Abstract
In the current paper, we compare the inter-day variability of the metabolite concentration of di(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DnBP) in 247 morning urine samples obtained from 19 probands of different age and sex with the metabolite concentration in morning urine obtained from 215 probands of the “Tübingen Survey” cross-sectional study. In the first longitudinal study the morning urine of seven volunteers was collected four times a year for seven consecutive days (course of the year study). In a second study the morning urine of 12 students of a boarding school was collected on five consecutive days (course of a week study). For participants of the two different longitudinal studies we obtained mean concentrations in first void morning urine for mono(2-ethyl-5-hydroxyhexyl) phthalate (5OH-MEHP) in the range from 21.3 to 110 µg/L, 10.5 to 35.6 µg/L for mono(2-ethyl-5-oxohexyl) phthalate (5oxo-MEHP), and 45.5 to 143 µg/L for mono(2-ethyl-5-carboxypentyl) phthalate (5cx-MEPP). The corresponding relative standard deviations (rel. Std.D in %) for these DEHP-metabolites vary between 45.2% and 262%. The 50th percentiles vary for 5OH-MEHP between 17.5 and 65.6 µg/L, for 5oxo-MEHP between 9.0 and 20.3 µg/L and for 5cx-MEPP between 42.5 and 82.0 µg/L. For participants of the “Tübingen Survey” cross-sectional study the means vary for 5OH-MEHP between 58.2 and 85.0 µg/L, between 33.6 and 38.7 µg/L for 5oxo-MEHP and between 110 and 158 µg/L for 5cx-MEPP with rel. standard deviations in a range between 86.5 to 175%. The corresponding 50th percentiles vary for 5OH-MEHP between 26.5 and 42.3 µg/L, for 5oxo-MEHP between 18.0 and 26.3 µg/L, and for 5cx-MEPP between 57.2 and 77.6 µg/L. In order to compare the data from the longitudinal studies with the data from the cross-sectional study, the frequency distribution of the results of both types of studies was compared first. In a second step, the results of a t-test (p-values) was used to check whether the results of the long-term studies differ statistically significantly from the results of the cross-sectional study (p < 0.05). The present data show that the frequency distributions of DEHP-metabolites are comparable. For most of the participants respectively subject groups t-test results prove that no statistical significant difference between results obtained from longitudinal studies compared to the results of the cross-sectional study are apparent. The available data on the exposure of individual subjects mirror the data obtained from cross-sectional studies of the general population and give hints to the risk of individual increased DEHP exposure. Results also highlight the importance of living conditions on the risk of increased DEHP exposure.
1. Introduction
The group of endocrine substances has long played a prominent role in the risk discussion on the effects of environmental pollutants on human health. Within this group diesters of orthophthalic acid (1,2-benzenedicarboxylic acid), in particular di(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), butylbenzyl phthalate (BBzP) and diisobutyl phthalate (DIBP) are considered critical due to their high production volumes and widespread use in the manufacture of consumer goods [1,2,3]. Additionally, other phthalates like DEHP and di-n-butyl phthalate (DnBP) have anti-androgenic effects in animal experiments. DEHP and MnBP “are so called endocrine disruptors [which] interfere with the complexly regulated hormonal processes that control sexual differentiation [2]”. Based on these risk assessments, the use of phthalates has been subject to regulation. Annex XVII to regulation 1907/2006 (REACH) restricts the use of DEHP, DBP and BBzP in toys and childcare products [4]. Regulation (EC) No. 1272/2008 on the classification, labelling and packaging of substances and mixtures [5] classifies DEHP as a reproductive toxic substance of category 1B. Following this decision, the remaining diesters were also classified as reproduction toxic substances (category 1B) in July 2017. Moreover, because of their endocrine disrupting effects, all were included in the list of substances of very high concern (article 57(f)), and added to Annex IV according to Article 59 (10) of the REACH regulation [6]. This implies that future uses of these four orthophthalates (DEHP, DBP, BBP and DIBP) on the EU market are limited to applications for which a case-specific authorization has been granted. These legal restrictions thus limit the oral and dermal intake of these mobile phthalates via the most important vectors of exposure.
These orthophthalates have a substantial migration potential and can be present in relatively high concentrations in many of the respective products [7,8,9,10], a remarkable yet highly variable exposure of the general population to them can be observed. Due to the substantial number of articles on the subject, it is referred to a number of selected publications [11,12,13,14,15,16,17,18,19,20].
A question that arises from the high inter-individual heterogeneity in exposure to DEHP is whether similar variations can be observed intra-individually over the course of time. Seminal investigations on the issue by Calafat [15], Fromme et al. [17], Hildenbrand et al. [18] and Preau et al. [19] show that inter-day exposure of subjects can likewise fluctuate substantially over time. Their results indicate that a significant proportion of exposure to DEHP is due to sources that are encountered sporadically rather than on a regular basis. Studies assessing the relative importance of food consumption and the use of consumer goods for exposure have generally found that, with the exception of small children, a majority of DEHP intake stems from food [21,22,23].
