Enhancement of Detoxification of Petroleum Hydrocarbons and Heavy Metals in Oil-Contaminated Soil by Using Glycine-β-Cyclodextrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Characteristics and Preparation of Contaminated Soil
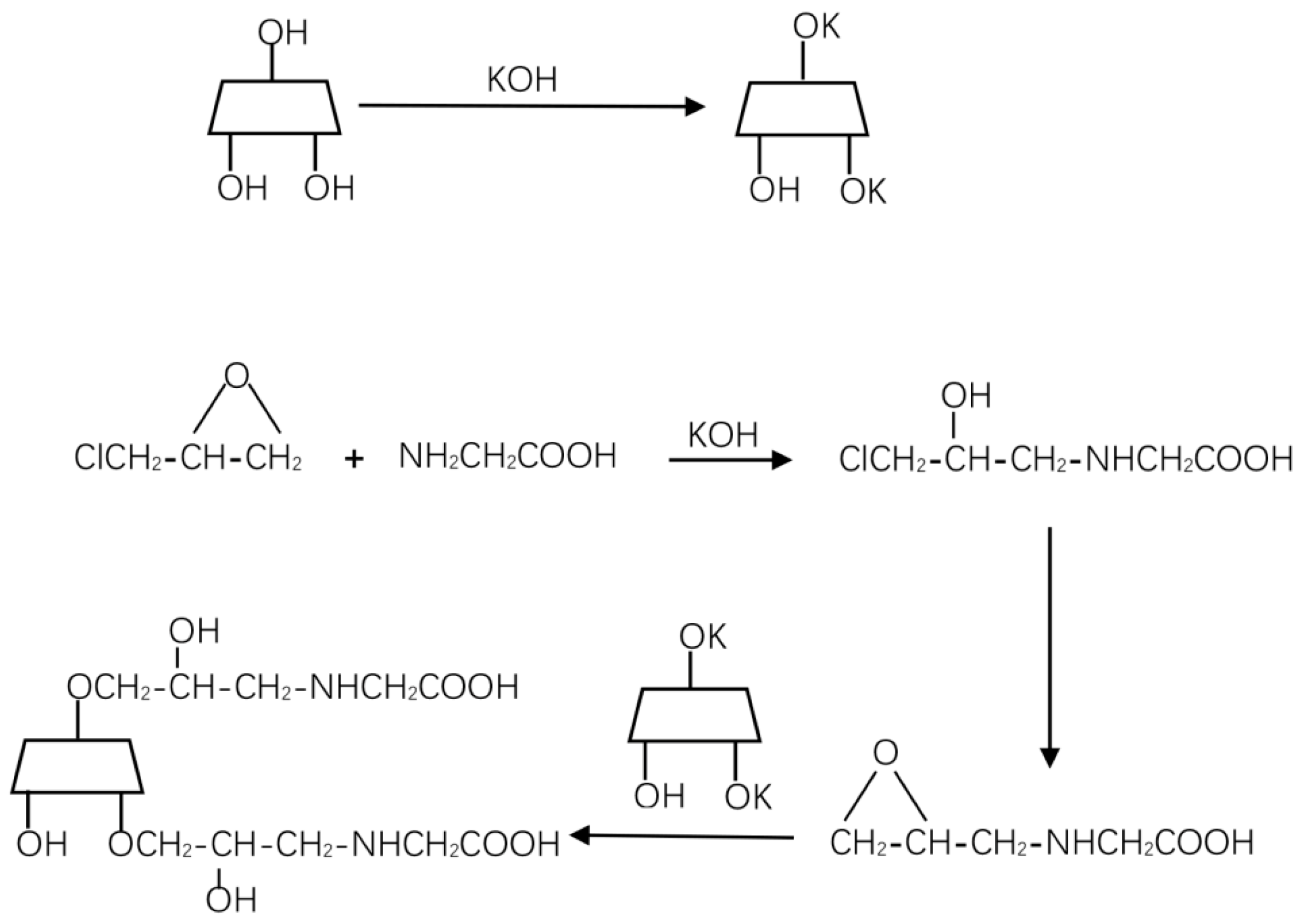
2.2. Modification of Cyclodextrin
2.3. Determination of Total Petroleum Hydrocarbon Content
2.4. Determination of Heavy Metal Content
2.5. Desorpion Experiment
2.5.1. Solubilization of Petroleum Hydrocarbons and Heavy Metals by β-CD Before and After Modification
2.5.2. Factors Influencing Desorption by G-β-CD
2.5.3. Variation of Heavy Metal Speciation in Soils Before and After Desorption with G-β-CD
2.5.4. Equation of Desorption Rate
3. Results and Discussion
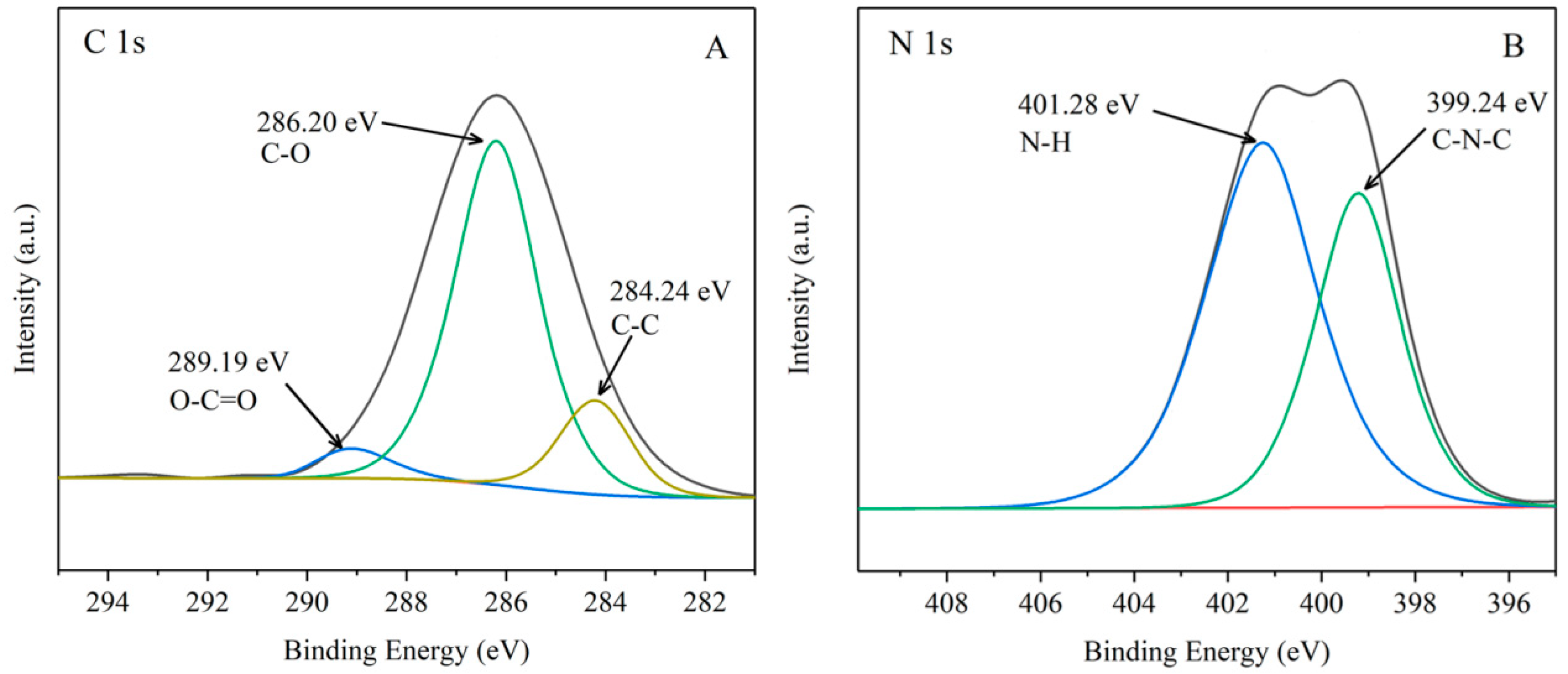
3.1. XPS Analysis of G-β-CD
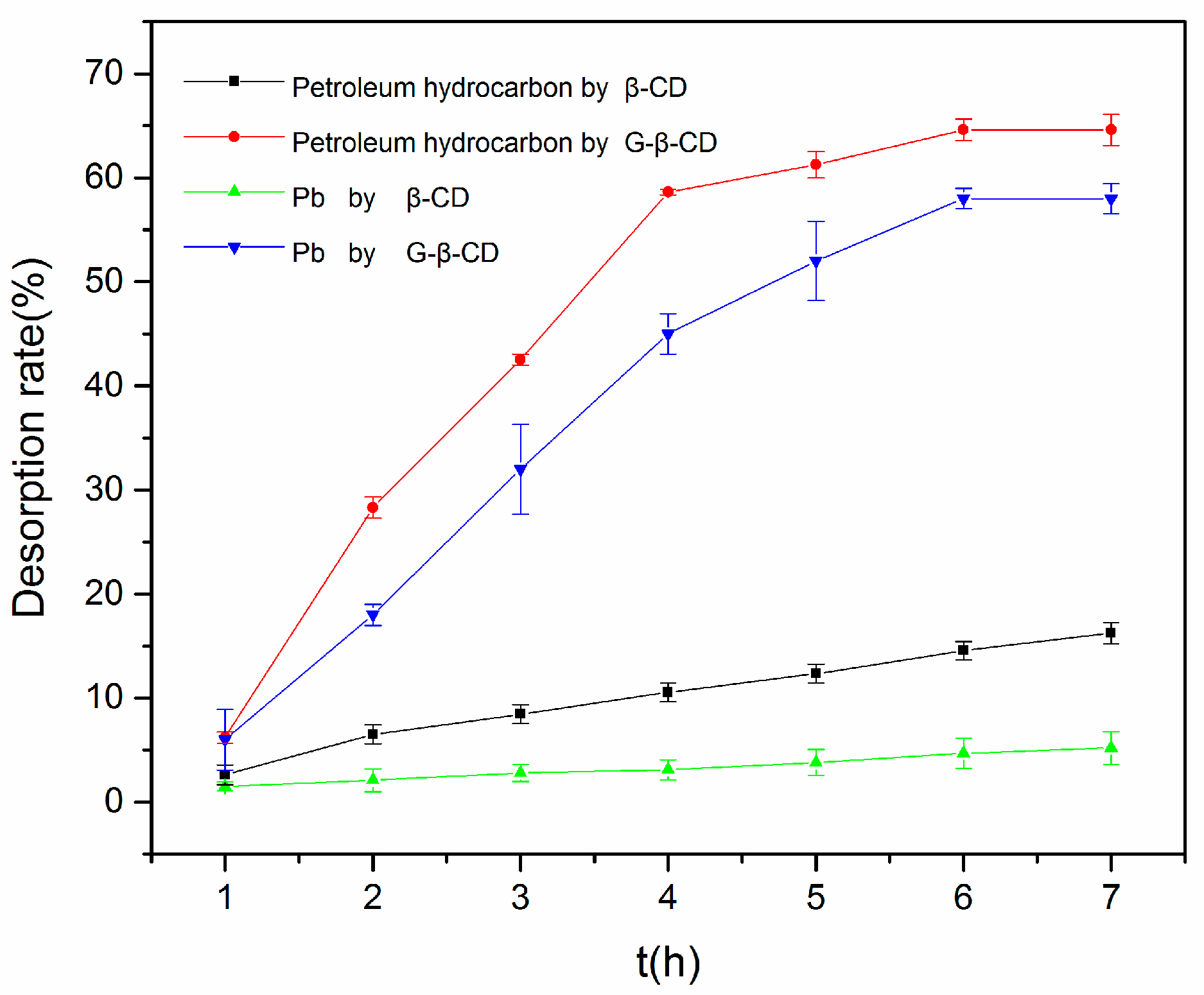
3.2. Effect of β-CD and G-β-CD on Solubilization of Petroleum Hydrocarbons and Heavy Metals
3.3. Factors Affecting Desorption
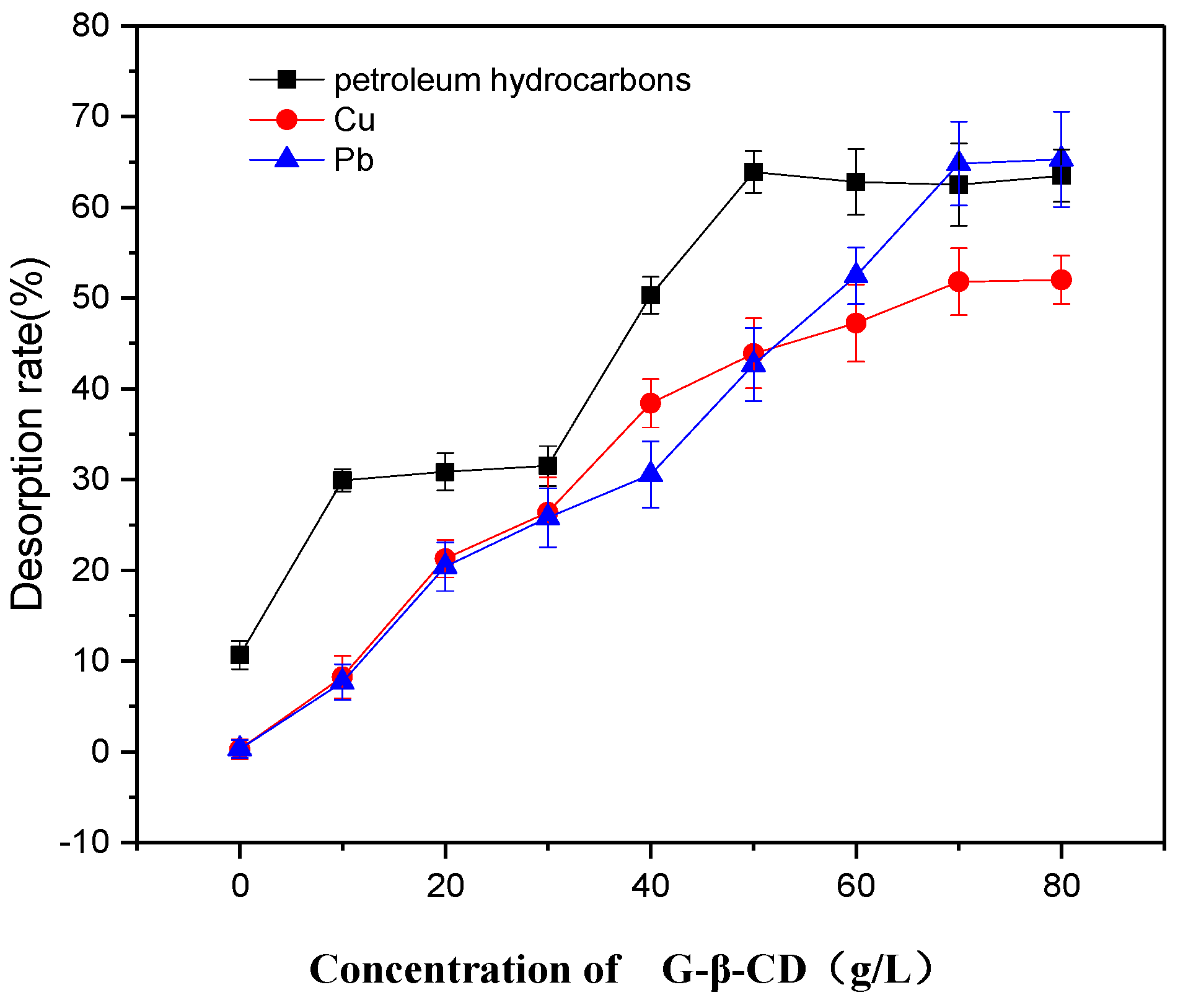
3.3.1. Effect of Initial Concentration of G-β-CD on Desorption
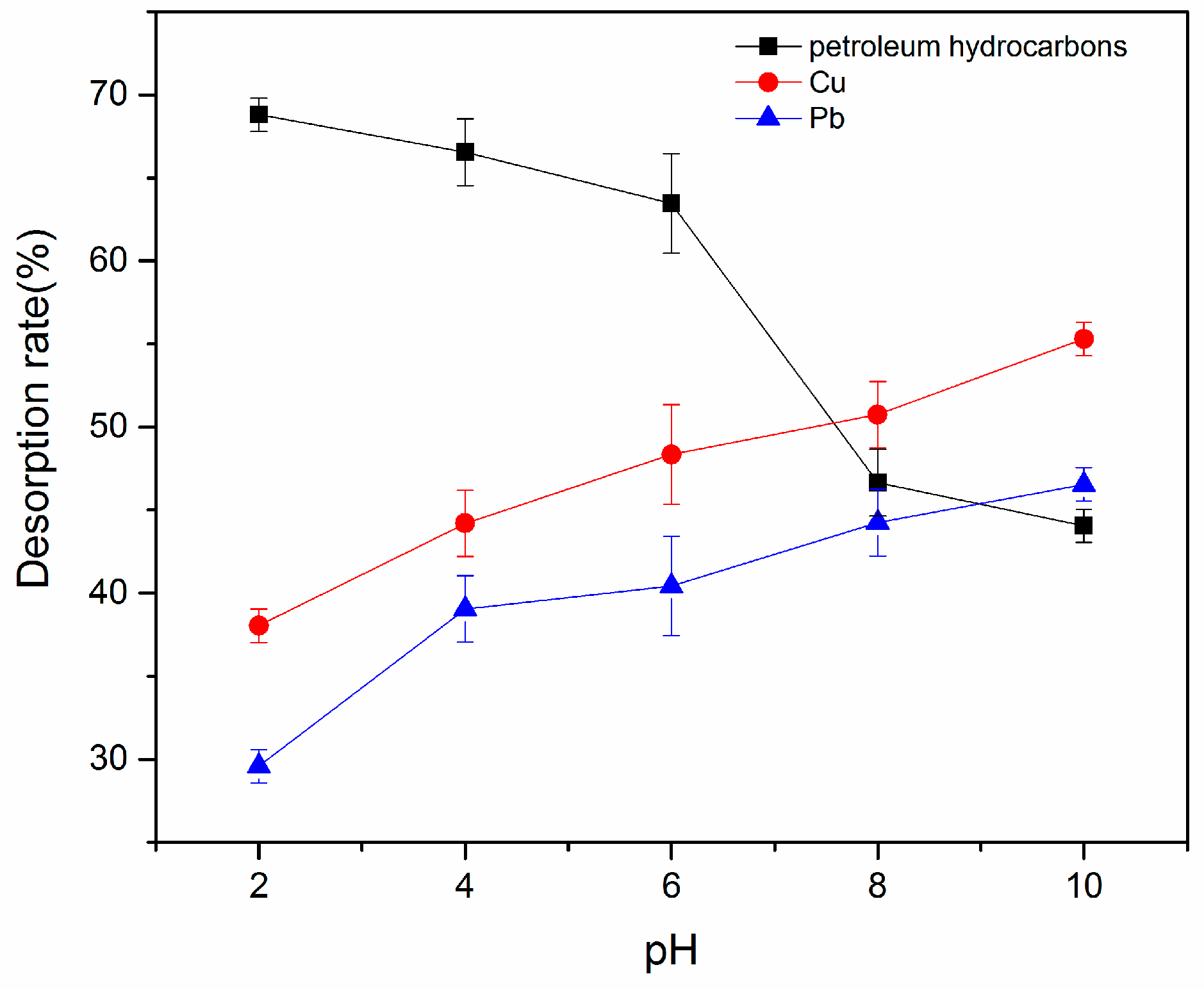
3.3.2. Effect of pH on Desorption
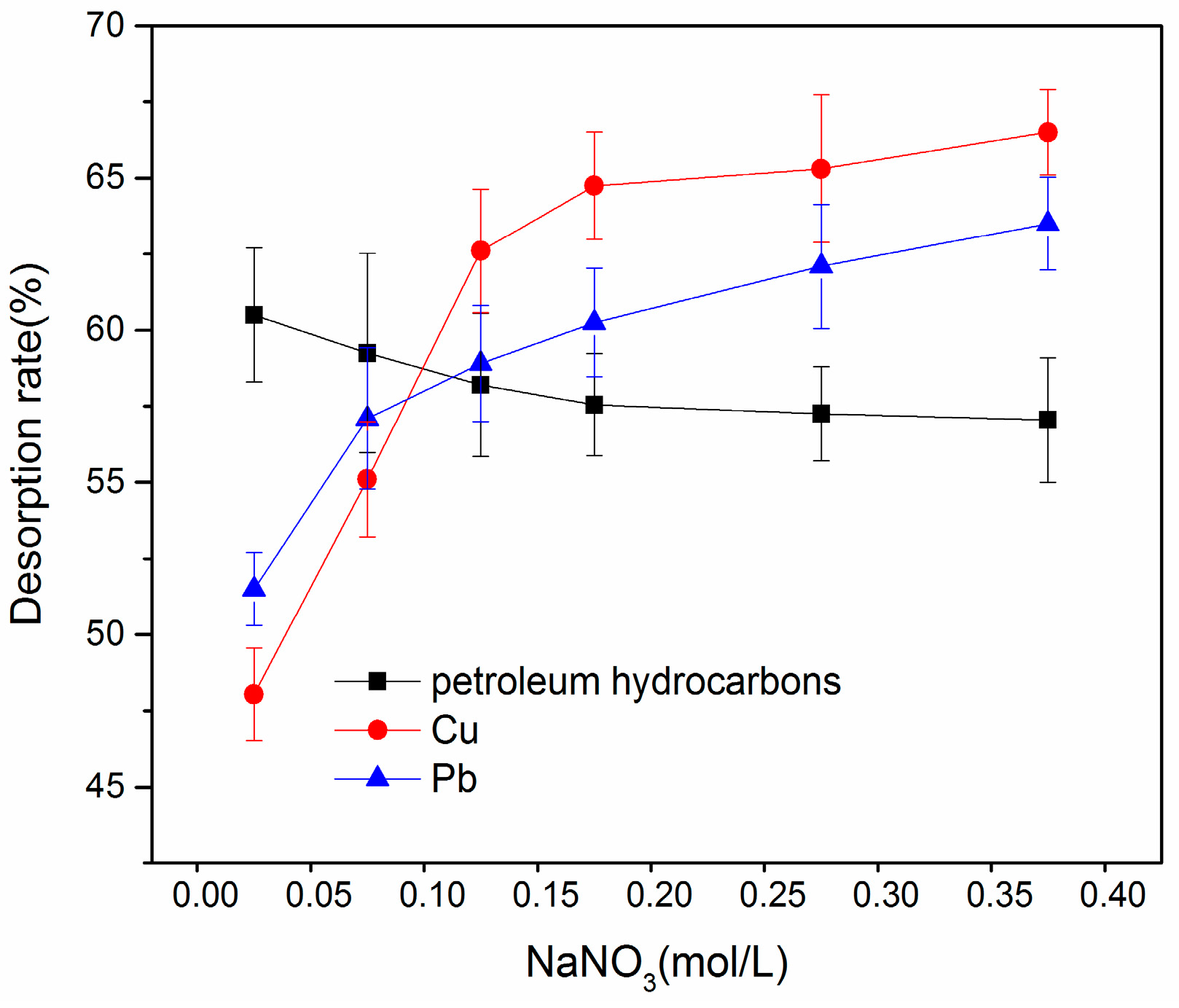
3.3.3. Effect of Ionic Strength on Desorption
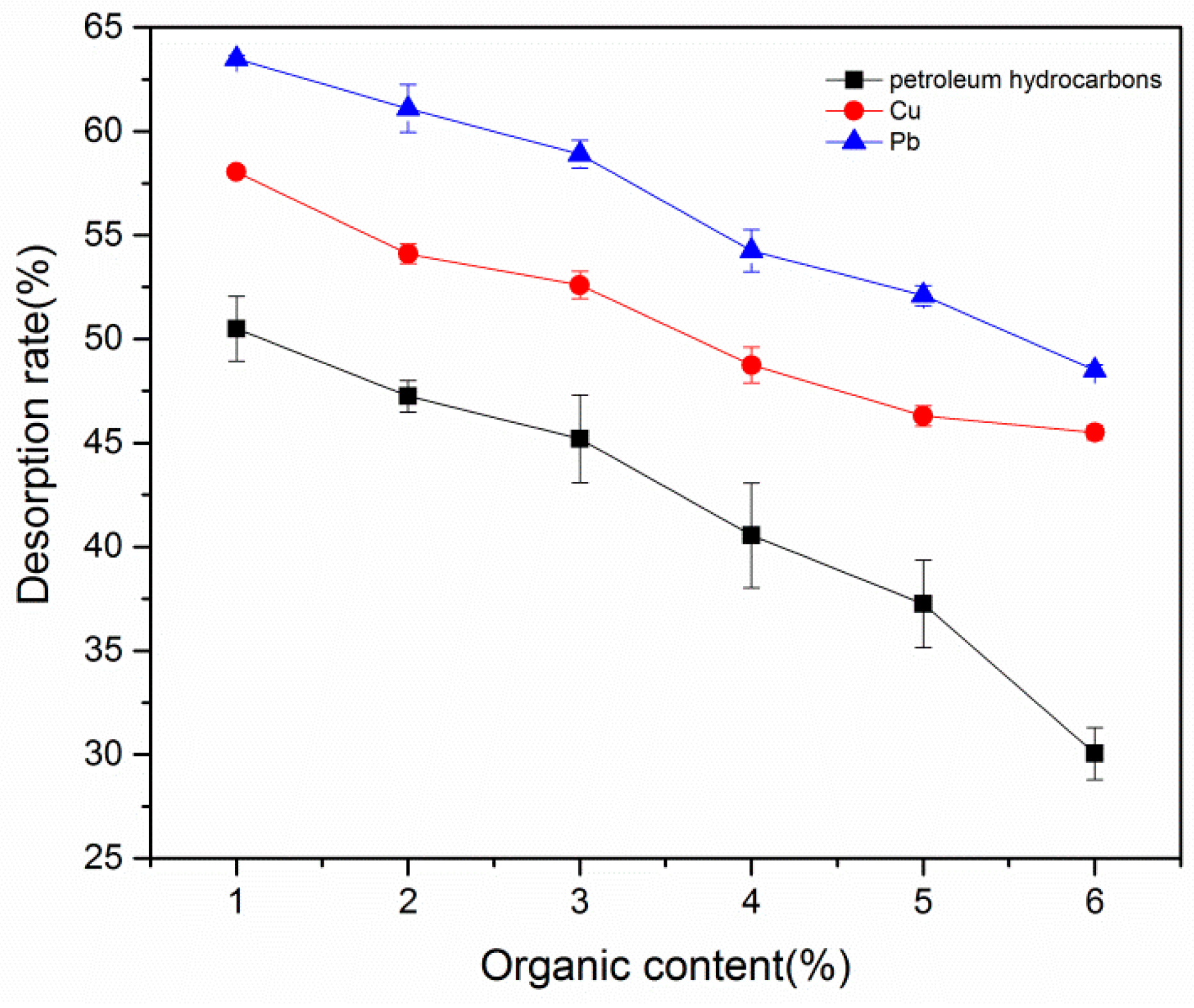
3.3.4. Effect of Organic Matter Concentration on Desorption
