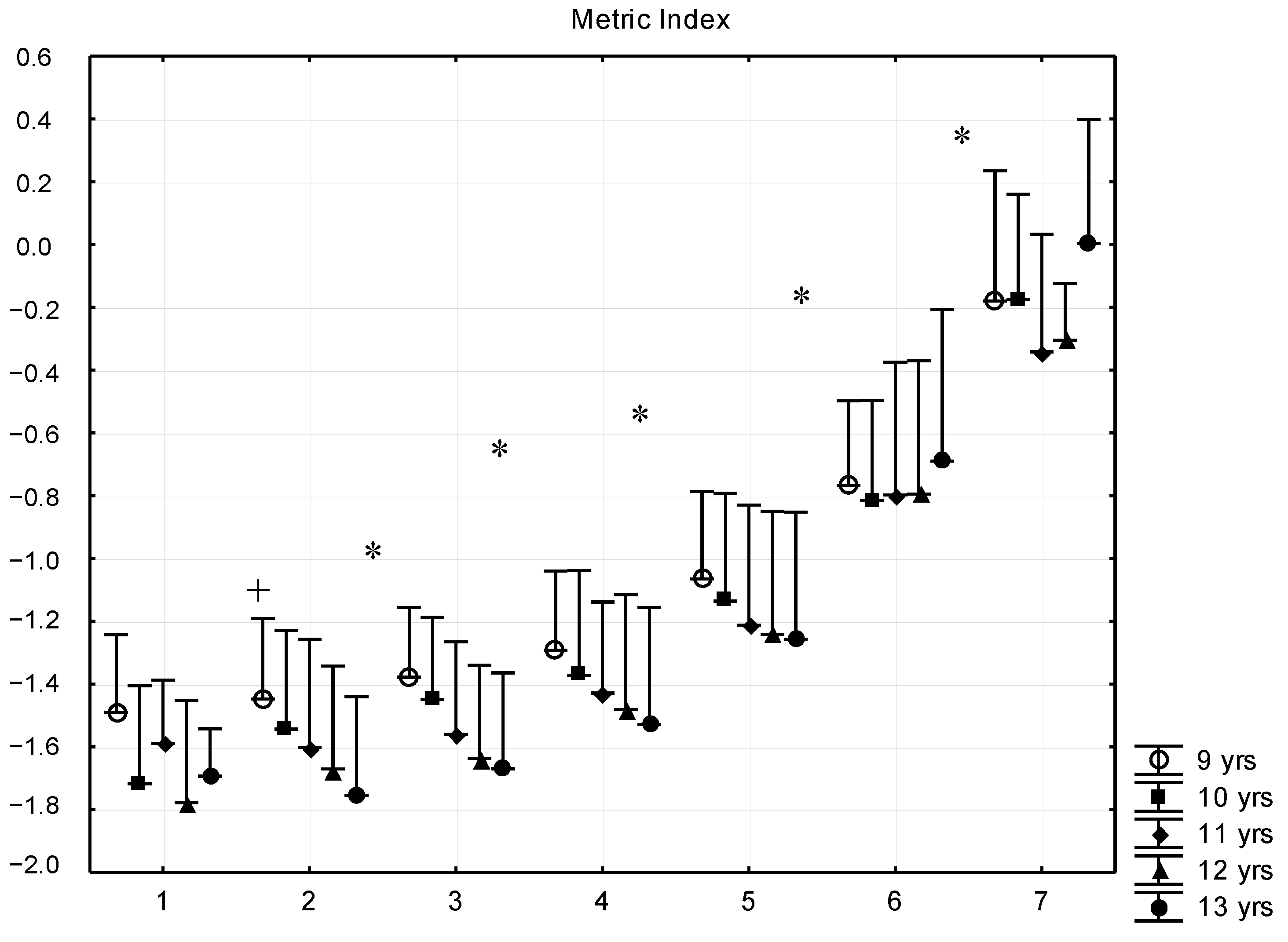
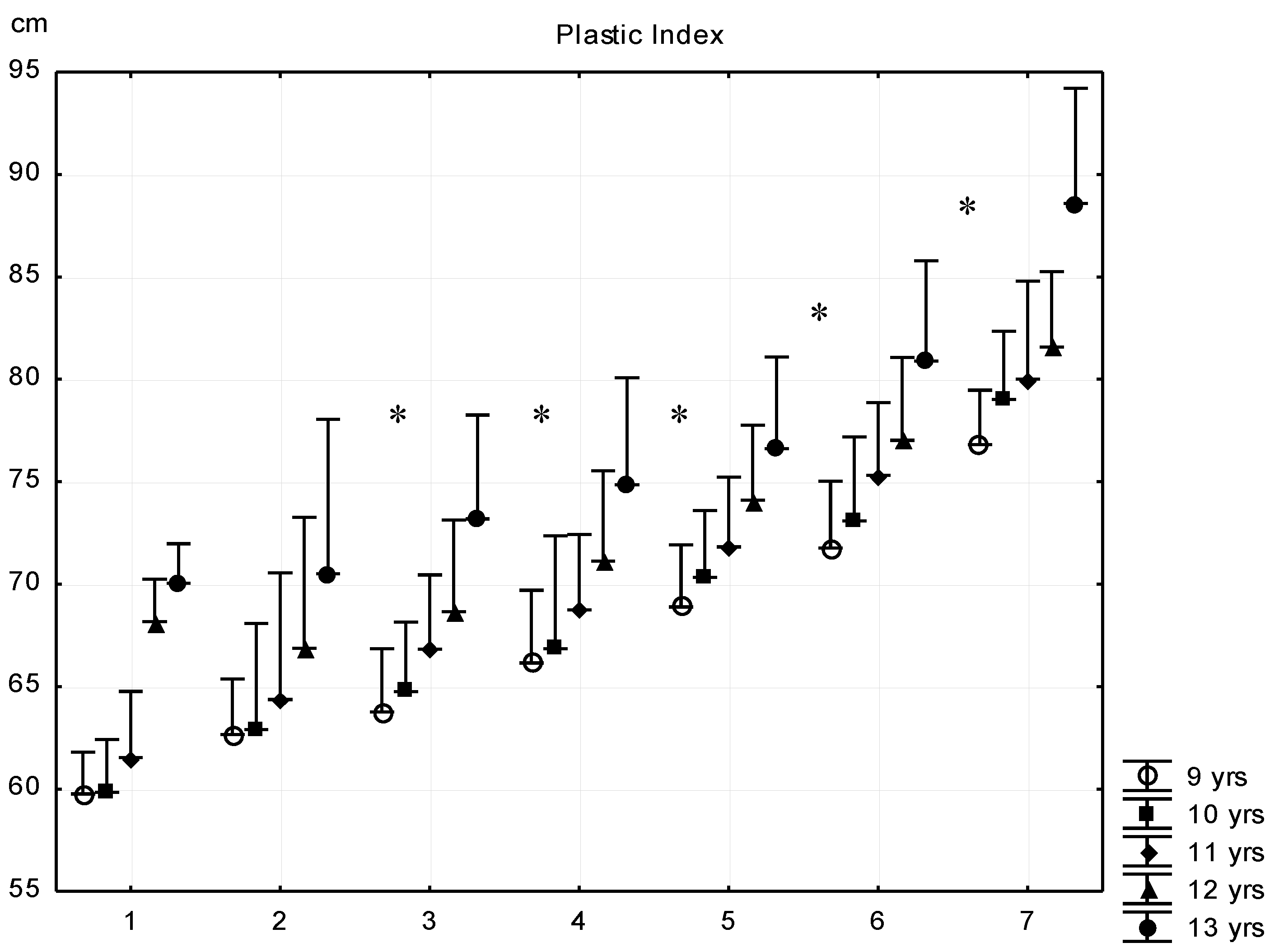
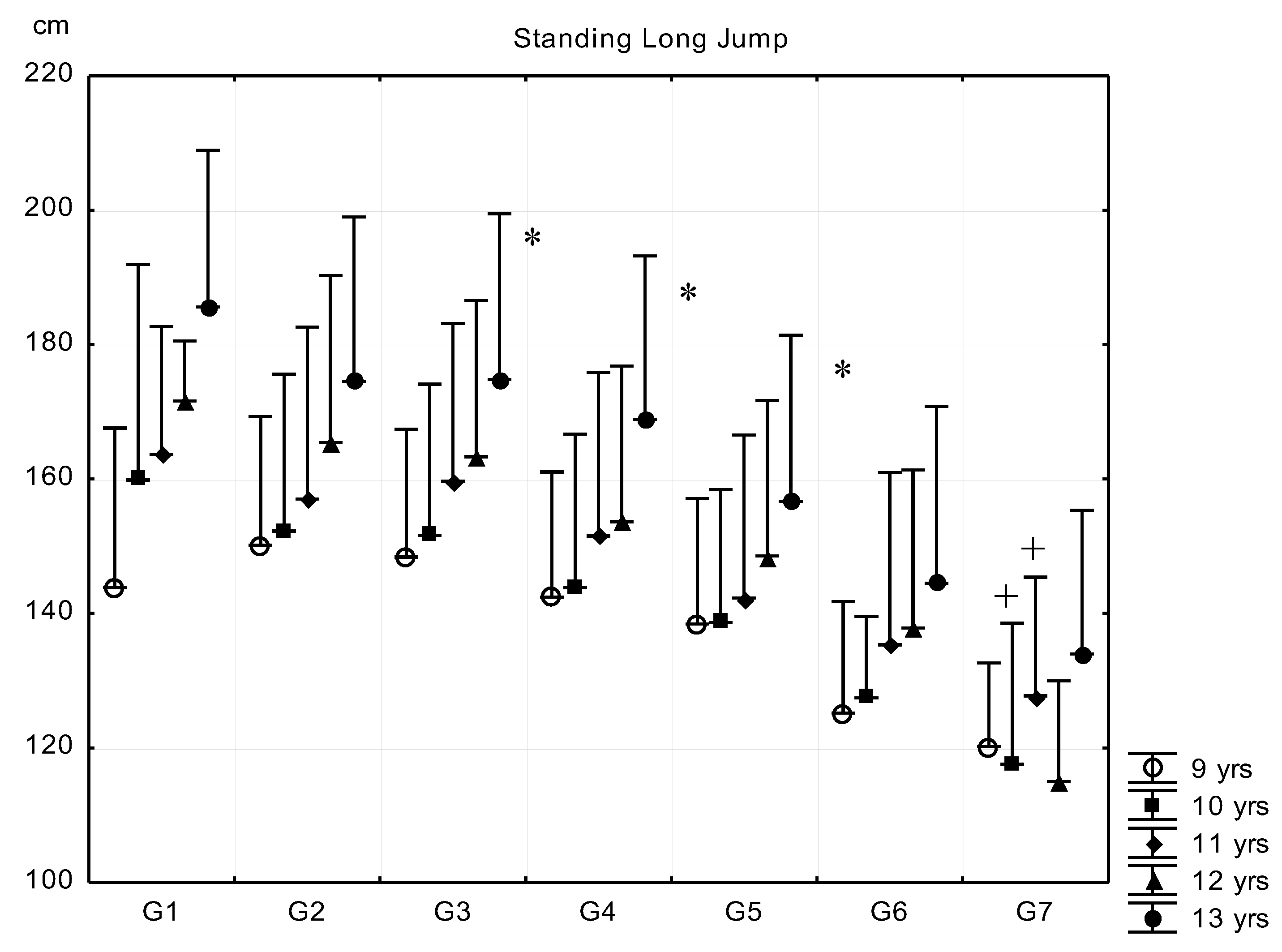

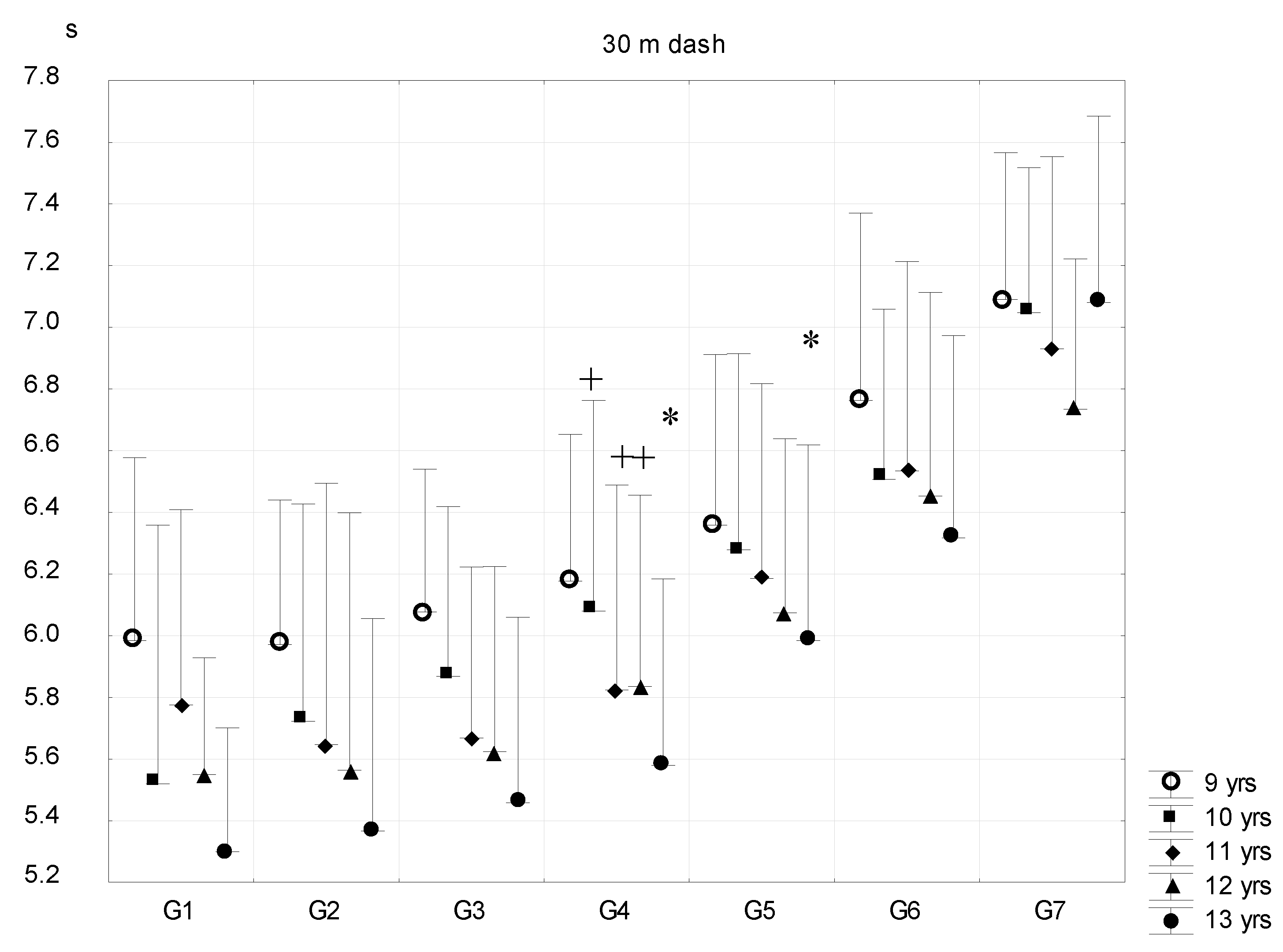
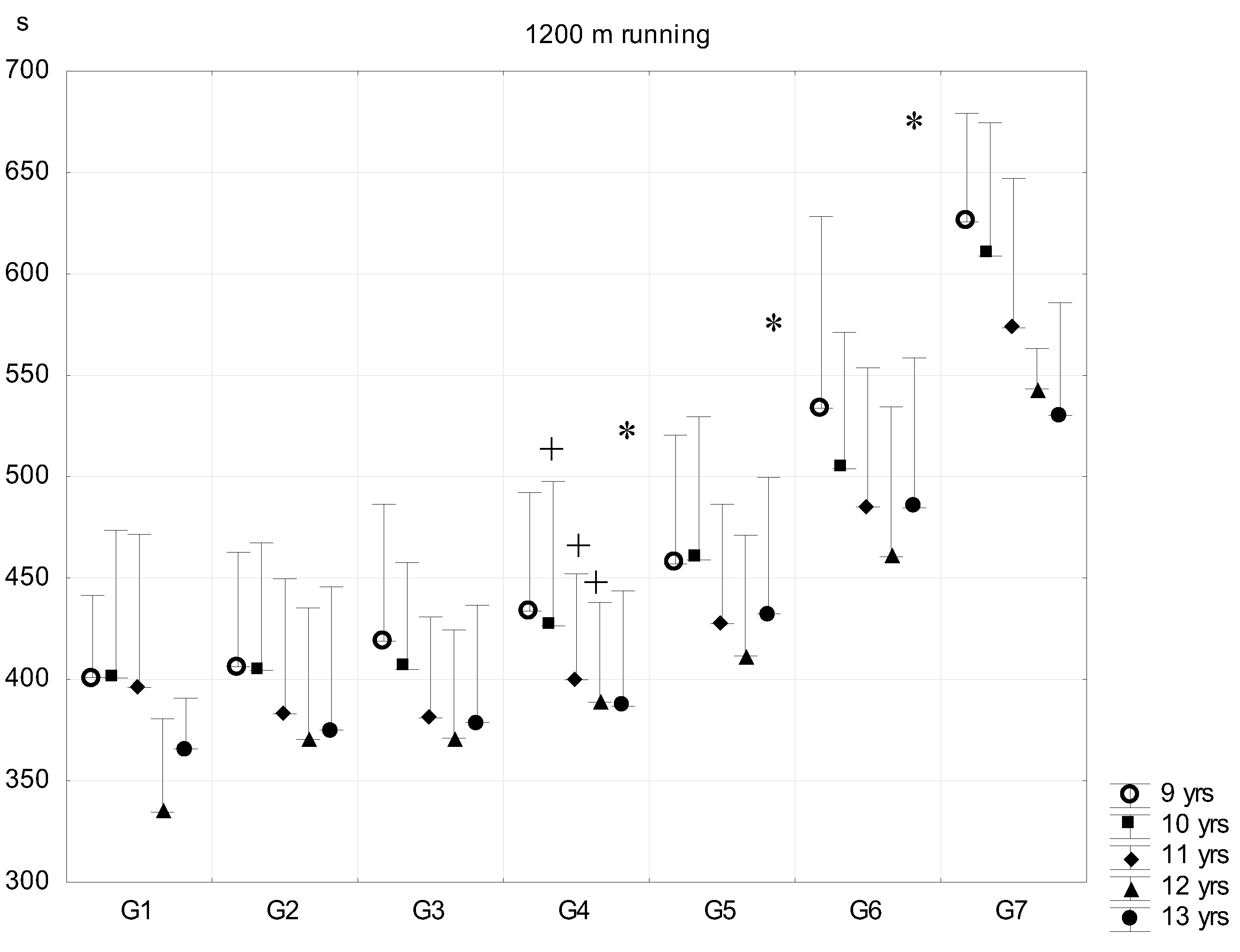
| Sub-Groups | Fat% | n (9 Years) | n (10 Years) | n (11 Years) | n (12 Years) | n (13 Years) |
|---|---|---|---|---|---|---|
| (Fat% Mean ± SD) | ||||||
| G1 | <8 | 7 (7.1 ± 0.8) | 8 (6.9 ± 0.9) | 9 (6.5 ± 0.4) | 5 (7.2 ± 0.3) | 5 (7.2 ± 0.4) |
| G2 | 8–11.99 | 181 (10.5 ± 1.1) | 223 (10.3 ± 1.4) | 139 (10.4 ± 1.5) | 132 (10.5 ± 1.5) | 121 (10.7 ± 1.5) |