In the present paper, we study inter-day variability in daily DEHP and DnBP exposure among different groups of subjects, and compare these findings to results of our conventional cross-sectional survey. For studying inter-day variability we analyzed first morning urine voids of seven subjects aged 19 to 58 from Southwestern Germany. These individuals were tracked for 4 × 7 days each. Additionally, we examine 60 morning urine samples collected for five consecutive weekdays from 12 boarding school students aged 17–20. The students lived in a boarding school also located in Southwestern Germany. Aside from insights into the temporal pattern of DEHP and DnBP intake for individual subjects, and the influence of environmental conditions e.g., boarding school, we are thus able to derive information on the comparability of longitudinal and cross-sectional results.
2. Materials and Methods
The present paper shows the results of concentration levels of mono-n-butyl phthalate (MnBP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (5OH-MEHP), mono(2-ethyl-5-oxohexyl) phthalate (5oxo-MEHP) and mono(2-ethyl-5-carboxypentyl) phthalate (5cx-MEPP) in morning urine (first morning void) for different subject groups obtained through either longitudinal or cross-sectional studies. In detail:
- Longitudinal 4 × 7 days study (“course of the year“): For this study first morning voids from seven male and female subjects were collected for seven consecutive days (Monday to Sunday) in the months January, April, July and October (and thus in the four seasons of the year) in total 187 urine samples. Sampling was carried out throughout the year 2005. Three test persons aged between 19 and 28 years (two male, one female) and four subjects (two men and two women) being 40 years or older. All participants in this study were employees from the three participating research institutes or their family members.
- Longitudinal 1 × 5 days study (“course of the week“): For this study 60 morning urine samples were collected from 12 boarding school students of both sexes aged between 17 and 20 years. The participants (students) were proposed in cooperation with the school management. The urine samples of each participant were sampled on five consecutive weekdays (Monday to Friday). They provided in total 60 urine samples in December 2005. The meals of this boarding school were prepared and delivered by a cantina kitchen. Additionally, at the time of examination, buying from shops outside the school compound was possible for the students of this group.
- The cross-sectional study (“Tübingen Survey”): This study consists of 215 male and female working age (16 to 64 years) test persons. The participants were employees from one of the research institutes of the University of Tübingen, as well as other persons and family members living in the area of Tübingen. From each subject one first morning void was collected during the year 1999. At this time, all test persons had been part of the general population of Southwestern Germany.
To facilitate comparison with longitudinal study subjects, these test persons were combined into two age groups—young adults between 15 and 30 years of age and adults 40 years and older. Each group consisted of subjects from both sexes.
At the time of sampling subjects from all studies lived across Southwestern Germany. Participation in all studies was on a voluntary basis. All studies were approved by the Ethics Commission at the State Medical Chamber of Baden-Württemberg project identification code 011-05-f, date of approval 1 March 2005 and the Ethics Commission of the Faculty of Medicine of the University Hospital Tübingen, project identification codes 80/99 and 288/99, dates of approval 9 June 1999 and 7 February 2000, respectively. t-Test comparability calculations were performed with the “graphpadquickcals” t-test.
All urine samples are first morning voids. They were collected in PVC-free urine cups. The urine samples were refrigerated and immediately transported to the laboratory where they were deep-frozen and stored at −20 °C prior to analysis. The 247 samples of the longitudinal studies were stored between 1 month and 2 years. From 215 urine samples of the cross-sectional study 77 were stored between 3 months and 2 years. Sixty-nine samples were stored about 2.4 years and 69 about 3.75 years. Following Watkins et al. [23] and Wittassek et al. [24], we measure metabolite concentrations by standardized volume rather than based on creatinine content. The metabolites used as standards such as MEHP, mono(n-octyl) phthalate (MOP), 5OH-MEHP, 5oxo-MEHP and 5cx-MEPP were synthesized at the Institute of Occupational and Social Medicine, University Hospital Tübingen working in collaboration with the University of Florence [25] and the University of Essen. MnBP was purchased from Chemos (Regenstauf, Germany). Following Mettang [26,27], urine samples were incubated with 50 units β-glucuronidase prior to the extraction to ensure enzymatic hydrolysis of the glucuronidated phthalate metabolites. The samples were acidified to pH 2–3 and extracted twice with ethylacetate. After drying and concentrating, the extracts were treated successively with diazomethane and BSTFA (N,O-bis(trimethylsilyl) trifluoroacetamide). The quantitative analysis was performed by selected ion monitoring (at m/z 148) gas chromatography/mass spectrometry (GC-MS) operating the mass spectrometer in a negative ion chemical ionization mode [26,27]. A 20-m capillary column (DB1, 0.18 mm inner diameter, 0.18 µm film thickness (J&W Scientific, Langen, Germany) was used. The column oven temperature was programmed as follows: 40 °C (1 min), 20 °C/min to 80 °C, 10°/min to 200 °C, 5 °C/min to 280 °C (5 min). Hydrogen was used as carrier gas (40 kPa). Quality of analysis was confirmed by external controls. In this set-up, the detection limit of MnBP, MEHP, 5OH-MEHP, 5oxo-MEHP, and cx-MEPP was 0.1 μg/L urine.