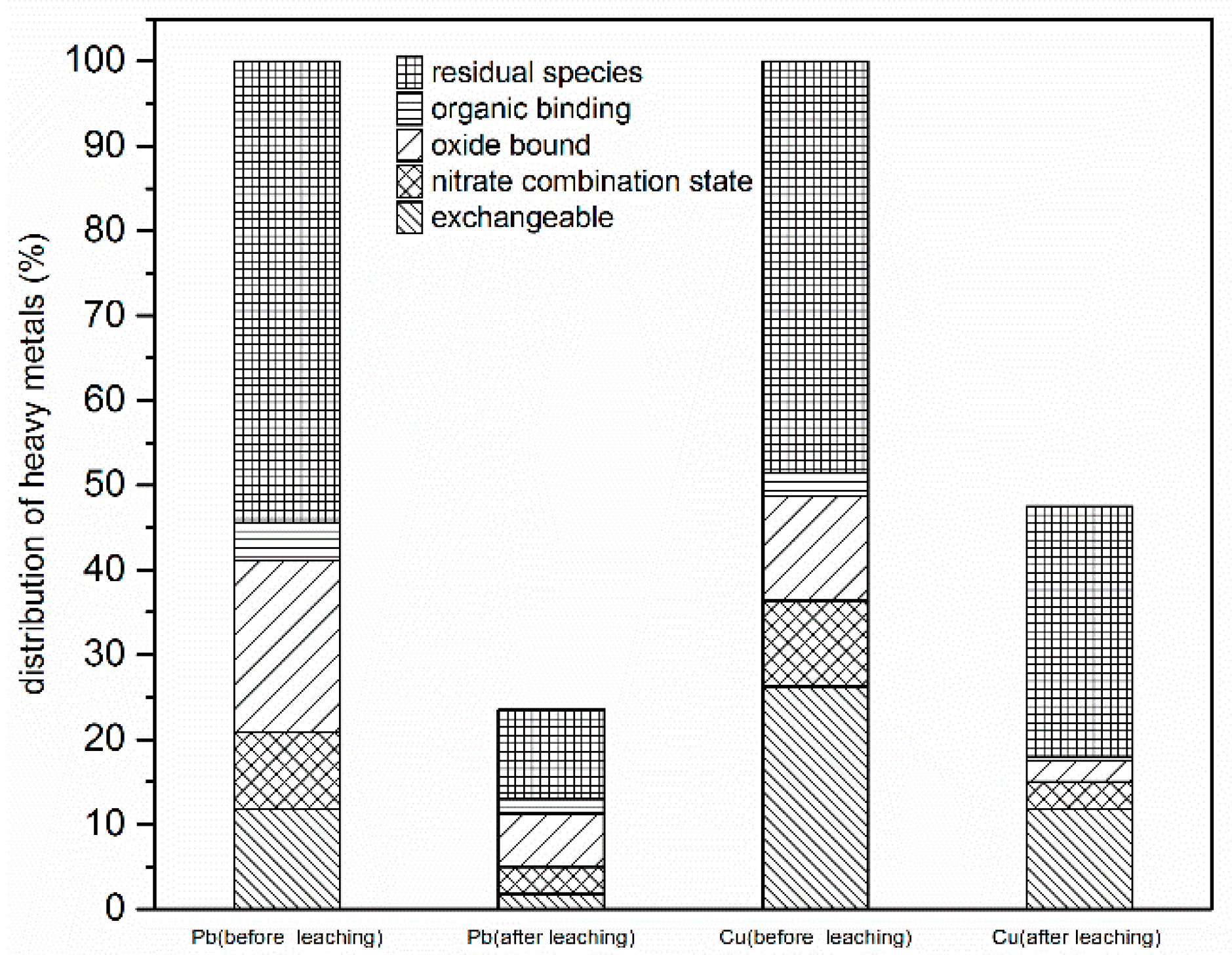
3.3.5. Speciation of Metal Ions in Oil-Contaminated Soil Before and After G-β-CD Desorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J. China’s unconventional oil: A review of its resources and outlook for long-term production. Energy 2015, 82, 31–42. [Google Scholar] [CrossRef]
- Xu, J.; Kong, F.X.; Song, S.H.; Cao, Q.Q.; Huang, T.L.; Cui, Y.W. Effect of Fenton pre-oxidation on mobilization of nutrients and efficient subsequent bioremediation of crude oil-contaminated soil. Chemosphere 2017, 180, 1–10. [Google Scholar] [CrossRef]
- Bai, L.P.; Luo, Y.; Shi, D.R.; Xie, X.F.; Liu, L.; Zhou, Y.Y.; Yan, Z.G.; Li, F.S. TOPSIS-based screening method of soil remediation technology for contaminated sites and its application. Soil. Sed. Contam. 2015, 24, 386–397. [Google Scholar] [CrossRef]
- Mouton, J.; Mercier, G.; Drogui, P. Experimental assessment of an innovative process for simultaneous PAHs and Pb removal from polluted soils. Sci. Total. Environ. 2009, 407, 5402–5410. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Luo, Y.M. Effects of soil amendment with different carbon sources and other factors on the bioremediation of an aged PAH-contaminated soil. Biodegradation 2010, 21, 167–178. [Google Scholar] [CrossRef]
