Management of Endocrinopathies in Pregnancy: A Review of Current Evidence
Abstract
1. Introduction
- Category A—Randomized control trials were not able to demonstrate any fetal risk in the first trimester of pregnancy, as well as no risk at all in the second and third trimester.
- Category B—Studies on experimental animals were not able to demonstrate a fetal risk but there are no well-controlled adequate studies in pregnant women. Most of the drugs are included in this category.
- Category C—Studies on experimental animals demonstrated an adverse effect upon fetus and there are no well-controlled adequate studies in pregnant women, but the potential benefits could justify the use of that drug in pregnant women, despite potential risks.
- Category D—There are important proofs showing the human fetal risks, starting from data of adverse reactions on experimental animals, or human studies, but the potential benefits could justify the use of drug in pregnant women despite potential risks.
- Category X—Studies on experimental animals and/or in humans from previously reported adverse effects or marketing studies demonstrate fetal anomalies and positive evidence on human-fetal risks. The risk of using the drug during pregnancy is far more than the benefits and women taking these drugs should avoid conception using contraceptive measures.
2. The Endocrine Disorders and Pregnancy
2.1. Thyroid Disorders and Pregnancy
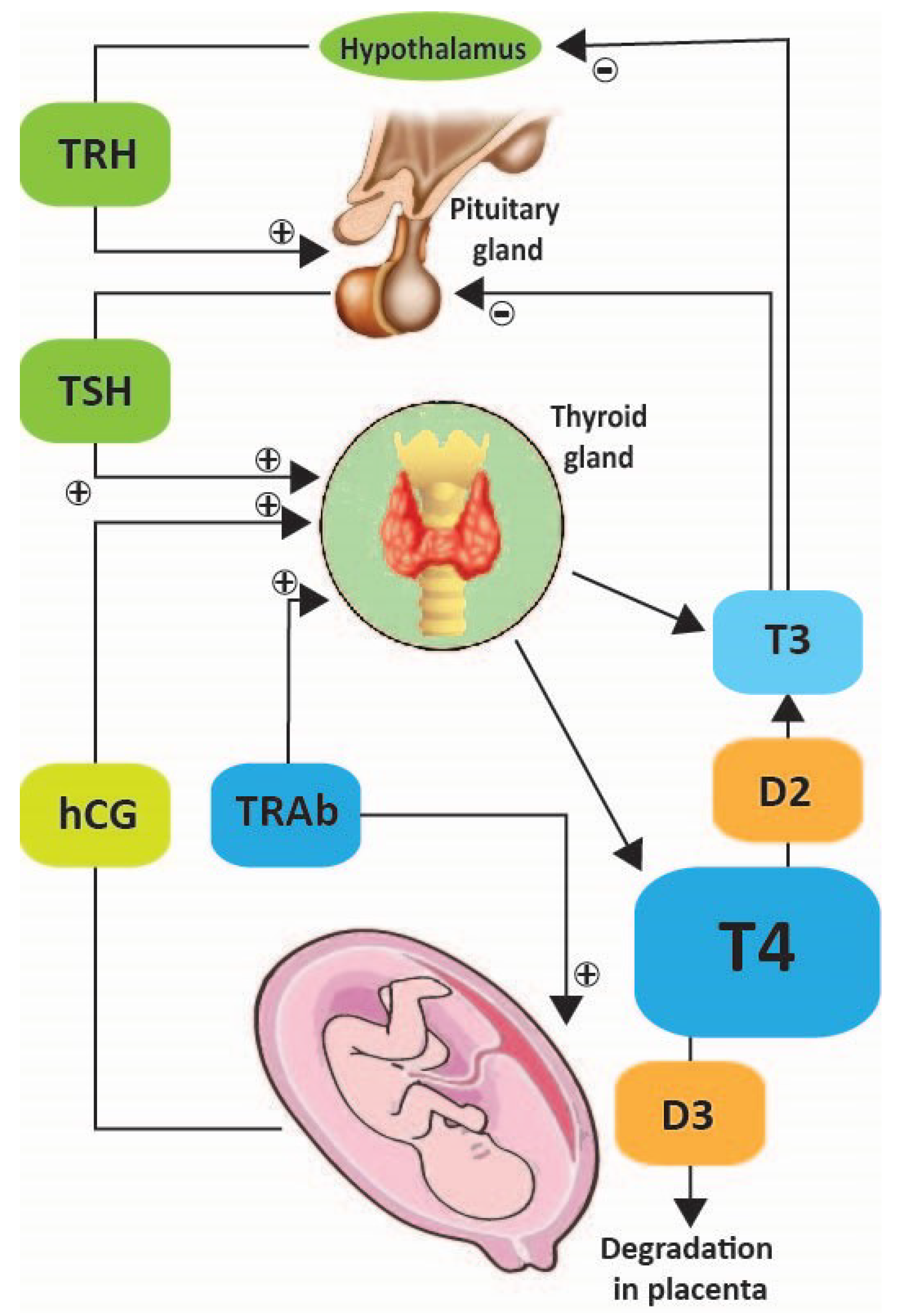
2.1.1. Physiology of Thyroid in Pregnancy
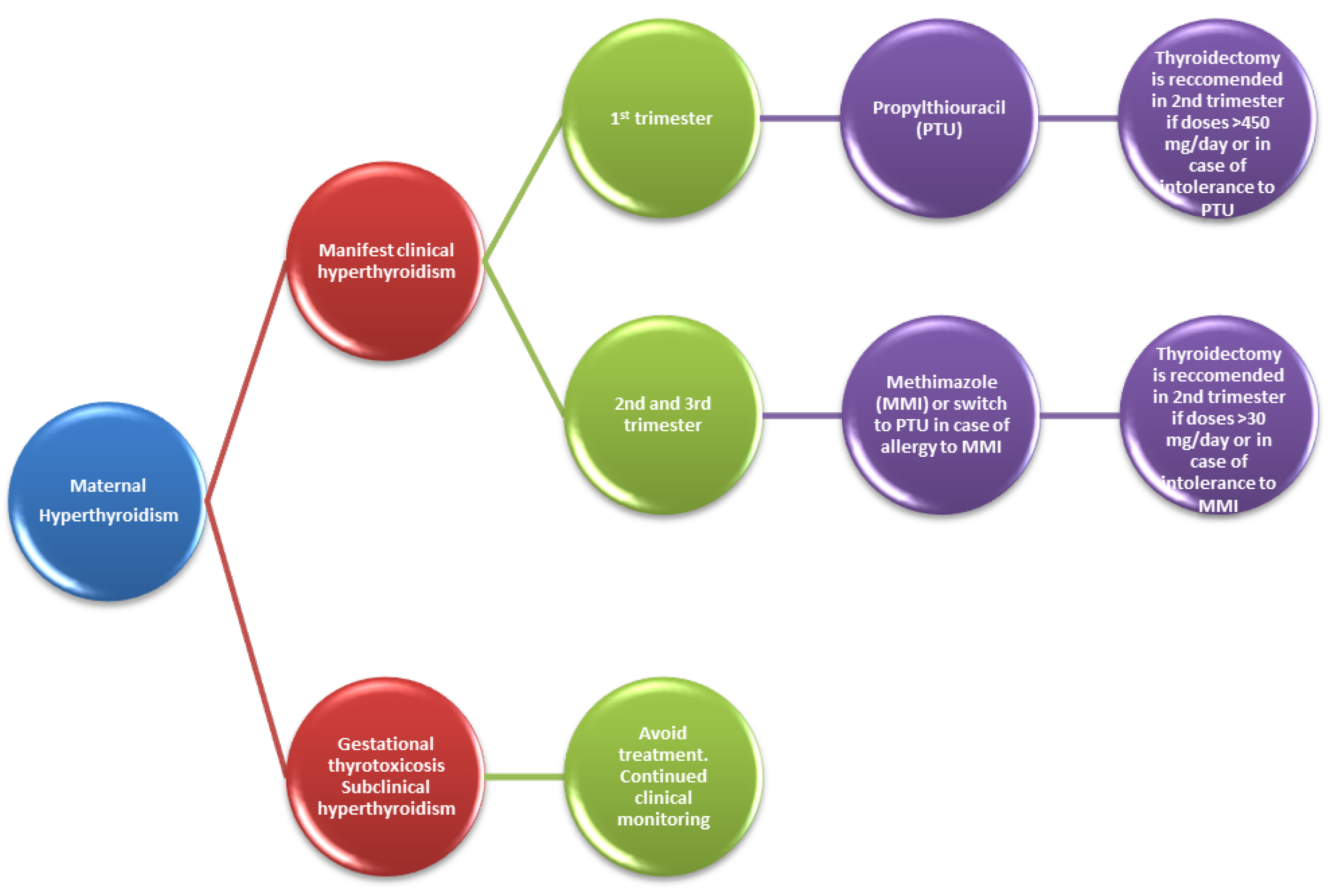
2.1.2. Hyperthyroidism
- Thiourea derivative propylthiouracil (PTU) classified by FDA in category D [4], inhibits thyroid peroxidase but also blocks the enzyme type I deiodinase, preventing conversion of T4 to T3, which is more biologically active.
- Imidazole derivatives methimazole (MMI) and carbimazole (CBZ), available in some countries, have the active metabolite methimazole. They inhibit thyroid peroxidase, reducing the synthesis of T4 and T3. Antithyroid drugs are the main pharmacological agents in the treatment of Graves’ disease in pregnant women [15]. Small amounts of PTU, MMI and CBZ cross the placenta and may decrease fetal thyroid function.
2.1.3. Hypothyroidism
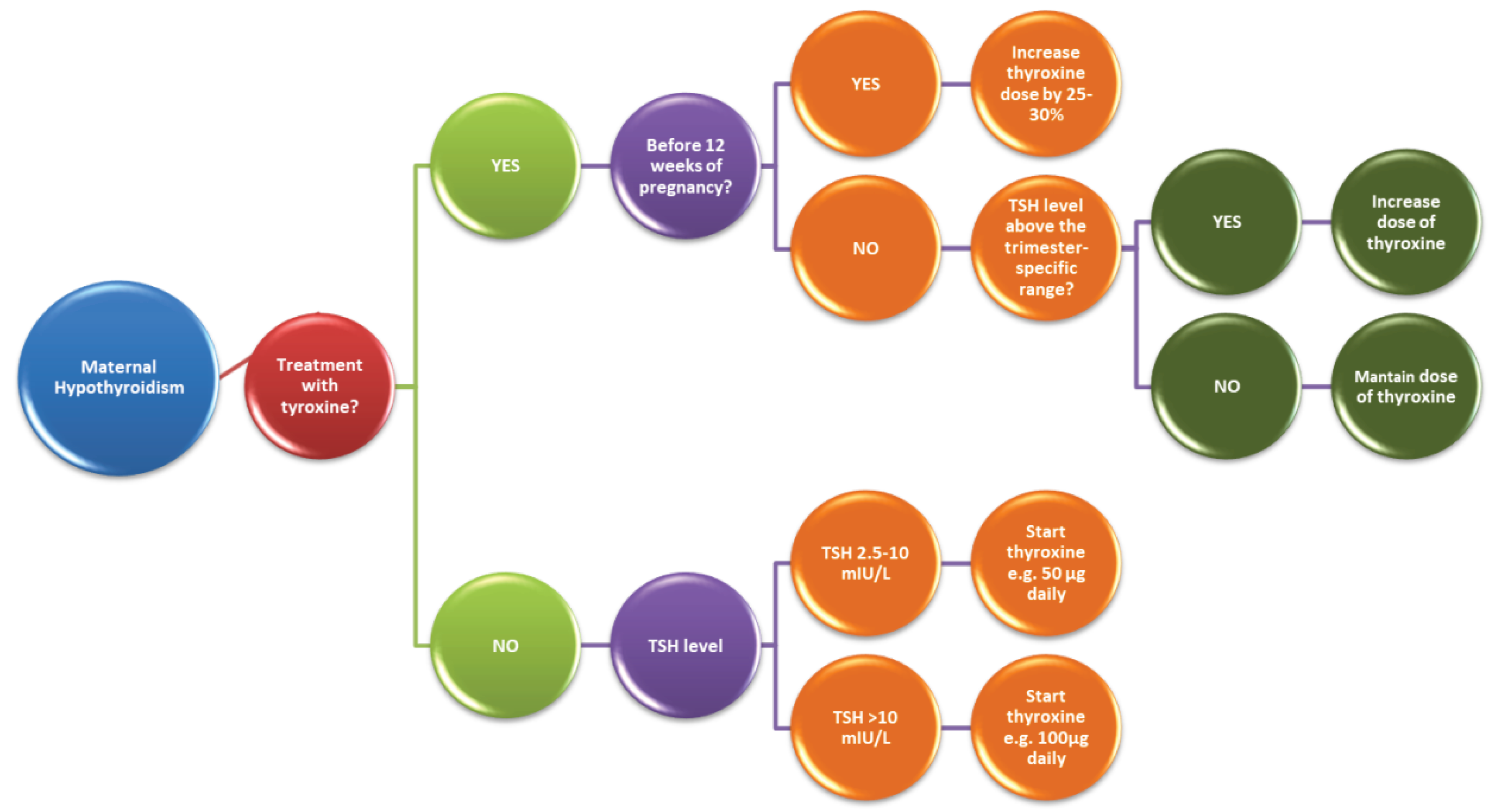
Manifest Clinical Hypothyroidism
- Presentation before 12 gestational weeks: patients will require increasing of their levothyroxine dose by 25–50% [18,24]; doses will remain constant after 16 to 20 weeks of gestation until delivery [28,29]. It is recommended to increase the dose of L-thyroxine from 7 therapeutic doses/week to nine therapeutic doses/week, immediately after confirmation of the pregnancy [18,29].