3. Results and Discussion
3.1. Di(2-ethylhexyl) Phthalate (DEHP) and Di-n-butyl Phthalate (DnBP)—Metabolites in Morning Urine
In a first overview the results of metabolites of DEHP and DnBP concentrations in morning urine from both longitudinal studies as well as the cross-sectional “Tübingen Survey” are presented in Table 1. As can be seen there, concentrations seem to differ substantially across age group, sex and living conditions. For instance, median values of 5OH-MEHP concentrations for female probands range from 17.5 µg/L for students from the boarding school up to 65.6 µg/L for female subjects in age group from 15 to 30 years from the 4 × 7 days (“course of the year”) study. The corresponding values of the 95th percentile in these two groups reach 58.9 µg/L and 426 µg/L. For 5cx-MEPP, we measure median concentrations between 47.0 µg/L among males aged 40 years or older from the 4 × 7 days (“course of the year”) study and 82.0 µg/L for males from the same study who fall into the age group between 15 and 30 years. Respectively the 95th percentiles take values of 443 µg/L in the older sub-sample and 493 µg/L in the younger (see Table 1). In summary, findings are characterized by a large dispersion of metabolite concentrations across individuals.

Table 1.
Minimum, median (50th percentile), 95th percentile, maximum for urinary concentrations of MEHP, 5OH-MEHP, 5oxo-MEHP, 5cx-MEPP, MnBP stratified by study design, age and sex.
This result becomes even apparent when considering the change in ∑(5OH-MEHP + 5oxo-MEHP) and 5cx-MEPP concentrations over time. Figure 1 shows inter-day variability of metabolite concentrations over the examination period for subjects aged 15 to 30 years from the 4 × 7 days (“course of the year”) longitudinal study.
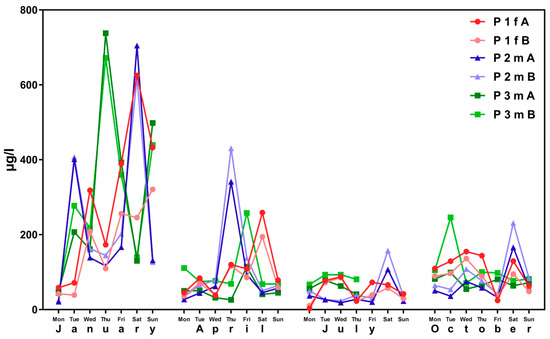
Figure 1.
Variability in urinary concentrations of ∑5OH-MEHP + 5oxo-MEHP (A) and 5cx-MEPP (B) in morning urine of one female (f) and two male (m) subjects at the age between 15 and 30 years (general population) over four periods of seven days each (one per season of the year). The number starting with “P” is the subject identifier.
Figure 2 shows corresponding results for the sub-sample of boarding school students in a similar age group (“course of the week“-longitudinal study). The main difference between the two groups is the degree of homogeneity in living conditions, which is higher among boarding school students. Figure 3 gives the results for participants aged between 15 and 30 obtained with the “Tübingen Survey” cross-sectional study. Finally, Figure 4 and Figure 5 give results for older participants (≥40 years) obtained from the 4 × 7 days longitudinal study and the “Tübingen Survey“.
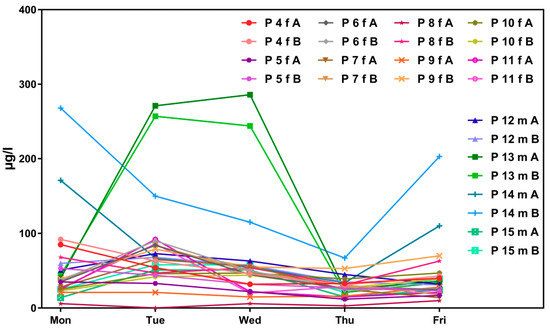
Figure 2.
Variability in urinary concentrations of ∑5OH-MEHP + 5oxo-MEHP (A) and 5cx-MEPP (B) in morning urine of eight female (f) and four male (m) subjects at the age between 17 and 20 years (students of a boarding school) over the course of a school week. The number starting with “P” is the subject identifier.
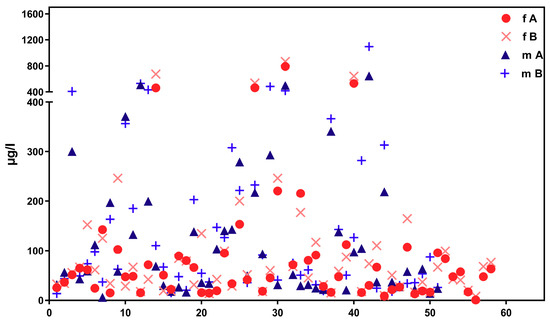
Figure 3.
Concentration distribution of ∑5OH-MEHP + 5oxo-MEHP (A) und 5cx-MEPP (B) in morning urine of 58 female (f, red symbols) and 51 male (m, blue symbols). Subjects aged between 15 and 30 years from the general population (“Tübingen Survey”). X-axis: urine samples (from female and male participants) in arbitrary order.

Figure 4.