- Inoue, Y.; Katayama, A. Two-scale evaluation of remediation technologies for a contaminated site by applying economic input-output life cycle assessment: Risk-cost, risk-energy consumption and risk-CO2 emission. J. Hazard. Mater. 2011, 192, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.; Hothem, S.; Howerton, H.; Larson, R.; Sanford, R. 9, 10-Phenanthrenequinone photoautocatalyzes its formation from phenanthrene, and inhibits biodegradation of naphthalene. J. Environ. Qual. 2005, 34, 462–468. [Google Scholar]
- Johnsen, A.R.; Wick, L.Y.; Harms, H. Princip les of microbial PAH-degradation in soil. Environ. Pollut. 2005, 133, 71–84. [Google Scholar] [CrossRef]
- Abuhamed, T.; Bayraktar, E.; Mehmetoglu, T. Substrate interactions during the biodegradation benzene, toluene and phenolmixtures. Process. Biochem. 2003, 39, 27–35. [Google Scholar]
- Cheng, M.; Zeng, G.M.; Huang, D.L.; Yang, C.P.; Lai, C.; Zhang, C.; Liu, Y. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds. Chem. Eng. J. 2017, 314, 98–113. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Ling, W.T.; Zhu, L.Z.; Zhao, B.W.; Zheng, Q.S. Surfactant-enhanced phytoremediation of soils contaminated with hydrophobic organic contaminants: Potential and assessment. Pedosphere 2007, 17, 409–418. [Google Scholar] [CrossRef]
- Andas, J.; Adam, F.; Rahman, I.A. Optimization and mechanistic study of the liquid-phase oxidation of naphthalene over biomass-derived iron catalyst. Chem. Eng. J. 2014, 252, 382–392. [Google Scholar] [CrossRef]
