Combination of In Situ Feeding Rate Experiments and Chemical Body Burden Analysis to Assess the Influence of Micropollutants in Wastewater on Gammarus pulex
Abstract
:1. Introduction
2. Material and Methods
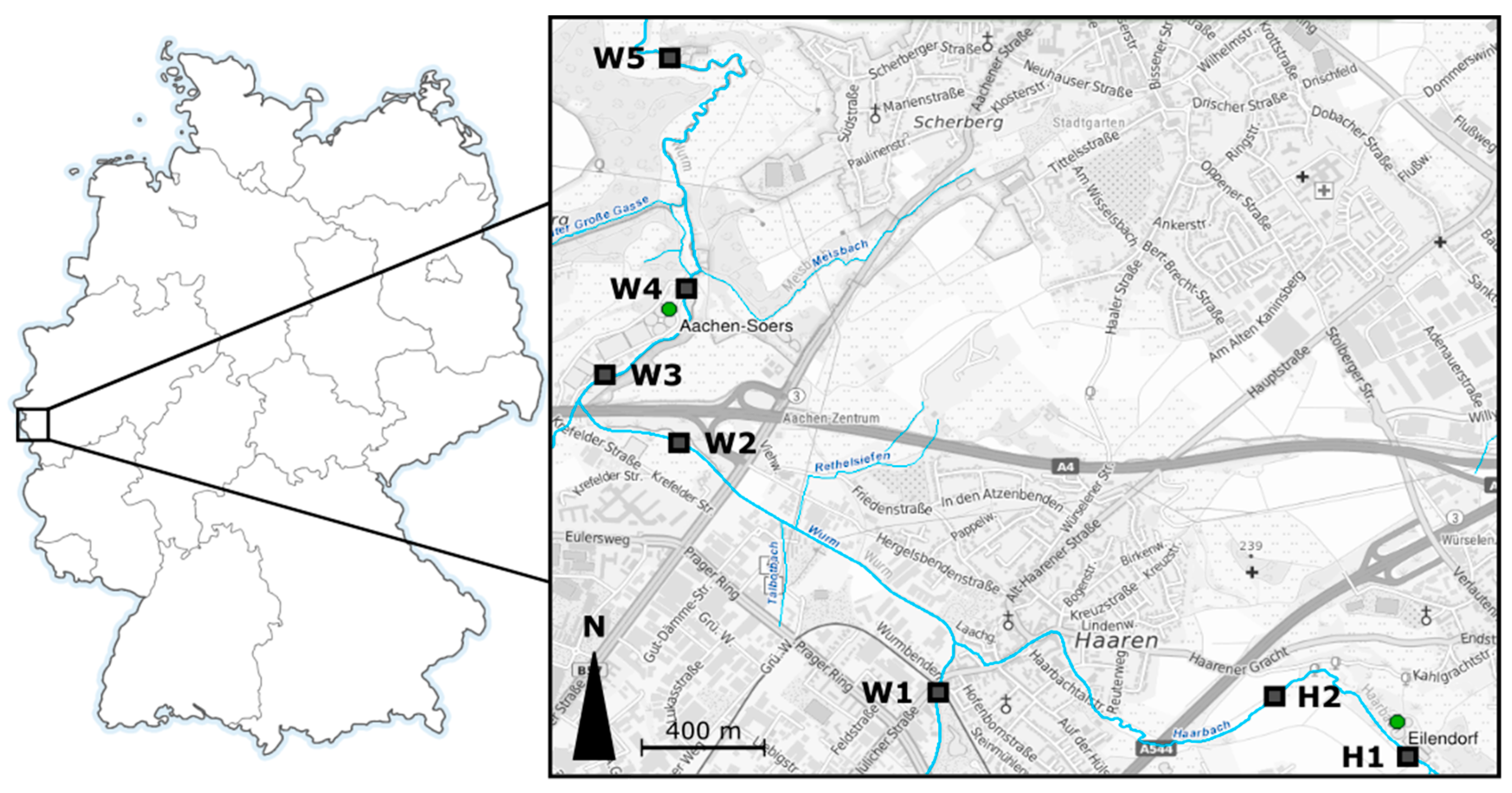
2.1. Study Area and Design
2.2. Feeding Rate Inhibition
2.2.1. Calculation of Feeding Rate
2.2.2. Statistical Analysis
2.3. Biota Analyses
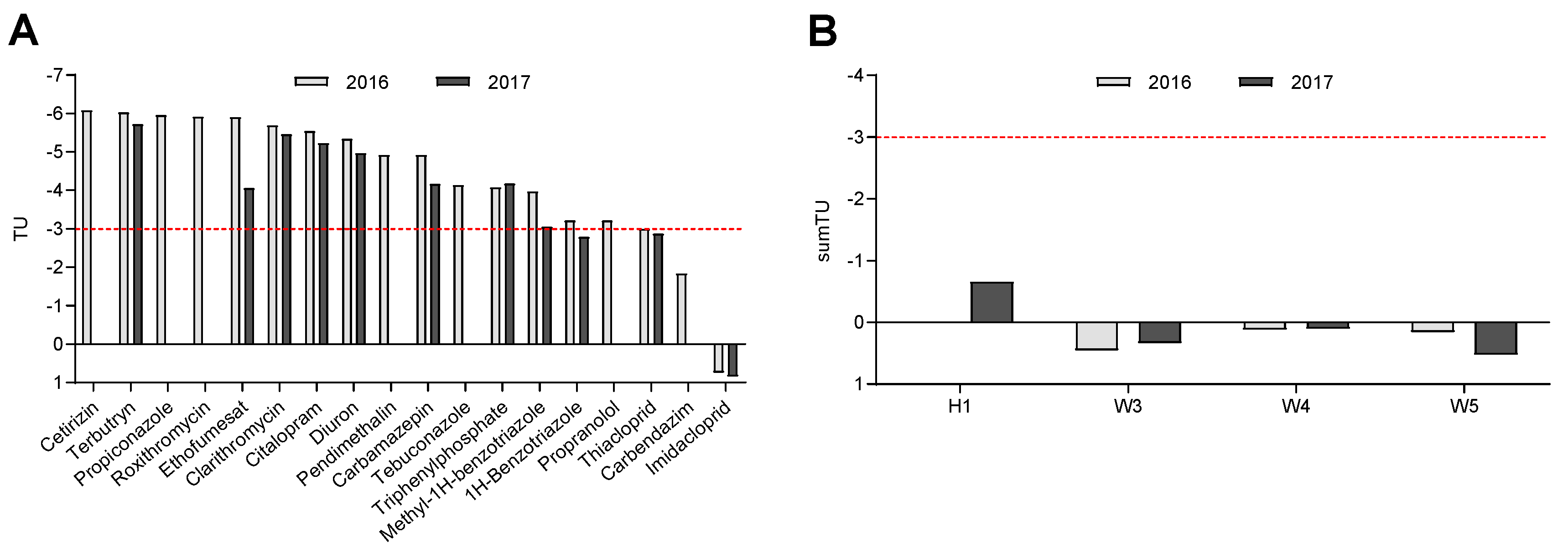
Toxic Pressure
3. Results
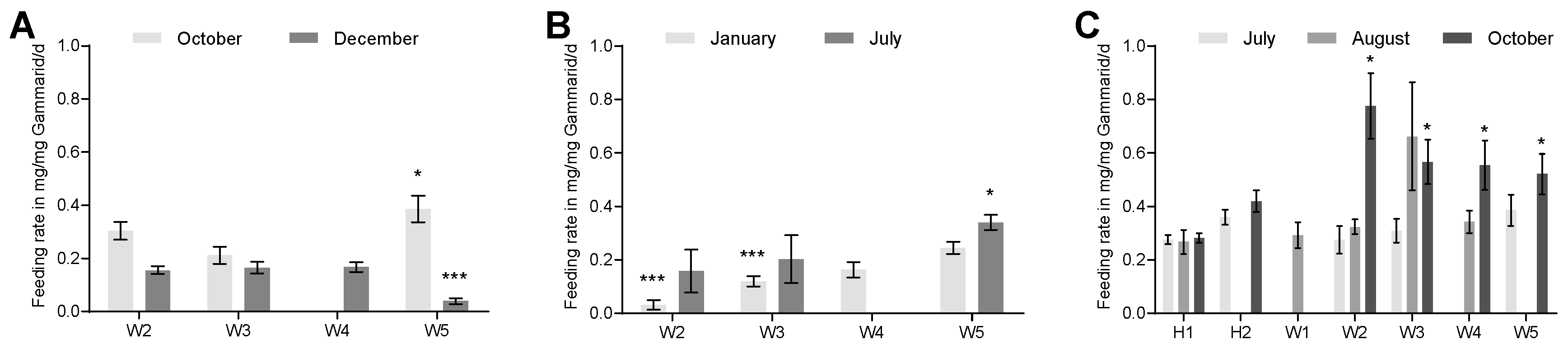
3.1. Feeding Rate Inhibition
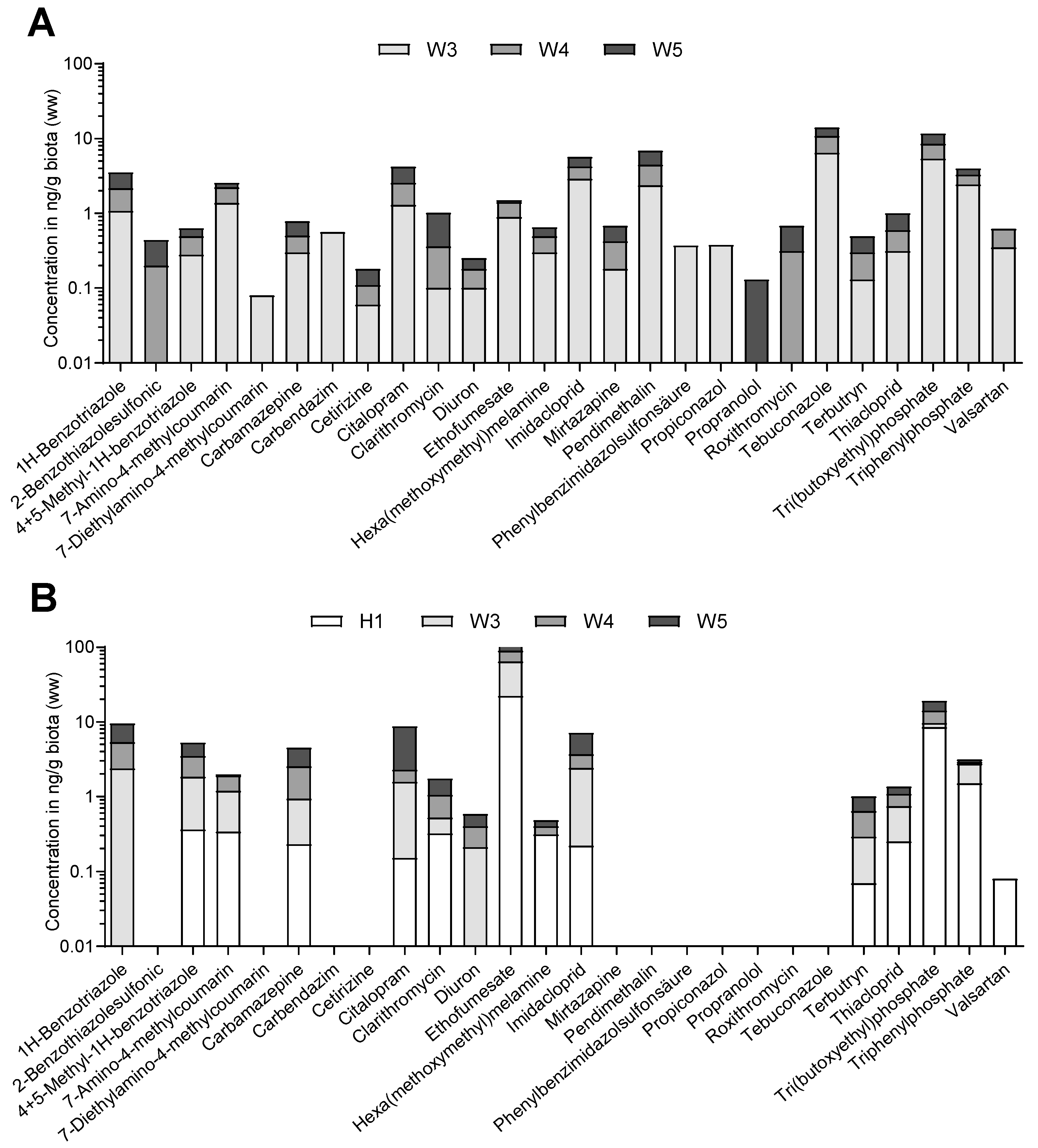
3.2. Biota Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaar, H.; Clara, M.; Gans, O.; Kreuzinger, N. Micropollutant removal during biological wastewater treatment and a subsequent ozonation step. Environ. Pollut. 2010, 158, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Dulio, V.; van Bavel, B.; Brorström-Lundén, E.; Harmsen, J.; Hollender, J.; Schlabach, M.; Slobodnik, J.; Thomas, K.; Koschorreck, J. Emerging pollutants in the EU: 10 years of NORMAN in support of environmental policies and regulations. Environ. Sci. Eur. 2018, 30, 5. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.; Joss, A. Human Pharmaceuticals, Hormones and Fragrances; IWA Publishing: London, UK, 2007. [Google Scholar]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; Mcardell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef] [PubMed]
- Brack, W.; Escher, B.I.; Müller, E.; Schmitt-Jansen, M.; Schulze, T.; Slobodnik, J.; Hollert, H. Towards a holistic and solution-oriented monitoring of chemical status of European water bodies: How to support the EU strategy for a non-toxic environment? Environ. Sci. Eur. 2018, 30, 33. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, M.; Schulz, R. Ozonation of secondary treated wastewater reduces ecotoxicity to Gammarus fossarum (Crustacea; Amphipoda): Are loads of (micro) pollutants responsible? Water Res. 2011, 45, 3999–4007. [Google Scholar] [CrossRef] [PubMed]