- If a TSH level is higher than normal for gestational age—the L-thyroxine dose should be increased;
- If a TSH level does not exceed the upper normal limit for gestational age—the L-thyroxine dose should not be changed;
- If the pregnant woman is diagnosed with hypothyroidism and is not being treated with L-thyroxine, thyroid function should be evaluated, and the dosage of L-thyroxine will be chosen based on TSH levels;
- TSH values >2.5 mIU/mL determine the administration of L-thyroxine in different doses. Therefore, TSH values between 2.5 and 10 mIU/L require 50 L-thyroxine µg daily. For TSH values > 10 mIU/L, the daily dose of L-thyroxine is 100 µg.
Subclinical Hypothyroidism
2.1.4. Gestational Thyrotoxicosis
2.1.5. Postpartum Thyroiditis
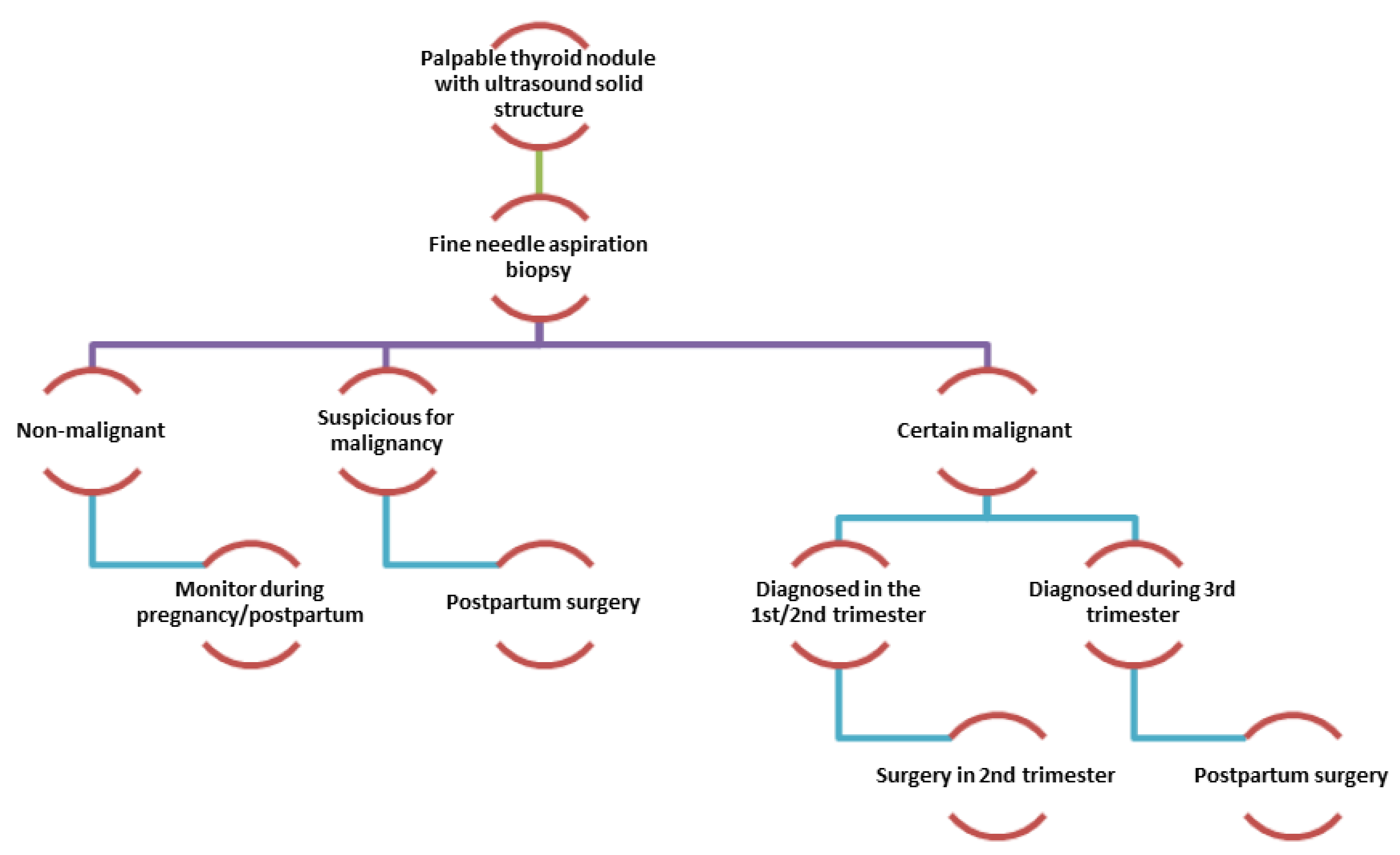
2.1.6. Nodular Thyroid Disease and Pregnancy
2.2. Parathyroid Disorders and Pregnancy
2.2.1. Calcium Homeostasis in Pregnancy
2.2.2. Hyperparathyroidism
Primary Hyperparathyroidism
Secondary Hyperparathyroidism
2.2.3. Hypoparathyroidism
2.3. Adrenal Disorders and Pregnancy
2.3.1. Physiology of Adrenal Glands in Pregnancy
2.3.2. Physiopathology of Adrenal Glands in Pregnancy
Genetic Disorders: Congenital Adrenal Hyperplasia
Adrenocortical Hypofunction: Addison’s Disease
Adrenocortical Hyperfunction: Cushing’s Syndrome and Conn Disease
Adrenomedullary Hyperfunction: Pheochromocytoma
2.4. Pituitary Disorders and Pregnancy
2.4.1. Pituitary’s Physiology in Pregnancy
2.4.2. Pityitary’s Physiopathology in Pregnancy
Tumoral Pathology of Pituitary and Pregnancy: Prolactinoma
Hyperactivity of Pituitary Gland and Pregnancy: Acromegaly
Hypoactivity of Pituitary Gland and Pregnancy
Diabetes Insipidus
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osorio, J. Endocrine disorders in pregnancy: Excessive maternal weight increases risk of infant overgrowth. Nat. Rev. Endocrinol. 2012, 8, 624. [Google Scholar] [CrossRef] [PubMed]
- Velegrakis, A.; Sfakiotaki, M.; Sifakis, S. Human placental growth hormone in normal and abnormal fetal growth. Biomed. Rep. 2017, 7, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Oude Rengerink, K.; Logtenberg, S.; Hooft, L.; Bossuyt, P.M.; Mol, B.W. Pregnant womens’ concerns when invited to a randomized trial: A qualitative case control study. BMC Pregnancy Childbirth 2015, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- FDA News Release. FDA Issues Final Rule on Changes to Pregnancy and Lactation Labeling Information for Prescription Drug and Biological Products. 2014. Available online: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM425398.pdf (accessed on 16 November 2018).