Variability in urinary-concentrations of ∑5OH-MEHP + 5oxo-MEHP (A) and 5cx-MEPP (B) concentrations in morning urine of two female (f) and two male (m) subjects aged 40 years or older (general population) over four periods of seven days each (one per season of the year). The number starting with “P” is the subject identifier.
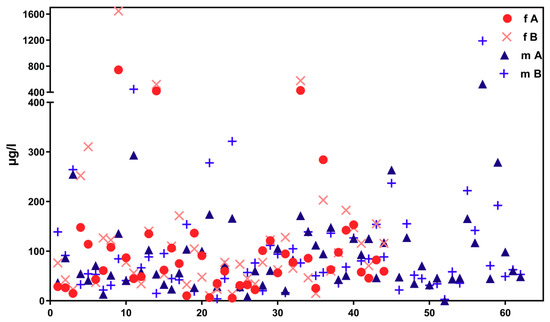
Figure 5.
Concentration distribution of ∑5OH-MEHP + 5oxo-MEHP (A) und 5cx-MEPP (B) in morning urine of 44 female (f, red symbols) and 62 male (m, blue symbols). Subjects aged 40 years or older from the general population (“Tübingen Survey”). X-axis: urine samples (from female and male participants) in arbitrary order.
3.2. Comparability of the Individual Inter-Day Variability of Phthalate Metabolites in Urine Obtained by Longitudinal Studies with the Results of the “Tübingen Survey” Cross-Sectional Study
Regarding the results of classical cross-sectional studies one of the essential characteristics of the exposure to DEHP and DnBP is a large variation in the metabolite urinary concentration across test subjects [2,11,12,13,14,15,16,17,23,24,25,26,27,28,29,30,31,32]. Table 1 and Figure 3 and Figure 5 confirm this statement for the results of the “Tübingen Survey” presented here. Table 1 as well as Figure 1, Figure 2 and Figure 4 indicate a first hint that the distribution of the concentration of urinary DEHP metabolites obtained from few participants over a longer period of time is not significantly different from the concentration levels of many subjects at a single time point. This raises the question of whether long-term studies with few subjects lead to comparable results with the results of cross-sectional studies. When examining the results more closely, either by comparing all obtained results of a certain subject group in ascending order (compare Figure 6) or calculating the distribution frequency (compare Figure 7), it can be shown that the overwhelming majority of individual samples of all subjects falls into a comparatively narrow bandwidth of metabolite exposure with low concentrations. Observations with concentrations of metabolites of DEHP beyond this range are rare (compare also Figure 6 and Figure 7). The similar frequency distribution for the sum of 5OH-MEHP + 5oxo-MEHP shown in Figure 7 is a clear indicator for the comparability of results obtained by the course of the year - longitudinal study with the present cross-sectional study.
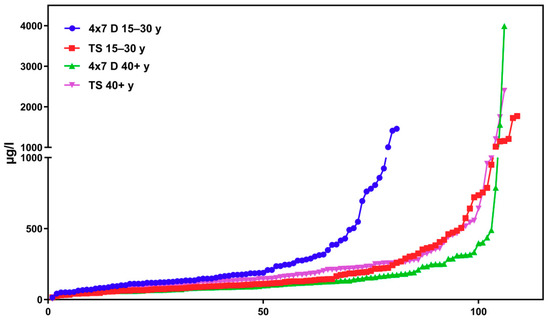
Figure 6.
Results for the sum of MEHP + 5OH-MEHP + 5oxo-MEHP + 5cx-MEPP in the morning urine in ascending order. All test subjects age >15 years. Comparison of longitudinal and cross-sectional studies. 4 × 7 d = course of the year study; TS = “Tübingen Survey” y = age in years. X-axis: urine samples in corresponding order.
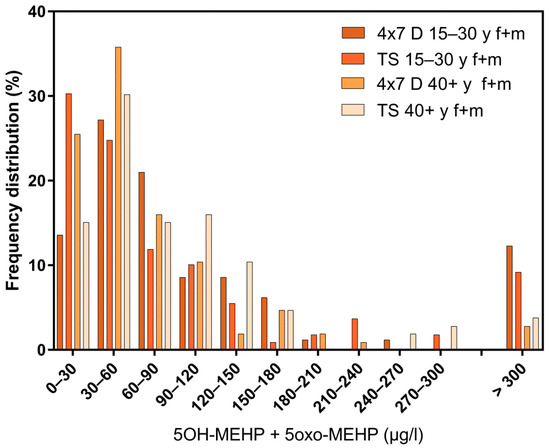
Figure 7.
Frequency distribution of the sum of 5OH-MEHP + 5oxo-MEHP in the morning urine of female (f) and male (m) participants. All test subjects age >15 years. Comparison of longitudinal and cross-sectional studies; 4 × 7 d = course of the year study; TS = “Tübingen Survey”; y = age in years. X-axis: categories for ∑5OH-MEHP+5oxo-MEHP in µg/L in morning urine.