- Hu, X.T.; Zhu, J.X.; Ding, Q. Environmental life-cycle comparisons of two polychlorinated biphenyl remediation technologies: Incineration and base catalyzed decomposition. J. Hazard. Mater. 2011, 191, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahian, M.; Emtiazi, G.; Cappello, S. Isolation and characterization of crude-oil-degrading bacteria from the Persian Gulf and the Caspian Sea. Mar. Pollut. Bull. 2012, 64, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.J. Polycyclic aromatic hydrocarbons sorbed on soils: A short review of chemical oxidation based treatments. J. Hazard. Mater. 2006, 138, 234–251. [Google Scholar] [CrossRef]
- Li, A.; Lin, R.J.; Lin, C.; He, B.Y.; Zheng, T.T.; Lu, L.B.; Cao, Y. An environment-friendly and multi-functional absorbent from chitosan for organic pollutants and heavy metal ion. Carbohyd. Polym. 2016, 148, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.B.; Lejon, T.; Jensen, P.E.; Ottosen, L.M. Simultaneous electrodialytic removal of PAH, PCB, TBT and heavy metals from sediments. J. Environ. Manag. 2017, 198, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.Y.; Zha, S.X.; Li, S.B.; Chen, Z.L. Simultaneous removal of mixed contaminants by organoclays-Amoxicillin and Cu(II) from aqueous solution. Appl. Clay. Sci. 2014, 102, 196–201. [Google Scholar] [CrossRef]