- Nelson-Piercy, C. Handbook of Obstetric Medicine, 5th ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Hershman, J.M. Physiological and pathological aspects of the effect of human chorionic gonadotropin on the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Ain, K.B.; Mori, Y.; Refetoff, S. Reduced clearance rate of thyroxine-binding globulin (TBG) with increased sialylation: A mechanism for estrogen-induced elevation of serum TBG concentration. J. Clin. Endocrinol. Metab. 1987, 65, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Emerson, C.H.; Braverman, L.E. Transfer and metabolism of thyroid-related substances in the placenta. Adv. Exp. Med. Biol. 1991, 299, 181–196. [Google Scholar] [PubMed]
- Roti, E.; Gnudi, A.; Braverman, L.E.; Robuschi, G.; Emanuele, R.; Bandini, P.; Benassi, L.; Pagliani, A.; Emerson, C.H. Human cord blood concentrations of thyrotropin, thyroglobulin, and iodothyronines after maternal administration of thyrotropin-releasing hormone. J. Clin. Endocrinol. Metab. 1981, 53, 813–817. [Google Scholar] [CrossRef]
- Lazarus, J.H. Management of hyperthyroidism in pregnancy. Endocrine 2014, 45, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.; Cunningham, F. Thyrotoxicosis and heart failure that complicate pregnancy. Am. J. Obstet. Gynecol. 2004, 190, 211–217. [Google Scholar] [CrossRef]
- Mannisto, T.; Mendola, P.; Reddy, U.; Laughon, S.K. Neonatal outcomes and birth weight in pregnancies complicated by maternal thyroid disease. Am. J. Epidemiol. 2013, 178, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Phoojaroenchanachai, M.; Sriussadaporn, S.; Peerapatdit, T.; Vannasaeng, S.; Nitiyanant, W.; Boonnamsiri, V.; Vichayanrat, A. Effect of maternal hyperthyroidism during late pregnancy on the risk of neonatal low birth weight. Clin. Endocrinol. (Oxf) 2001, 54, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Chiovato, L.; Pinchera, A. Graves’ disease. In Endocrinology, 5th ed.; De Groot, L.J., Jameson, J.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2006; pp. 1995–2028. [Google Scholar]
- Vita, R.; Lapa, D.; Vita, G.; Trimarchi, F.; Benvenga, S. A patient with stress-related onset and exacerbations of Graves disease. Nat. Clin. Pract. Endocrinol. Metab. 2009, 5, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Barbero, P.; Valdez, R.; Rodriguez, H.; Tiscornia, C.; Mansilla, E.; Allons, A.; Coll, S.; Liascovich, R. Choanal atresia associated with maternal hyperthyroidism treated with methimazole: A case-control study. Am. J. Med. Genet. A 2008, 146A, 2390–2395. [Google Scholar] [CrossRef] [PubMed]
- Bahn, R.S.; Burch, H.S.; Cooper, D.S.; Garber, J.R.; Greenlee, C.M.; Klein, I.L.; Laurberg, P.; McDougall, I.R.; Rivkees, S.A.; Ross, D.; et al. The Role of Propylthiouracil in the Management of Graves’ Disease in Adults: Report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid 2009, 19, 673–674. [Google Scholar] [CrossRef] [PubMed]
- De Groot, L.; Abalovich, M.; Alexander, E.K.; Amino, N.; Barbour, L.; Cobin, R.H.; Eastman, C.J.; Lazarus, J.H.; Luton, D.; Mandel, S.J.; et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 2543–2565. [Google Scholar] [CrossRef] [PubMed]
- Kohler, S.; Senn, O.; Saleh, L.; Wass, J.; Schmid, C. Timing of Thyroxine Dose Adjustment in Hypothyroid Patients: When are TSH Levels Stable? J. Thyroid Disord. Ther. 2014, 3, 161. [Google Scholar]
- Cooper, D.S. Propylthiouracil levels in hyperthyroid patients unresponsive to large doses. Evidence of poor patient compliance. Ann. Intern. Med. 1985, 102, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.J.; Cooper, D.S. The use of antithyroid drugs in pregnancy and lactation. J. Clin. Endocrinol. Metab. 2001, 86, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Mutharasan, P.; Oatis, W.; Kwaan, H.; Molitch, M. Delayed anithyroid drug-induced agranulocytosis. Endocr. Pract. 2012, 18, e69–e72. [Google Scholar] [CrossRef] [PubMed]
- Stoffer, S.S.; Hamburger, J.I. Inadvertent 131I therapy for hyperthyroidism in the first trimester of pregnancy. J. Nucl. Med. 1976, 17, 146–149. [Google Scholar] [PubMed]
- Stagnaro-Green, A.; Abalovich, M.; Alexander, E.; Azizi, F.; Mestman, J.; Negro, R.; Nixon, A.; Pearce, E.N.; Soldin, O.P.; Sullivan, S.; et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011, 21, 1081–1125. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.R.; Cobin, R.H.; Gharib, H.; Hennessey, J.V.; Klein, I.; Mechanick, J.I.; Pessah-Pollack, R.; Singer, P.A.; Woeber, K.A. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 2012, 22, 1200–1235. [Google Scholar] [CrossRef] [PubMed]
- Saki, F.; Dabbaghmanesh, M.H.; Ghaemi, S.Z.; Forouhari, S.; Ranjbar Omrani, G.; Bakhshayeshkaram, M. Thyroid function in pregnancy and its influences on maternal and fetal outcomes. Int. J. Endocrinol. Metab. 2014, 12, e19378. [Google Scholar] [CrossRef] [PubMed]
- Hollowell, J.G., Jr.; Garbe, P.L.; Miller, D.T. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 1999, 341, 2016–2017. [Google Scholar] [PubMed]
- Alexander, E.K.; Marqusee, E.; Lawrence, J.; Jarolim, P.; Fischer, G.A.; Larsen, P.R. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N. Engl. J. Med. 2004, 351, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Yassa, L.; Marqusee, E.; Fawcett, R.; Alexander, E.K. Thyroid hormone early adjustment in pregnancy (the THERAPY) trial. J. Clin. Endocrinol. Metab. 2010, 95, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Obican, S.G.; Jahnke, G.D.; Soldin, O.P.; Scialli, A.R. Teratology public affairs committee position paper: Iodine deficiency in pregnancy. Birth Defects Res. A Clin. Mol. Teratol. 2012, 94, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.L.; Pearce, E.N. Screening for maternal thyroid dysfunction in pregnancy: A review of the clinical evidence and current guidelines. J. Thyroid Res. 2013, 2013, 851326. [Google Scholar] [CrossRef] [PubMed]