In contrast, only the results for female students of the boarding school show a significantly smaller variation of metabolite concentration in urine (compare Figure 2 and Table 2). Further, two more detailed possibilities to assess possible differences between results obtained from longitudinal studies compared to results obtained from cross-sectional-studies are discussed. One is given by determining p-values with the t-test to show significant differences between two data rows based on means and standard deviations. In Table 2 means and standard deviations for all subject groups are presented. The results of t-testing show, that based on the sum of 5OH-MEHP and 5oxo-MEHP only the results for female and male students of the boarding school (p = 0.0015; confidence interval 95%) and the results for female subjects of the “Tübingen Survey” of the age group ≥40 years versus the results for female subjects of the course of the year study differ significantly (p = 0.0396; confidence interval 95%). The comparison of the results of all other subjects groups prove that there is no significant difference between the results obtained from longitudinal versus cross-sectional studies.

Table 2.
Comparison of longitudinal and cross-sectional studies; Mean and standard deviation (Std.D) absolute (abs.) in µg/L and relative (rel.) in % for urinary concentration of 5OH-MEHP, 5oxo-MEHP, 5cx-MEPP, the sum of 5OH-MEHP + 5oxo-MEHP and MnBP stratified by study design, age and sex.
In regard to MnBP in urine, the results are less clear. Significant differences can be detected by comparing the results of both age groups of male participants of the “Tübingen Survey” with the results of the course of the year study (p = 0.0185; confidence interval 95% for age group 15–30 years; p = 0.0005 for age group ≥40 years; confidence interval 95%). Significant differences can also be detected by comparing the results for female and male participants, age group ≥40 years of the course of the year study (p = 0.0046; confidence interval 95%) and by comparing the results for female participants of the course of the year study with the participants of “Tübingen Survey” regarding the age group ≥40 years (p = 0.0414; confidence interval 95%).
Figure 8 shows quite clearly, that the 50th percentile (median) and the mean of the different subject groups from the “course of the year”-longitudinal study do not differ significantly from 50th percentile (median) and the mean from the “Tübingen Survey”-cross-sectional study (compare also Table 1 and Table 2).
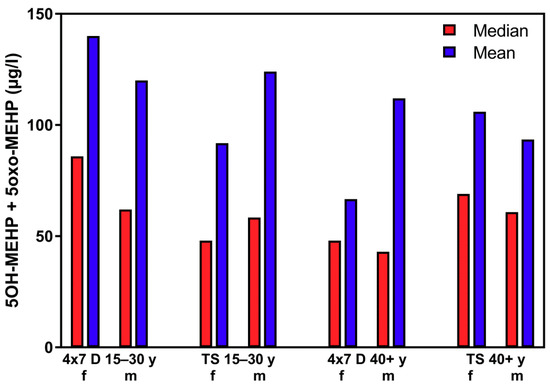
Figure 8.
Mean and 50th percentile (median) of the sum of 5OH-MEHP + 5oxo-MEHP for female (f) and male (m) participants aged 15–30 years and ≥40 years for the “course of the years”-longitudinal study (4 × 7 d) and the “Tübingen Survey” cross-sectional study.
Another possibility to assess comparability is to consider the share of values exceeding the given guide-line values detected either by longitudinal or cross-sectional studies. In Germany Human BioMonitoring (HBM) values are implemented by the German “Human Biomonitoring Commission”. These HBM values are derived from toxicological and epidemiological studies [28]. For DEHP respectively the sum of 5OH-MEHP + 5oxo-MEHP age and gender specific different HBM I values exist [28]. These HBM values are useful to assess a higher body burden with metabolites of DEHP. Due to the age and gender specific different HBM I values female and male participants are assessed separately. For the group of female subjects of the course of a week and of the course of the year longitudinal studies four of 161 samples exceeded this HBM I value. The results of the “Tübingen Survey” show that five of 152 samples obtained from females exceeded the HBM I value. For male subjects two of 126 samples of both of the longitudinal studies exceeded HBM I while none of the samples obtained from male participants of “Tübingen Survey” exceeded HBM I.
In describing the exposure of the general population to the plasticizers DnBP and DEHP, the literature commonly uses characteristics of an empirical distribution, such as the median, the range, or the 95th percentile, of MnBP and DEHP metabolites in urine samples [2,11,12,13,14,15,16,17,23,29,30,31,32]. In the present paper, we take a longitudinal perspective by repeatedly measuring concentration values of these metabolites among a small number of subjects over time and contrast results of these studies with findings from a cross-sectional survey (see Table 1 and Table 2). We discuss whether the exposure of the general population is reflected in the results from the two longitudinal studies. A comparison must take into account a number of factors. For example, the age structure of the studies used for comparison often differs. Moreover, there has been a reduction in the burden of phthalates on the general population in recent years [12,13,14]. Another limitation of the study is perhaps, that some of the urine samples of the “Tübingen Survey”cross-sectional study were stored at −20 °C longer than two years. However, data from the literature [33] show that urine samples used for quality control are stable for at least two years.
With respect to the large variation in the metabolite urinary concentrations and the limitations mentioned above, Table 3 shows exemplary, that median, 95P and maximum for the age group of young adults (15–30 years) determined with both of the longitudinal studies in general do not differ significantly from data reported in literature for adolescents and young adults (11–21 years).