- Baudin, C.; Pean, C.; Perly, B.; Gosselin, P. Inclusion of organic pollutants in cyclodextrin and derivative. Int. J. Environ. An. Ch. 2000, 77, 233–242. [Google Scholar] [CrossRef]
- Badr, T.; Hanna, K.; Brauer, C. Enhanced solubilization and removal of naphthalene and phenanthrene by cyclodextrins from two contaminated soils. J. Hazard. Mater. 2004, 112, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Petitgirard, A.; Djehiche, M. PAH contaminated soil remediation by reusing an aqueous solution of cyclodextrins. Chemosphere 2009, 75, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.; Anderson, P.P.; Schubert, C.M.; Gault, M.B.; Blanford, W.J.; Sandrin, T.R. Carboxymethyl-β-cyclodextrin mitigates toxicity of cadmium, cobalt, and copper during naphthalene biodegradation. Bioresour. Technol. 2010, 101, 2672–2677. [Google Scholar] [CrossRef]
- Bhattarai, B.; Muruganandham, M.; Suri, R.P.S. Development of high efficiency silica coated β-cyclodextrin polymeric adsorbent for the removal of emerging contaminants of concern from water. J. Hazard. Mater. 2014, 273, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, S.; Prasher, S.O.; Marshall, W.D. Simultaneous mobilization of heavy metals and polychlorinated biphenyl (PCB) compounds from soil with cyclodextrin and EDTA in admixture. Chemosphere 2007, 68, 150–158. [Google Scholar] [CrossRef]