- Maletz, S.; Floehr, T.; Beier, S.; Klümper, C.; Brouwer, A.; Behnisch, P.; Higley, E.; Giesy, J.P.; Hecker, M.; Gebhardt, W. In vitro characterization of the effectiveness of enhanced sewage treatment processes to eliminate endocrine activity of hospital effluents. Water Res. 2013, 47, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Brack, W. Effect-directed analysis: A promising tool for the identification of organic toxicants in complex mixtures? Anal. Bioanal. Chem. 2003, 377, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Brack, W.; Altenburger, R.; Schüürmann, G.; Krauss, M.; Herráez, D.L.; van Gils, J.; Slobodnik, J.; Munthe, J.; Gawlik, B.M.; van Wezel, A. The solutions project: Challenges and responses for present and future emerging pollutants in land and water resources management. Sci. Total Environ. 2015, 503, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.; Delaney, P.; Mainstone, C.; Clarke, S. Measurement by in situ bioassay of water quality in an agricultural catchment. Water Res. 1995, 29, 2441–2448. [Google Scholar] [CrossRef]
- Wernersson, An.; Carere, M.; Maggi, C.; Tusil, P.; Soldan, P.; James, A.; Sanchez, W.; Dulio, V.; Broeg, K.; Reifferscheid, G. The European technical report on aquatic effect-based monitoring tools under the water framework directive. Environ. Sci. Eur. 2015, 27, 7. [Google Scholar] [CrossRef]
- Crossland, N.O. The role of mesocosm studies in pesticide registration. In Proceedings of the Brighton Crop Protection Conference, Pests and Diseases, Brighton, UK, 19–22 November 1990; British Crop Protection Council Publications: Alton, UK, 1990; Volume 2. [Google Scholar]
- Graney, R.L.; Giesy, J.P.; DiToro, D. Mesocosm experimental design strategies: Advantages and disadvantages in ecological risk assessment. In Using Mesocosms to Assess the Aquatic Ecological Risk of Pesticides: Theory and Practice; Entomological Society of America: Lanham, MD, USA, 1989; pp. 74–88. [Google Scholar]
- Hopkin, S.P. In situ biological monitoring of pollution in terrestrial and aquatic ecosystems. In Handbook of Ecotoxicology; Blackwell Scientific: Hoboken, NJ, USA, 1993; pp. 397–427. [Google Scholar]