Table 3.
5OH-MEHP, 5oxo-MEHP in morning urine for subjects aged 15 to 30 years. This paper results compared with published data from cross-sectional studies.
Participants aged between 15 and 30 from the presented longitudinal studies differ in one important aspect. Test persons from the 4 × 7 days study (“course of the year”) are individuals living a normal life in Southwestern Germany. They have been randomly selected and correspond to the general population. In contrast, subjects from the 1 × 5 days study (“course of the week”) are comprised of students from a full-time boarding school, implying that this group lives, attends classes and eats together. A comparison of Figure 1 and Figure 2 (age group 15–30 years) demonstrates this influence on the metabolites of DEHP. Especially for female students the mean and standard deviation of the DEHP metabolites 5OH-MEHP, 5oxo-MEHP and 5cx-MEPP are lower among female boarding school students than among all other groups of test persons (see Table 2). t-Test also proves, that the results for this subject group differs significantly to results obtained from individuals living a so called “normal” life. For all other subject groups no significant difference between results for DEHP metabolites obtained in longitudinal studies to results obtained in the cross-sectional study are detectable. For MnBP we found that data obtained in longitudinal studies differ for many subject groups from results gained with the presented cross-sectional study. Thus the available data do not permit to establish a significant effect of life style on exposure to DnBP.
As noted earlier, results presented in Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5 show that individual exposure to DEHP tends to be characterized by a small number of events with high loads. This finding corresponds well to concentration profiles of monoethyl phthalate (MEP) and 5OH-MEHP (respectively MEHHP) exposure reported by Preau et al. and Calafat [15,19].
In general we find that median DEHP metabolite concentrations in first morning urine voids are similar between the longitudinal studies which are based on a limited number of test persons, and the more common cross-sectional surveys if no special circumstances exist. In contrast to these surveys, however, longitudinal studies provide some indications on the frequency by which individual subjects are or were exposed to the risk of increased DEHP intake. Intra-individual sampling also suggests, that individual high urine concentrations observed in cross-sectional studies are not necessarily an indication of a health risk. The present data prove, that high concentrations of metabolites of DEHP in urine normally are rare outliers and the body burden with DEHP returns to a lower level within 1 or 2 days.
In summary, results suggest that longitudinal studies, even when based on a limited number of test persons, are a useful complement to the common cross-sectional surveys. In particular, because they provide information on the frequency by which individual members of the general population experience increased levels of DEHP exposure. Moreover, our findings highlight the importance of living conditions on these incidence rates.
4. Conclusions
Results from the present study show that the substantial heterogeneity in DEHP exposure characteristic for the general population, is reflected in the intra-individual variation of test persons whose exposure level is repeatedly measured over time. For DnBP the correlation of longitudinal versus cross-sectional studies is less clear. That is, when tracking individual exposure over time, the resulting series is usually characterized by low to moderate levels of intake, infrequently punctuated by high exposure events. These events are commonly short-lived such that measured loads rapidly regress to pre-event levels. As a consequence, measuring exposure to DEHP and DnBP among comparatively few individuals who are followed over time yields empirical load distributions that are comparable to the ones obtained from a larger cross-sectional sample of the same population. Nevertheless, average (median respectively mean) exposure levels can change from one population to the next, as they depend to a substantial degree on living conditions and dietary customs. Our results suggest that homogenous living conditions, such as those found in the boarding school, for example, lead to a significant influence in the variation of daily exposure to DEHP. For DnBP a comparable relation cannot be seen. Longitudinal studies thus complement the more classical cross-sectional surveys by providing information on the frequency of high-exposure events—at least during the time of examination.
Author Contributions
S.H. conceived, designed and administrated the cross-sectional study. S.H., T.G. and G.V. conceived and designed the longitudinal studies. T.G. has done the project administration, project monitoring and sampling surveillance for them. S.H. was substantially involved in data acquisition and supervision. G.V. and S.H. have done the data interpretation. Original draft preparation and writing was done by G.V. and S.H. All authors critically revised the manuscript and gave final approval of the version to be published.
Funding
This research was funded by Lieselotte und Karl Otto Winkler-Stiftung für Arbeitsmedizin, project number TS 226/01.004/99 and Landesstiftung Baden-Württemberg gGmbH, project number P-LS-E2/19.
Acknowledgments
We would like to sincerely thank administrators from the boarding school for their support and assistance, and express our deepest gratitude to all participants for their patience and cooperation. We thank Dr. Reiner Kimmel for his support in recruiting participants in the cross-sectional study and in collecting the samples. Also, we would like to thank Roman Wodarz and Isolde Wodarz for their excellent assistance and technical support in laboratory work. The work of the Institute of Occupational and Social Medicine and Health Services Research is supported by an unrestricted grant from the Baden-Wuerttemberg Employers’ Association of the Metal and Electric Industry (Südwestmetall). We acknowledge support in publishing funding by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Phthalates and Cumulative Risk Assessment: The Tasks Ahead, National Research Council (US) Committee on the Health Risks of Phthalates; National Academies Press (US): Washington, DC, USA, 2008; ISBN 0-309-12842-0.