| G3 | 12–15.99 | 399 (13.9 ± 1.1) | 515 (14.0 ± 1.1) | 412 (14.1 ± 1.1) | 443 (14.2 ± 1.0) | 419 (14.1 ± 1.1) |
| G4 | 16–19.99 | 264 (17.9 ± 1.2) | 391 (17.8 ± 3.9) | 400 (17.9 ± 1.1) | 387 (17.9 ± 1.1) | 344 (17.7 ± 1.1) |
| G5 | 20–23.99 | 184 (21.9 ± 1.1) | 322 (22.0 ± 1.2) | 302 (22.0 ± 1.2) | 270 (21.8 ± 1.2) | 218 (21.9 ± 1.2) |
| G6 | 24–27.99 | 72 (25.5 ± 1.1) | 162 (25.4 ± 1.1) | 210 (25.7 ± 1.1) | 187 (25.5 ± 0.9) | 122 (25.5 ± 1.1) |
| G7 | >28 | 9 (28.8 ± 0.7) | 22 (29.3 ± 1.0) | 24 (29.0 ± 1.2) | 5 (28.2 ± 0.2) | 5 (29.0 ± 0.7) |
| 1116 (16.5 ± 4.6) | 1643 (17.3 ± 4.9) | 1496 (18.2 ± 5.0) | 1429 (17.8 ± 4.6) | 1235 (17.3 ± 4.5) | ||
| Total | N = 6919 (17.5 ± 4.8) | |||||
| r | Fistball Throw (m) | Standing Long Jump (cm) | 30 m Dash (m/s) | 1200 m Running (m/s) |
|---|---|---|---|---|
| Fat%—total sample | −0.13 *** | −0.34 *** | −0.39 *** | −0.44 *** |
| Fat%—9 years | −0.10 * | −0.33 *** | −0.37 *** | −0.42 *** |
| Fat%—10 years | −0.14 *** | −0.38 *** | −0.42 *** | −0.48 *** |