- Schulz, R.; Liess, M. Validity and ecological relevance of an active in situ bioassay using Gammarus pulex and Limnephilus lunatus. Environ. Toxicol. Chem. 1999, 18, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.; Burton, G.A.; Culp, J.M.; Greenberg, M.S.; Munkittrick, K.R.; Ribeiro, R.; Salazar, M.H.; St-Jean, S.D. Review of aquatic in situ approaches for stressor and effect diagnosis. Integr. Environ. Assess. Manag. 2007, 3, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Maltby, L.; Clayton, S.A.; Wood, R.M.; McLoughlin, N. Evaluation of the Gammarus pulex in situ feeding assay as a biomonitor of water quality: Robustness, responsiveness, and relevance. Environ. Toxicol. Chem. 2002, 21, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Hellawell, J.M. Biological Indicators of Freshwater Pollution and Environmental Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Resh, V.H.; Rosenberg, D.M. Freshwater Biomonitoring and Benthic Macroinvertebrates; Chapman & Hall: New York, NY, USA, 1993. [Google Scholar]
- Coulaud, R.; Geffard, O.; Xuereb, B.; Lacaze, E.; Quéau, H.; Garric, J.; Charles, S.; Chaumot, A. In situ feeding assay with Gammarus fossarum (Crustacea): Modelling the influence of confounding factors to improve water quality biomonitoring. Water Res. 2011, 45, 6417–6429. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Plant Protection Products and their Residues. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge-of-field surface waters. EFSA J. 2013, 11, 3290. [Google Scholar]
- Baird, D.J.; Brown, S.S.; Lagadic, L.; Liess, M.; Maltby, L.; Moreira-Santos, M.; Schulz, R.; Scott, G.I. In situ-based effects measures: Determining the ecological relevance of measured responses. Integr. Environ. Assess. Manag. 2007, 3, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Barata, C.; Damasio, J.; Lopez, M.A.; Kuster, M.; de Alda, M.L.; Barcelo, D.; Riva, M.C.; Raldua, D. Combined use of biomarkers and in situ bioassays in Daphnia magna to monitor environmental hazards of pesticides in the field. Environ. Toxicol. Chem. 2007, 26, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Maltby, L. Studying stress: The importance of organism-level responses. Ecol. Appl. 1999, 9, 431–440. [Google Scholar] [CrossRef]
- Gerhardt, A.; Koster, M.; Lang, F.; Leib, V. Active in situ biomonitoring of pesticide pulses using Gammarus spp. In small tributaries of lake constance. J. Environ. Prot. 2012, 3, 573. [Google Scholar] [CrossRef]