- Stoffmonographie für Phthalate—Neue und aktualisierte Referenzwerte für Monoester und oxidierte Metabolite im Urin von Kindern und Erwachsenen. Stellungnahme der Kommission Human-Biomonitoring des Umweltbundesamtes. Gesundheitsforsch. Gesundheitsschutz 2011, 54, 770–785. [CrossRef] [PubMed]
- Opinion of the French Agency for Food. Environmental and Occupational Health & Safety on the Development of a Chronic Toxicity Reference Value for di(2-ethylhexyl) phthalate (DEHP) via the Oral Route ANSES Opinion No 2012–SA-0180, 14. 11. 2012. Available online: https://www.anses.fr/fr/system/files/CHIM2012sa0180EN.pdf (accessed on 2 July 2018).
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC–Annex XVII-Official Journal of the European Union L 396 of 30 December 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02006R1907-20140410&from=DE (accessed on 2 July 2018).
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006 Official Journal of the European Union L 353/1 31.12.2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272 (accessed on 2 July 2018).
- Commission Implementing Decision of 4.7.2017 on the identification of bis(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), benzyl butyl phthalate (BBP) and diisobutyl phthalate (DIBP) as substances of very high concern according to Article 57(f) of Regulation (EC) No 1907/2006 of the European Parliament and of the Council. Notified under document C(2017) 4462 Official Journal of the European Union L 173/35 06.07.2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017D1210 (accessed on 2 July 2018).
- Wormuth, M. Consumer Exposure to Chemical Substances with Diverse Applications—Contributions from Retail Products, Building Materials, and Diffuse Sources. Ph.D. Thesis, ETH Zürich, Zürich, Switzerland, 2005. Available online: https://www.research-collection.ethz.ch/handle/20.500.11850/116444 (accessed on 2 July 2018).
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbühler, K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, K.; Kappenstein, O.; Ebner, I.; Würtz, A. Phthalat-Belastung der Bevölkerung in Deutschland: Expositionsrelevante Quellen, Aufnahmepfade und Toxikokinetik am Beispiel von DEHP und DINP Band II: Ergänzende Messungen von DEHP, DINP und DiNCH in Lebensmitteln und Migrationsmessungen in Verbraucherprodukten, UBA-FB 001637/2. Schriftenreihe Umwelt und Gesundheit Umweltbundesamt Berlin, Deutschland. 2012. Available online: http://www.uba.de/uba-info-medien/4392.html (accessed on 2 July 2018).
- Volland, G. Phthalate. In Handbuch Gebäude-Schadstoffe und Gesunde Innenraumluft; Zwiener, G., Lange, F.-M., Eds.; Erich Schmidt Verlag: Berlin, Germany, 2012; pp. 467–484. ISBN 978-3-503-12990-4. [Google Scholar]
- Koch, H.M.; Calafat, A.M. Human body burdens of chemicals used in plastic manufacture. Phil. Trans. R. Soc. B 2009, 364, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Rüther, M.; Schütze, A.; Conrad, A.; Pälmke, C.; Apel, P.; Brüning, T.; Kolossa-Gehring, M. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int. J. Hyg. Environ. Health 2017, 220 Pt A, 130–141. [Google Scholar] [CrossRef]
- Fourth National Report on Human Exposure to Environmental Chemicals 2009, Department of Health and Human Services, Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/exposurereport/pdf/FourthReport.pdf (accessed on 3 July 2018).
- Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables January 2017, Department of Health and Human Services, Center for Disease Control and Prevention, Volume One. Available online: https://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Volume1_Jan2017.pdf (accessed on 3 July 2018).
- Calafat, A.M. Measuring Phthalate Metabolites in Biological Matrices—Workshop on Quality of Analytical Data in HBM Nov. 30, 2011 lecture Brussels, Belgium. Available online: http://www.eu-hbm.info/democophes/workshops-hbm-week/HBManalytics_MeasuringPhthalateMetabolitesinBiologicalMatrices_Calafat.pdf (accessed on 4 July 2018).
- Becker, K.; Göen, T.; Seiwert, M.; Conrad, A.; Pick-Fuss, H.; Müller, J.; Wittassek, M.; Schulz, C.; Kolossa-Gehring, M. GerES IV: Phthalate metabolites and bisphenol A in urine of German children. Int. J. Hyg. Environ. Health 2009, 212, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Bolte, G.; Koch, H.M.; Angerer, J.; Boehmer, S.; Drexler, H.; Mayer, R.; Liebl, B. Occurrence and daily variation of phthalate metabolites in urine of an adult population. J. Int. J. Hyg. Environ. Health 2007, 210, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, S.; Wodarz, R.; Gabrio, T.; Volland, G. Biomonitoring of the di(2-ethylhexyl) phthalate metabolites mono(2-ethyl-5-hydroxyhexyl) phthalate and mono(2-ethyl-5-oxohexyl) phthtalate in children and adults during the course of time and seasons. J. Int. J. Hyg. Environ. Health 2009, 216, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Preau, J.L., Jr.; Wong, L.-Y.; Silva, M.J.; Needham, L.L.; Calafat, A.M. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: An observational study. Environ. Health Perspect. 2010, 118, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Heinemeyer, G.; Heiland, A.; Sommerfeld, C.; Springer, A.; Hausdörfer, S.; Treutz, M.; Lindtner, O.; Rüdiger, T. Phthalat-Belastung der Bevölkerung in Deutschland: Expositionsrelevante Quellen, Aufnahmepfade und Toxikokinetik am Beispiel von DEHP und DINP Band I: Exposition durch Verzehr von Lebensmitteln und Anwendung von Verbraucherprodukten. UBA-FB 001637/1 Umweltbundesamt Schriftenreihe Umwelt und Gesundheit: Berlin, Germany, 2012. Available online: http://www.uba.de/uba-info-medien/4391.html (accessed on 4 July 2018).