- Ajmani, S.N.; Aggarwal, D.; Bhatia, P.; Sharma, M.; Sarabhai, V.; Paul, M. Prevalence of overt and subclinical thyroid dysfunction among pregnant women and its effect on maternal and fetal outcome. J. Obstet. Gynaecol. India 2014, 64, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Brabant, G.; Peeters, R.P.; Chan, S.Y.; Bernal, J.; Bouchard, P.; Salvatore, D.; Boelaert, K.; Laurberg, P. Management of subclinical hypothyroidism in pregnancy: Are we too simplistic? Eur. J. Endocrinol. 2015, 173, P1–P11. [Google Scholar] [CrossRef] [PubMed]
- Maraka, S.; Ospina, N.M.; O’Keeffe, D.T.; Espinosa De Ycaza, A.E.; Gionfriddo, M.R.; Erwin, P.J.; Coddington, C.C., 3rd; Stan, M.N.; Murad, M.H.; Montori, V.M. Subclinical Hypothyroidism in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.; Brown, R.S.; Daumerie, C.; Hubalewska-Dydejczyk, A.; Negro, R.; Vaidya, B. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur. Thyroid J. 2014, 3, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Boelaert, K. Optimal management of hypothyroidism, hypothyroxinaemia and euthyroid TPO antibody positivity preconception and in pregnancy. Clin. Endocrinol. (Oxf) 2015, 82, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Negro, R.; Formoso, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: Effects on obstetrical complications. J. Clin. Endocrinol. Metab. 2006, 91, 2587–2591. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Y.; Loh, K.C.; Yeo, G.S.; Chee, Y.C. Transient hyperthyroidism of hyperemesis gravidarum. BJOG 2002, 109, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.M.; Mestman, J.H. Transient non-autoimmune hyperthyroidism of early pregnancy. J. Thyroid Res. 2011, 2011, 142413. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.S.; Laurberg, P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013, 1, 238–249. [Google Scholar] [CrossRef]
- Labadzhyan, A.; Brent, G.A.; Hershman, J.M.; Leung, A.M. Thyrotoxicosis of Pregnancy. J. Clin. Transl. Endocrinol. 2014, 1, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Mannisto, T.; Mendola, P.; Grewal, J.; Xie, Y.; Chen, Z.; Laughon, S.K. Thyroid diseases and adverse pregnancy outcomes in a contemporary US cohort. J. Clin. Endocrinol. Metab. 2013, 98, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Glinoer, D. The regulation of thyroid function in pregnancy: Pathways of endocrine adaptation from physiology to pathology. Endocr. Rev. 1997, 18, 404–433. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.P.; Khoo, D.H.; Eng, P.H.; Tan, H.K.; Yo, S.L.; Jacob, E. Prevalence of gestational thyrotoxicosis in Asian women evaluated in the 8th to 14th weeks of pregnancy: Correlations with total and free beta human chorionic gonadotrophin. Clin. Endocrinol. (Oxf) 2001, 55, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Polonsky, K.S.; Larsen, P.R.; Kronenberg, H.M. William’s Textbook of Endocrinology; Elsevier Saunders: Philadeplphia, PA, USA, 2012. [Google Scholar]
- Sun, S.; Qiu, X.; Zhou, J. Clinical analysis of 65 cases of hyperemesis gravidarum with gestational transient thyrotoxicosis. J. Obstet. Gynaecol. Res. 2014, 40, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro-Green, A. Clinical review 152: Postpartum thyroiditis. J. Clin. Endocrinol. Metab. 2002, 87, 4042–4047. [Google Scholar] [CrossRef]
- Benvenga, S.; Vigo, M.T.; Metro, D.; Granese, R.; Vita, R.; Le Donne, M. Type of fish consumed and thyroid autoimmunity in pregnancy and postpartum. Endocrine 2016, 52, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.H.; Parkes, A.B.; Premawardhana, L.D. Postpartum thyroiditis. Autoimmunity 2002, 35, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Di Bari, F.; Vita, R.; Le Donne, M.; Triolo, O.; Granese, R.; Borrielli, I.; Sole, G.; Floridia, M.; Genovesi, F.; et al. Relatively high rate of postpartum thyroiditis in the Straits of Messina area. Predictivity of both postpartum thyroiditis and permanent hypothyroidism by performing, in the first trimester of gestation, thyroid ultrasonography and measurement of serum thyroperoxidase and thyroglobulin autoantibodies. J. Clin. Transl. Endocrinol. 2019, 15, 12–18. [Google Scholar]
- Lambert, N.C.; Evans, P.C.; Hashizumi, T.L.; Maloney, S.; Gooley, T.; Furst, D.E.; Nelson, J.L. Cutting edge: Persistent fetal microchimerism in T lymphocytes is associated with HLA-DQA1*0501: Implications in autoimmunity. J. Immunol. 2000, 164, 5545–5548. [Google Scholar] [CrossRef]
- Samuels, M.H. Subacute, Silent Thyroiditis, Postpartum. Med. Clin. North Am. 2012, 96, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.H.; Ammari, F.; Oretti, R.; Parkes, A.B.; Richards, C.J.; Harris, B. Clinical aspects of recurrent postpartum thyroiditis. Br. J. Gen. Pract. 1997, 47, 305–308. [Google Scholar]
- Kuijpens, J.L.; Pop, V.J.; Vader, H.L.; Drexhage, H.A.; Wiersinga, W.M. Prediction of post partum thyroid dysfunction: Can it be improved? Eur. J. Endocrinol. 1998, 139, 36–43. [Google Scholar] [CrossRef]
- Abalovich, M.; Amino, N.; Barbour, L.A.; Cobin, R.H.; De Groot, L.J.; Glinoer, D.; Mandel, S.J.; Stagnaro-Green, A. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2007, 92 (8 Suppl.), S1–S47. [Google Scholar] [CrossRef]
- Azizi, F. The occurrence of permanent thyroid failure in patients with subclinical postpartum thyroiditis. Eur. J. Endocrinol. 2005, 153, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Khaled, H.; Al Lahloubi, N.; Rashad, N. A review on thyroid cancer during pregnancy: Multitasking is required. J. Adv. Res. 2016, 7, 565–570. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Zhou, Z.; Qian, M.F.; Gong, T.; Wang, J.D. Association of thyroid carcinoma with pregnancy: A meta-analysis. Mol. Clin. Oncol. 2015, 3, 341–346. [Google Scholar] [CrossRef]