- Macedo-Sousa, J.A.; Pestana, J.L.T.; Gerhardt, A.; Nogueira, A.J.A.; Soares, A.M.V.M. Behavioural and feeding responses of Echinogammarus meridionalis (Crustacea, Amphipoda) to acid mine drainage. Chemosphere 2007, 67, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Bousfield, E.L. Shallow-Water Gammaridean Amphipoda of New England; Comstock Publishing Associates: Ithaca, NY, USA, 1973. [Google Scholar]
- MacNeil, C.; Dick, J.T.A.; Elwood, R.W. The trophic ecology of freshwater Gammarus spp.(Crustacea: Amphipoda): Problems and perspectives concerning the functional feeding group concept. Biol. Rev. 1997, 72, 349–364. [Google Scholar] [CrossRef]
- Burton, G.A.; Nelson, M.K.; Ingersoll, C.G. Freshwater benthic toxicity tests. In Sediment Toxicity Assessment; Lewis Publishers: Boca Raton, FL, USA, 1992; pp. 213–240. [Google Scholar]
- Tlili, K.; Labadie, P.; Bourges, C.; Desportes, A.; Chevreuil, M. Bioaccumulation of polybrominated diphenyl ethers by the freshwater benthic amphipod Gammarus pulex. Arch. Environ. Contam. Toxicol. 2012, 63, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Blaha, M.; Kumar, V.; Cerveny, D.; Fedorova, G.; Randak, T. Presence of pharmaceuticals in benthic fauna living in a small stream affected by effluent from a municipal sewage treatment plant. Water Res. 2015, 72, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, K.L.; McNeill, S. Bioaccumulation of heavy metals by aquatic macro-invertebrates of different feeding guilds: A review. Sci. Total Environ. 1999, 229, 1–19. [Google Scholar] [CrossRef]
- Vrana, B.; Allan, I.J.; Greenwood, R.; Mills, G.A.; Dominiak, E.; Svensson, K.; Knutsson, J.; Morrison, G. Passive sampling techniques for monitoring pollutants in water. TrAC Trends Anal. Chem. 2005, 24, 845–868. [Google Scholar] [CrossRef]
- Ashauer, R.; Caravatti, I.; Hintermeister, A.; Escher, B.I. Bioaccumulation kinetics of organic xenobiotic pollutants in the freshwater invertebrate gammarus pulex modeled with prediction intervals. Environ. Toxicol. Chem. 2010, 29, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Scherbaum, E.; Bertsch, D. Validation of a simple and rapid multiresidue method (QuEChERS) and its implementation in routine pesticide analysis. In Proceedings of the MGPR Symposium, Aix en Provence, France, 20–24 May 2003. [Google Scholar]