- Wang, G.H.; Zhou, Y.M.; Wang, X.G. Simultaneous removal of phenanthrene and lead from artificially contaminated soils with glycine-cyclodextrin. J. Hazard. Mater. 2010, 184, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Paiga, P.; Mendes, L.; Albergaria, J.T.; Delerue-Matos, C.M. Determination of total petroleum hydrocarbons in soil from different locations using infrared spectrophotometry and gas chromatography. Chem. Pap. 2012, 66, 711–721. [Google Scholar] [CrossRef]
- Liu, J.Y.; Sun, S.Y.; Zhang, R.X.; Zhong, S. Determination and Distribution of Heavy Metals in the Municipal Waste Incineratnce. App. Mech. Mater. 2011, 55, 229–232. [Google Scholar]
- Tessier, A.; Campbell, P.G.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Wang, M.E.; Markert, B.; Chen, W.P. Identification of heavy metal pollutants using multivariate analysis and effects of land uses on their accumulation in urban soils in Beijing, China. Environ. Monit. Assess. 2012, 184, 5889–5897. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, Y.G.; Gu, Y.L.; Liu, S.B.; Zeng, G.M.; Cai, X.X.; Hu, X.J.; Wang, H.; Liu, S.M.; Jiang, L.H. Biochar pyrolyzed from MgAl-layered double hydroxides pre-coated ramie biomass (Boehmeria nivea (L.) Gaud.): Characterization and application for crystal violet removal. J. Environ. Manag. 