- Kung, A.W.; Chau, M.T.; Lao, T.T.; Tam, S.C.; Low, L.C. The effect of pregnancy on thyroid nodule formation. J. Clin. Endocrinol. Metab. 2002, 87, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Galofré, J.C.; Riesco-Eizaguirre, G.; Álvarez-Escolá, C. Clinical guidelines for management of thyroid nodule and cancer during pregnancy. Endocrinol. Nutr. 2014, 61, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Messuti, I.; Corvisieri, S.; Bardesono, F.; Rapa, I.; Giorcelli, J.; Pellerito, R.; Volante, M.; Orlandi, F. Impact of pregnancy on prognosis of differentiated thyroid cancer: Clinical and molecular features. Eur. J. Endocrinol. 2014, 170, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Imran, S.A.; Rajaraman, M. Management of differentiated thyroid cancer in pregnancy. J. Thyroid Res. 2011, 2011, 549609. [Google Scholar] [CrossRef]
- Pitkin, R.M. Calcium metabolism in pregnancy and the perinatal period: A review. Am. J. Obstet. Gynecol. 1985, 151, 99–109. [Google Scholar] [CrossRef]
- World Health Organization. e-Library of Evidence for Nutrition Actions (eLENA). Calcium Supplementation during Pregnancy to Reduce the Risk of Pre-Eclampsia. 2013. Available online: https://www.who.int/elena/titles/calcium_pregnancy/en (accessed on 25 November 2018).
- Krysiak, R.; Wilk, M.; Okopien, B. Recurrent pancreatitis induced by hyperparathyroidism in pregnancy. Arch. Gynecol. Obstet. 2011, 284, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.; Turnbull, H. Hyperparathyroidism: Generalized osteitis fibrosa with observations upon bones, parathyroid tumors and the normal parathyroid glands. Br. J. Surg. 1931, 19, 203. [Google Scholar] [CrossRef]
- Friderichsen, C. Tetany in a suckling with latent osteitis fibrosa in the mother. Lancet 1939, 233, 85–86. [Google Scholar] [CrossRef]
- Hacker, A.N.; Fung, E.B.; King, J.C. Role of calcium during pregnancy: Maternal and fetal needs. Nutr. Rev. 2012, 70, 397–409. [Google Scholar] [CrossRef]
- Dochez, V.; Ducarme, G. Primary hyperparathyroidism during pregnancy. Arch. Gynecol. Obstet. 2014, 291, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Politz, D.; Politz, L. Hyperparathyroidism during pregnancy and the effect of rising calcium on pregnancy loss: A call for earlier intervention. Clin. Endocrinol. (Oxf) 2009, 71, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.W.; Musselman, L. The treatment of tetany in pregnancy. Am. J. Obstet. Gynecol. 1942, 43, 547–567. [Google Scholar] [CrossRef]
- McLean, M.; Smith, R. Corticotrophin-releasing hormone and human parturition. Reproduction 2001, 121, 493–501. [Google Scholar] [CrossRef]
- Demey-Ponsart, E.; Foidart, J.M.; Sulon, J.; Sodoyez, J.C. Serum CBG, free and total cortisol and circadian patterns of adrenal function in normal pregnancy. J. Steroid Biochem. 1982, 16, 165–169. [Google Scholar] [CrossRef]
- Kamoun, M.; Mnif, M.F.; Charfi, N.; Kacem, F.H.; Naceur, B.B.; Mnif, F.; Dammak, M.; Rekik, N.; Abid, M. Adrenal diseases during pregnancy: Pathophysiology, diagnosis and management strategies. Am. J. Med. Sci. 2014, 347, 64–73. [Google Scholar] [CrossRef]
- Abdelmannan, D.; Aron, D.C. Adrenal disorders in pregnancy. Endocrinol. Metab. Clin. North Am. 2011, 40, 779–794. [Google Scholar] [CrossRef]
- Wilson, M.; Morganti, A.A.; Zervoudakis, I.; Letcher, R.L.; Romney, B.M.; Von Oeyon, P.; Papera, S.; Sealey, J.E.; Laragh, J.H. Blood pressure, the renin-aldosterone system and sex steroids throughout normal pregnancy. Am. J. Med. 1980, 68, 97–104. [Google Scholar] [CrossRef]
- Wajnrajch, M.P.; New, M.I. Chapter 103: Defects of adrenal steroidogenesis. In Endocrinology, Adult and Pediatric, 6th ed.; Jameson, J.L., De Groot, L.J., Eds.; Elsevier Saunders: Philadeplhia, PA, USA, 2010; pp. 1897–1920. [Google Scholar]
- Marumudi, E.; Khadgawat, R.; Surana, V.; Shabir, I.; Joseph, A.; Ammini, A.C. Diagnosis and management of classical congenital adrenal hyperplasia. Steroids 2013, 78, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Auchus, R.J.; Arlt, W. Approach to the patient: The adult with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2013, 98, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Forest, M.G.; David, M.; Morel, Y. Prenatal diagnosis and treatment of 21-hydroxylase deficiency. J. Steroid Biochem. Mol. Biol. 1993, 45, 75–82. [Google Scholar] [CrossRef]
- Garner, P.R. Management of congenital adrenal hyperplasia during pregnancy. Endocr. Pract. 1996, 2, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Dalaker, K.; Jorde, R.; Berge, L.N. Addison’s disease and pregnancy. Acta Obstet. Gynecol. Scand. 1989, 68, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, B.; Barbetta, L.; Morricone, L. Diagnosis and management of Addison’s disease during pregnancy. J. Endocrinol. Invest. 2003, 26, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.B.; McConahey, W.M. Pregnancy associated with diseases of the adrenal glands. Am. J. Obstet. Gynecol. 1953, 66, 970–987. [Google Scholar] [CrossRef]
- O’Shaughnessy, R.W.; Hackett, K.J. Maternal Addison’s disease and fetal growth retardation. A case report. J. Reprod. Med. 1984, 29, 752–756. [Google Scholar] [PubMed]
- Trainer, P.J. Corticosteroids and pregnancy. Semin. Reprod. Med. 2002, 20, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Van der Spuy, Z.M.; Jacobs, H.S. Management of endocrine disorders in pregnancy. Part II. Pituitary, ovarian and adrenal disease. Postgrad. Med. J. 1984, 60, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, I.; Demirci, F.; Yucel, O.; Simsek, E.; Yildiz, I. A case of primary Addison’s disease with hyperemesis gravidarum and successful pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Jadoul, M.; Ferrant, A.; De Plaen, J.F.; Crabbe, J. Mineralocorticoids in the management of primary adrenocortical insufficiency. J. Endocrinol. Invest. 1991, 14, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Cabassi, A.; Rocco, R.; Berretta, R.; Regolisti, G.; Bacchi-Modena, A. Eplerenone use in primary aldosteronism during pregnancy. Hypertension 2012, 59, e18–e19. [Google Scholar] [CrossRef] [PubMed]