- Payá, P.; Anastassiades, M.; Mack, D.; Sigalova, I.; Tasdelen, B.; Oliva, J.; Barba, A. Analysis of pesticide residues using the quick easy cheap effective rugged and safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and tandem mass spectrometric detection. Anal. Bioanal. Chem. 2007, 389, 1697–1714. [Google Scholar] [CrossRef] [PubMed]
- MULNV. Flussgebiete Nrw: Steckbriefe für das Gebiet Maas-Süd (rur), ab s. 110 Planungseinheit „Wurm“. 2013. Available online: https://www.flussgebiete.nrw.de/system/files/atoms/files/pe-stb_maas_sued_entwurf_20141222.pdf (accessed on 11 March 2019).
- Gualtieri, M.; Andrioletti, M.; Vismara, C.; Milani, M.; Camatini, M. Toxicity of tire debris leachates. Environ. Int. 2005, 31, 723–730. [Google Scholar] [CrossRef] [PubMed]
- NRW MUNLV. Steckbriefe der Planungseinheiten in den Nordrheinwestfälischen Anteilen von Rhein, Weser, Ems Und Maas: Oberflächengewässer und Grundwasser–teileinzugsgebiet Rhein/sieg Nrw, Düsseldorf. 2009. Available online: https://www.flussgebiete.nrw.de/system/files/atoms/files/pe-stb_rheingraben-nord_entwurf_20141222.pdf (accessed on 11 March 2019).
- Maltby, L.; Clayton, S.A.; Yu, H.; McLoughlin, N.; Wood, R.M.; Yin, D. Using single-species toxicity tests, community-level responses, and toxicity identification evaluations to investigate effluent impacts. Environ. Toxicol. Chem. 2000, 19, 151–157. [Google Scholar] [CrossRef]
- Inostroza, P.A.; Wicht, An.; Huber, T.; Nagy, C.; Brack, W.; Krauss, M. Body burden of pesticides and wastewater-derived pollutants on freshwater invertebrates: Method development and application in the Danube river. Environ. Pollut. 2016, 214, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Inostroza, P.A.; Vera-Escalona, I.; Wicht, A.; Krauss, M.; Brack, W.; Norf, H. Anthropogenic stressors shape genetic structure: Insights from a model freshwater population along a land use gradient. Environ. Sci. Technol. 2016, 50, 11346–11356. [Google Scholar] [CrossRef] [PubMed]