| Fat%—11 years | −0.17 *** | −0.35 *** | −0.42 *** | −0.52 *** |
| Fat%—12 years | −0.15 *** | −0.36 *** | −0.43 *** | −0.45 *** |
| Fat%—13 years | −0.16 *** | −0.39 *** | −0.44 *** | −0.47 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szmodis, M.; Szmodis, I.; Farkas, A.; Mészáros, Z.; Mészáros, J.; Kemper, H.C.G. The Relationship between Body Fat Percentage and Some Anthropometric and Physical Fitness Characteristics in Pre- and Peripubertal Boys. Int. J. Environ. Res. Public Health 2019, 16, 1170. https://doi.org/10.3390/ijerph16071170
Szmodis M, Szmodis I, Farkas A, Mészáros Z, Mészáros J, Kemper HCG. The Relationship between Body Fat Percentage and Some Anthropometric and Physical Fitness Characteristics in Pre- and Peripubertal Boys. International Journal of Environmental Research and Public Health. 2019; 16(7):1170. https://doi.org/10.3390/ijerph16071170
Chicago/Turabian StyleSzmodis, Márta, Iván Szmodis, Anna Farkas, Zsófia Mészáros, János Mészáros, and Han C.G. Kemper. 2019. "The Relationship between Body Fat Percentage and Some Anthropometric and Physical Fitness Characteristics in Pre- and Peripubertal Boys" International Journal of Environmental Research and Public Health 16, no. 7: 1170. https://doi.org/10.3390/ijerph16071170
APA StyleSzmodis, M., Szmodis, I., Farkas, A., Mészáros, Z., Mészáros, J., & Kemper, H. C. G. (2019). The Relationship between Body Fat Percentage and Some Anthropometric and Physical Fitness Characteristics in Pre- and Peripubertal Boys. International Journal of Environmental Research and Public Health, 16(7), 1170. https://doi.org/10.3390/ijerph16071170