- Guilhaume, B.; Sanson, M.L.; Billaud, L.; Bertagna, X.; Laudat, M.H.; Luton, J.P. Cushing’s syndrome and pregnancy: Aetiologies and prognosis in twenty-two patients. Eur. J. Med. 1992, 1, 83–89. [Google Scholar] [PubMed]
- Nieman, L.K.; Biller, B.M.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M. The diagnosis of Cushing’s syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef] [PubMed]
- Abelove, W.A.; Rupp, J.J.; Paschkis, K.E. Acromegaly and pregnancy. J. Clin. Endocrinol. Metab. 1954, 14, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.H.; Torpy, D.J.; Jeffries, W.S. The medical management of Cushing’s syndrome during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hana, V.; Dokoupilova, M.; Marek, J.; Plavka, R. Recurrent ACTH-independent Cushing’s syndrome in multiple pregnancies and its treatment with metyrapone. Clin. Endocrinol. 2001, 54, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Boronat, M.; Marrero, D.; Lopez-Plasencia, Y.; Barber, M.; Schamann, Y.; Novoa, F.J. Successful outcome of pregnancy in a patient with Cushing’s disease under treatment with ketoconazole during the first trimester of gestation. Gynecol. Endocrinol. 2011, 27, 675–677. [Google Scholar] [CrossRef] [PubMed]
- McClamrock, H.D.; Adashi, E.Y. Gestational hyperandrogenism. Fertil. Steril. 1992, 57, 257–274. [Google Scholar] [PubMed]
- Rossi, G.P. Prevalence and diagnosis of primary aldosteronism. Curr. Hypertens. Rep. 2010, 12, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, N.A.; El-Sandabesee, D.; Steel, S.A.; Roland, J.M. Conn’s syndrome in pregnancy successfully treated with amiloride. J. Obstet. Gynaecol. 2007, 27, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, M.; Bemporad, D. Diagnosis and management of pheochromocytoma during pregnancy. J. Endocrinol. Invest. 2002, 25, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Biggar, M.A.; Lennard, T.W. Systematic review of phaeochromocytoma in pregnancy. Br. J. Surg. 2013, 100, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; Angelos, P.; Kaplan, E.; Bakris, G. Pheochromocytoma in pregnancy: A case series and review. Hypertension 2010, 55, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Lata, I.; Sahu, S. Management of paroxysmal hypertension due to incidental pheochromocytoma in pregnancy. J. Emerg. Trauma Shock 2011, 4, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.M.; Lee, K.O. Endocrine emergencies in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Woodmansee, W.W. Pituitary Disorders in Pregnancy. Neurol. Clin. 2019, 37, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Glezer, A.; Bronstein, M.D. Chapter 13: The Pituitary Gland in Pregnancy. In The Pituitary, 4th ed.; Melmed, S., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 397–411. [Google Scholar]
- Regal, M.; Paramo, C.; Sierra, S.M.; Garcia-Mayor, R.V. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin. Endocrinol. (Oxf) 2001, 55, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E. Prolactinoma in pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E. Endocrinology in pregnancy: Management of the pregnant patient with a prolactinoma. Eur. J. Endocrinol. 2015, 172, R205–R213. [Google Scholar] [CrossRef] [PubMed]
- Christin-Maitre, S.; Delemer, B.; Touraine, P.; Young, J. Prolactinoma and estrogens: Pregnancy, contraception and hormonal replacement therapy. Ann. Endocrinol. (Paris) 2007, 68, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Motivala, S.; Gologorsky, Y.; Kostandinov, J.; Post, K.D. Pituitary disorders during pregnancy. Endocrinol. Metab. Clin. North Am. 2011, 40, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Rjosk, H.K.; Fahlbusch, R.; von Werder, K. Influence of pregnancies on prolactinomas. Acta Endocrinol. 1982, 100, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Glezer, A.; Bronstein, M.D. Prolactinomas, cabergoline, and pregnancy. Endocrine 2014, 47, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, S.K.; Bajwa, S.J.; Mohan, P.; Singh, A. Management of prolactinoma with cabergoline treatment in a pregnant woman during her entire pregnancy. Indian J. Endocrinol. Metab. 2011, 15 (Suppl. 3), S267–S270. [Google Scholar] [CrossRef]
- Domingue, M.E.; Devuyst, F.; Alexopoulou, O.; Corvilain, B.; Maiter, D. Outcome of prolactinoma after pregnancy and lactation: A study on 73 patients. Clin. Endocrinol. (Oxf) 2014, 80, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Crosignani, P.G.; Mattei, A.M.; Scarduelli, C.; Cavioni, V.; Boracchi, P. Is pregnancy the best treatment for hyperprolactinaemia? Hum. Reprod. 1989, 4, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, M.D.; Salgado, L.R.; de Castro Musolino, N.R. Medical management of pituitary adenomas: The special case of management of the pregnant woman. Pituitary 2002, 5, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.B. Medical therapy for acromegaly. Endocrinol. Metab. Clin. North Am. 1999, 28, 171–190. [Google Scholar] [CrossRef]
- Maffei, P.; Tamagno, G.; Nardelli, G.B.; Videau, C.; Menegazzo, C.; Milan, G.; Calcagno, A.; Martini, C.; Vettor, R.; Epelbaum, J.; et al. Effects of octreotide exposure during pregnancy in acromegaly. Clin. Endocrinol. (Oxf) 2010, 72, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Brian, S.R.; Bidlingmaier, M.; Wajnrajch, M.P.; Weinzimer, S.A.; Inzucchi, S.E. Treatment of acromegaly with pegvisomant during pregnancy: Maternal and fetal effects. J. Clin. Endocrinol. Metab. 2007, 92, 3374–3377. [Google Scholar] [CrossRef] [PubMed]