- Ashauer, R.; Boxall, A.; Brown, C. Uptake and elimination of Chlorpyrifos and Pentachlorophenol into the freshwater amphipod Gammarus pulex. Arch. Environ. Contam. Toxicol. 2006, 51, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Warne, M.S.J.; Hawker, D.W. The number of components in a mixture determines whether synergistic and antagonistic or additive toxicity predominate: The funnel hypothesis. Ecotoxicol. Environ. Saf. 1995, 31, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Liess, M.; Schäfer, R.B.; Schriever, C.A. The footprint of pesticide stress in communities—Species traits reveal community effects of toxicants. Sci. Total Environ. 2008, 406, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, M.; Schulz, R. Population response to ozone application in wastewater: An on-site microcosm study with Gammarus fossarum (Crustacea: Amphipoda). Ecotoxicology 2011, 20, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Englert, D.; Zubrod, J.P.; Schulz, R.; Bundschuh, M. Effects of municipal wastewater on aquatic ecosystem structure and function in the receiving stream. Sci. Total Environ. 2013, 454, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Chaumot, A.; Geffard, O.; Armengaud, J.; Maltby, L. Gammarids as reference species for freshwater monitoring. In Aquatic Ecotoxicology; Academic Press: Cambridge, MA, USA, 2015; pp. 253–280. [Google Scholar]
- Crane, M.; Maltby, L. The lethal and sublethal responses of Gammarus pulex to stress: Sensitivity and sources of variation in an in situ bioassay. Environ. Toxicol. Chem. 1991, 10, 1331–1339. [Google Scholar] [CrossRef]
- Alonso, Á.; de Lange, H.J.; Peeters, E.T.H.M. Development of a feeding behavioural bioassay using the freshwater amphipod Gammarus pulex and the multispecies freshwater biomonitor. Chemosphere 2009, 75, 341–346. [Google Scholar] [CrossRef] [PubMed]
- De Lange, H.J.; Noordoven, W.; Murk, A.J.; Lürling, M.F.; Peeters, E.T. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat. Toxicol. 2006, 78, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Inostroza, P.A.; Vera-Escalona, I.; Wild, R.; Norf, H.; Brauns, M. Tandem action of natural and chemical stressors in stream ecosystems: Insights from a population genetic perspective. Environ. Sci. Technol. 2018, 52, 7962–7971. [Google Scholar] [CrossRef] [PubMed]