2016, 184, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.Y.; Liu, Y.G.; Li, B.; Liu, S.B.; Zeng, G.M.; Zeng, Z.W.; Wang, X.H.; Ning, Q.M.; Zheng, B.H.; Yang, C.P. Effect of Cu(II) ions on the enhancement of tetracycline adsorption by Fe3O4@SiO2-Chitosan/graphene oxide nanocomposite. Carbohyd. Polym. 2017, 157, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Rawling, M.C. The influence of salting out on the sorption of neutral organic compounds in estuaries. Water. Res. 2001, 35, 4379–4389. [Google Scholar] [CrossRef]
- Huang, J.H.; Yuan, F.; Zeng, G.M.; Li, X.; Gu, Y.L.; Shi, L.X.; Liu, W.C.; Shi, Y.H. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.G.; Liu, S.B.; Li, Z.W.; Tan, X.F.; Huang, X.X.; Zeng, G.M.; Zhou, Y.Y.; Zheng, B.H.; Cai, X.X. Competitive removal of Cd (II) and Pb (II) by biochars produced from water hyacinths: Performance and mechanism. RSC. Adv. 2016, 6, 5223–5232. [Google Scholar]
| pH | Moisture Content (%) | Soil Respiration Intensity (mg CO2/g) | Organic Matter Content (%) | Petroleum Hydrocarbon Content (mg/kg) | Total Lead Content (mg/kg) | Total Copper Content (mg/kg) |
|---|---|---|---|---|---|---|