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health 2014, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Völkel, W.; Filser, J.G.; Kessler, W. Phthalat-Belastung der Bevölkerung in Deutschland: Expositionsrelevante Quellen, Aufnahmepfade und Toxikokinetik am Beispiel von DEHP und DINP Band III: Humane Toxikokinetikstudie. UBA-FB 001637/3 Umweltbundesamt Schriftenreihe Umwelt und Gesundheit: Berlin, Germany, 2012. Available online: http://www.uba.de/uba-info-medien/4393.html (accessed on 4 July 2018).
- Watkins, D.J.; Eliot, M.; Sathyanarayana, S.; Calafat, A.M.; Yolton, K.; Lanphear, B.P.; Braun, J.M. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ. Sci. Technol. 2014, 48, 8881–8890. [Google Scholar] [CrossRef] [PubMed]
- Wittassek, M.; Heger, W.; Koch, H.M.; Becker, K.; Angerer, J.; Kolossa-Gehring, M. Daily intake of di(2-ethylhexyl) phthalate (DEHP) by German children—A comparison of two estimation models based on urinary DEHP metabolite levels. Int. J. Hyg. Environ. Health 2007, 210, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nuti, F.; Hildenbrand, S.; Chelli, M.; Wodarz, R.; Papini, A.M. Synthesis of DEHP metabolites as biomarkers for GC-MS evaluation of phthalates as endocrine disrupters. Bioorg. Med. Chem. 2005, 13, 3461–3465. [Google Scholar] [CrossRef] [PubMed]
- Mettang, T.; Thomas, S.; Kiefer, T.; Fischer, F.P.; Kuhlmann, U.; Wodarz, R.; Rettenmeier, A.W. Uraemic pruritus and exposure to di(2-ethylhexyl) phthalate (DEHP) in haemodialysis patients. Nephrol. Dial. Transpl. 1996, 11, 2439–2443. [Google Scholar] [CrossRef]
- Mettang, T.; Alscher, D.M.; Pauli-Magnus, C.; Dunst, R.; Kuhlmann, U.; Rettenmeier, A.W. Phthalic acid is the main metabolite of the plasicizer di(2-ethylhexyl) phthalate in peritoneal dialysis patients. Adv. Perit. Dial. 1999, 15, 229–232. [Google Scholar] [PubMed]
- Beurteilungswerte der HBM-Kommission (Umweltbundesamt 2017). Available online: hbm-werte_dt_stand_2017_02_06.pdf http://www.umweltbundesamt.de/themen/gesundheit/kommissionen-arbeitsgruppen/kommission-human-biomonitoring/beurteilungswerte-der-hbm-kommission (accessed on 4 July 2018).
- Koch, H.M.; Drexler, H.; Angerer, J. Internal exposure of nursery-school children and their parents and teachers to di(2-ethylhexyl) phthalate (DEHP). J. Int. J. Hyg. Environ. Health 2004, 207, 15–22. [Google Scholar] [CrossRef]
- Myridakis, A.; Fthenou, E.; Balaska, E.; Vakinti, M.; Kogevinas, M.; Stephanou, E.G. Phthalate esters, parabens and bisphenol-A exposure among mothers and their children in Greece (Rhea cohort). Environ. Int. 2015, 83, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.; Jorgensen, N.; Andersson, A.-M.; Calafat, A.M.; Silva, M.J.; Redmon, J.B.; Sparks, A.; Drobnis, E.Z.; Wang, C.; Liu, F.; et al. Associations between urinary metabolites of di(2-ethylhexyl) phthalate and reproductive hormones in fertile men. Int. J. Androl. 2011, 34, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kho, Y.L.; Kim, S.K.; Choi, K.; Hwang, S.H.; Jeong, J.; Kim, P. DEHP, DEP and DBP exposure analysis using urinary metabolites of Gyongi Province university students. Korean J. Environ. Health Sci. 2013, 39, 408–417. [Google Scholar] [CrossRef]
- Koch, H.M. Untersuchung der Di(-2-ethylhexyl)phthalat (DEHP)-Belastung der Allgemeinbevölkerung—Durchführung eines Human-Biomonitorings. 2006. Available online: https://opus4.kobv.de/opus4-fau/files/393/HolgerMKochDissertation.pdf (accessed on 12 December 2018).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).