- Hague, W.M. Diabetes insipidus in pregnancy. Obstet. Med. 2009, 2, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Bulow, B.; Hagmar, L.; Mikoczy, Z.; Nordstrom, C.H.; Erfurth, E.M. Increased cerebrovascular mortality in patients with hypopituitarism. Clin. Endocrinol. (Oxf) 1997, 46, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Karaca, Z.; Tanriverdi, F.; Unluhizarci, K.; Kelestimur, F. Pregnancy and pituitary disorders. Eur. J. Endocrinol. 2010, 162, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Pop, V.J.; Brouwers, E.P.; Vader, H.L.; Vulsma, T.; van Baar, A.L.; de Vijlder, J.J. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: A 3-year follow-up study. Clin. Endocrinol. 2003, 59, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Wakasugi, T.; Yagi, K.; Ohnishi, A.; Ito, N.; Takeda, Y.; Yamagishi, M. Successful pregnancy and delivery in a patient with adult GH deficiency: Role of GH replacement therapy. Endocr. J. 2011, 58, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G. DDAVP use during pregnancy: An analysis of its safety for mother and child. Obstet. Gynecol. Surv. 1998, 53, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Zargar, A.H.; Singh, B.; Laway, B.A.; Masoodi, S.R.; Wani, A.I.; Bashir, M.I. Epidemiologic aspects of postpartum pituitary hypofunction (Sheehan’s syndrome). Fertil. Steril. 2005, 84, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Shinar, S.; Many, A.; Maslovitz, S. Questioning the role of pituitary oxytocin in parturition: Spontaneous onset of labor in women with panhypopituitarism—A case series. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Tonda, C.; Rizvi, A.A. Headache, pituitary lesion and panhypopituitarism in a pregnant woman: Tumor, apoplexy or hypophysitis? Am. J. Med. Sci. 2011, 342, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Shankar, R.; Black, K.; Rochelson, B. Reset osmostat in pregnancy: A case report. J. Matern. Fetal. Neonatal. Med. 2014, 27, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Van der Post, J.A.; van Buul, B.J.; Hart, A.A.; van Heerikhuize, J.J.; Pesman, G.; Legros, J.J.; Steegers, E.A.; Swaab, D.F.; Boer, K. Vasopressin and oxytocin levels during normal pregnancy: Effects of chronic dietary sodium restriction. J. Endocrinol. 1997, 152, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Monson, J.P.; Williams, D.J. Osmoregulatory adaptation in pregnancy and its disorders. J. Endocrinol. 1992, 132, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, S. Diabetes insipidus in pregnancy: Etiology, evaluation, and management. Endocr. Pract. 2009, 15, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Kalelioglu, I.; Kubat Uzum, A.; Yildirim, A.; Ozkan, T.; Gungor, F.; Has, R. Transient gestational diabetes insipidus diagnosed in successive pregnancies: Review of pathophysiology, diagnosis, treatment, and management of delivery. Pituitary 2007, 10, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Vande Walle, J.; Stockner, M.; Raes, A.; Norgaard, J.P. Desmopressin 30 years in clinical use: A safety review. Curr. Drug. Saf. 2007, 2, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Galiveeti, S.; Bichet, D.G.; Roth, J. Diabetes insipidus: Celebrating a century of vasopressin therapy. Endocrinology 2014, 155, 4605–4621. [Google Scholar] [CrossRef] [PubMed]
- Durr, J.A.; Hoggard, J.G.; Hunt, J.M.; Schrier, R.W. Diabetes insipidus in pregnancy associated with abnormally high circulating vasopressinase activity. N. Engl. J. Med. 1987, 316, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Oiso, Y.; Robertson, G.L.; Norgaard, J.P.; Juul, K.V. Clinical review: Treatment of neurohypophyseal diabetes insipidus. J. Clin. Endocrinol. Metab. 2013, 98, 3958–3967. [Google Scholar] [CrossRef] [PubMed]
- Karaca, Z.; Kelestimur, F. Pregnancy and other pituitary disorders (including GH deficiency). Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Kallen, B.A.; Carlsson, S.S.; Bengtsson, B.K. Diabetes insipidus and use of desmopressin (Minirin) during pregnancy. Eur. J. Endocrinol. 1995, 132, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Chanson, P.; Salenave, S. Treatment of neurogenic diabetes insipidus. Ann. Endocrinol. (Paris) 2011, 72, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, I.; Hizuka, N.; Takano, K. Oral DDAVP is a good alternative therapy for patients with central diabetes insipidus: Experience of five-year treatment. Endocr. J. 2003, 50, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Wallia, A.; Bizhanova, A.; Huang, W.; Goldsmith, S.L.; Gossett, D.R.; Kopp, P. Acute diabetes insipidus mediated by vasopressinase after placental abruption. J. Clin. Endocrinol. Metab. 2013, 98, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Castillo, E.; Magee, L.A.; Bichet, D.; Halperin, M. Hereditary nephrogenic diabetes insipidus: A major conundrum during labour and delivery. NDT Plus 2009, 2, 482–484. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calina, D.; Docea, A.O.; Golokhvast, K.S.; Sifakis, S.; Tsatsakis, A.; Makrigiannakis, A. Management of Endocrinopathies in Pregnancy: A Review of Current Evidence. Int. J. Environ. Res. Public Health 2019, 16, 781. https://doi.org/10.3390/ijerph16050781
Calina D, Docea AO, Golokhvast KS, Sifakis S, Tsatsakis A, Makrigiannakis A. Management of Endocrinopathies in Pregnancy: A Review of Current Evidence. International Journal of Environmental Research and Public Health. 2019; 16(5):781. https://doi.org/10.3390/ijerph16050781
Chicago/Turabian StyleCalina, Daniela, Anca Oana Docea, Kirill Sergeyevich Golokhvast, Stavros Sifakis, Aristides Tsatsakis, and Antonis Makrigiannakis. 2019. "Management of Endocrinopathies in Pregnancy: A Review of Current Evidence" International Journal of Environmental Research and Public Health 16, no. 5: 781. https://doi.org/10.3390/ijerph16050781
APA StyleCalina, D., Docea, A. O., Golokhvast, K. S., Sifakis, S., Tsatsakis, A., & Makrigiannakis, A. (2019). Management of Endocrinopathies in Pregnancy: A Review of Current Evidence. International Journal of Environmental Research and Public Health, 16(5), 781. https://doi.org/10.3390/ijerph16050781