| 6.30 | 1.92% | 0.132 | 1.0 | 327.805 | 10.792 | 9.204 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Liu, Y.-g.; Tan, X.-f.; Zeng, G.-m.; Gong, J.-l.; Lai, C.; Niu, Q.-y.; Tang, Y.-q. Enhancement of Detoxification of Petroleum Hydrocarbons and Heavy Metals in Oil-Contaminated Soil by Using Glycine-β-Cyclodextrin. Int. J. Environ. Res. Public Health 2019, 16, 1155. https://doi.org/10.3390/ijerph16071155
Zhang W, Liu Y-g, Tan X-f, Zeng G-m, Gong J-l, Lai C, Niu Q-y, Tang Y-q. Enhancement of Detoxification of Petroleum Hydrocarbons and Heavy Metals in Oil-Contaminated Soil by Using Glycine-β-Cyclodextrin. International Journal of Environmental Research and Public Health. 2019; 16(7):1155. https://doi.org/10.3390/ijerph16071155
Chicago/Turabian StyleZhang, Wei, Yun-guo Liu, Xiao-fei Tan, Guang-ming Zeng, Ji-lai Gong, Cui Lai, Qiu-ya Niu, and Yuan-qiang Tang. 2019. "Enhancement of Detoxification of Petroleum Hydrocarbons and Heavy Metals in Oil-Contaminated Soil by Using Glycine-β-Cyclodextrin" International Journal of Environmental Research and Public Health 16, no. 7: 1155. https://doi.org/10.3390/ijerph16071155
APA StyleZhang, W., Liu, Y.-g., Tan, X.-f., Zeng, G.-m., Gong, J.-l., Lai, C., Niu, Q.-y., & Tang, Y.-q. (2019). Enhancement of Detoxification of Petroleum Hydrocarbons and Heavy Metals in Oil-Contaminated Soil by Using Glycine-β-Cyclodextrin. International Journal of Environmental Research and Public Health, 16(7), 1155. https://doi.org/10.3390/ijerph16071155