- Munz, N.A.; Fu, Q.; Stamm, C.; Hollender, J. Internal concentrations in gammarids reveal increased risk of organic micropollutants in wastewater-impacted streams. Environ. Sci. Technol. 2018, 52, 10347–10358. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.; Dammel, S.; Ploessl, F.; Bracher, F.; Laforsch, C. Effects of a pharmaceutical mixture at environmentally relevant concentrations on the amphipod Gammarus fossarum. Mar. Freshw. Res. 2010, 61, 196–203. [Google Scholar] [CrossRef]
- Bundschuh, M.; Zubrod, J.P.; Klemm, P.; Elsaesser, D.; Stang, C.; Schulz, R. Effects of peak exposure scenarios on Gammarus fossarum using field relevant pesticide mixtures. Ecotoxicol. Environ. Saf. 2013, 95, 137–143. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Könemann, S.; Müller, Y.; Tschentscher, D.; Krauss, M.; Inostroza, P.A.; Brückner, I.; Pinnekamp, J.; Schiwy, S.; Hollert, H. Combination of In Situ Feeding Rate Experiments and Chemical Body Burden Analysis to Assess the Influence of Micropollutants in Wastewater on Gammarus pulex. Int. J. Environ. Res. Public Health 2019, 16, 883. https://doi.org/10.3390/ijerph16050883
Könemann S, Müller Y, Tschentscher D, Krauss M, Inostroza PA, Brückner I, Pinnekamp J, Schiwy S, Hollert H. Combination of In Situ Feeding Rate Experiments and Chemical Body Burden Analysis to Assess the Influence of Micropollutants in Wastewater on Gammarus pulex. International Journal of Environmental Research and Public Health. 2019; 16(5):883. https://doi.org/10.3390/ijerph16050883
Chicago/Turabian StyleKönemann, Sarah, Yvonne Müller, Daniel Tschentscher, Martin Krauss, Pedro A. Inostroza, Ira Brückner, Johannes Pinnekamp, Sabrina Schiwy, and Henner Hollert. 2019. "Combination of In Situ Feeding Rate Experiments and Chemical Body Burden Analysis to Assess the Influence of Micropollutants in Wastewater on Gammarus pulex" International Journal of Environmental Research and Public Health 16, no. 5: 883. https://doi.org/10.3390/ijerph16050883
APA StyleKönemann, S., Müller, Y., Tschentscher, D., Krauss, M., Inostroza, P. A., Brückner, I., Pinnekamp, J., Schiwy, S., & Hollert, H. (2019). Combination of In Situ Feeding Rate Experiments and Chemical Body Burden Analysis to Assess the Influence of Micropollutants in Wastewater on Gammarus pulex. International Journal of Environmental Research and Public Health, 16(5), 883. https://doi.org/10.3390/ijerph16050883




