Response of Freshwater Biofilms to Antibiotic Florfenicol and Ofloxacin Stress: Role of Extracellular Polymeric Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Reagents
2.2. Biofilms Cultivation and Exposure Experiments
2.3. Determination of Biofilm Chlorophyll a Content, Enzyme Activities, and EPS Properties
2.4. Batch Sorption Experiment
2.5. Determination of Antibiotics
2.6. Data Analysis
3. Results and Discussion
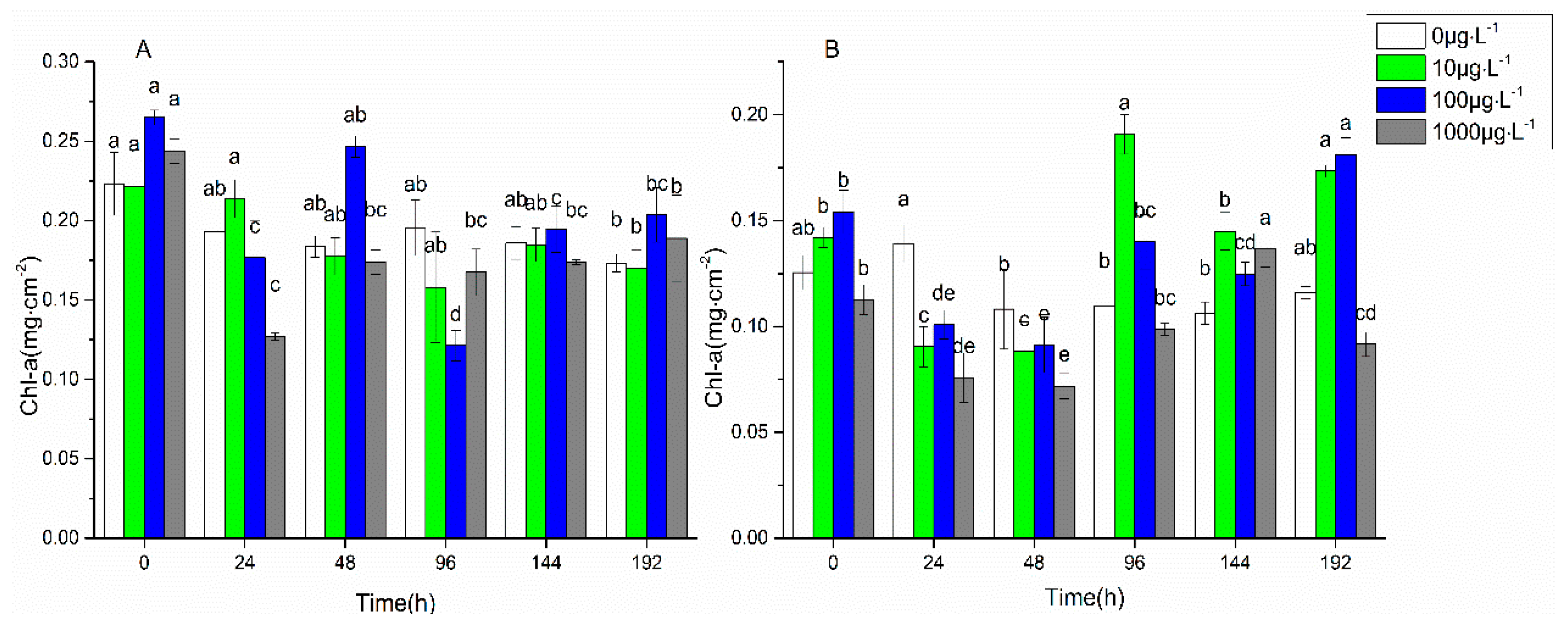
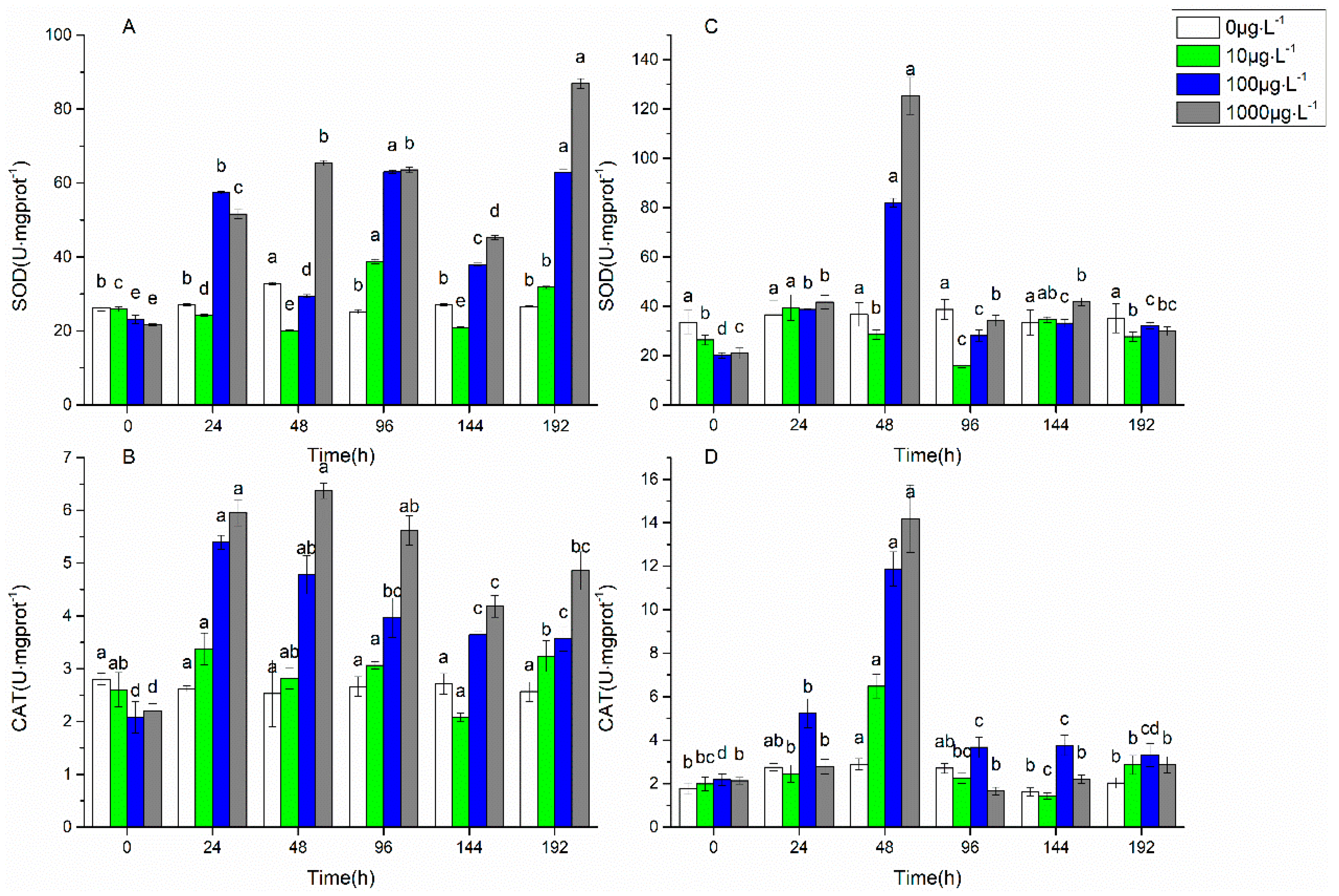
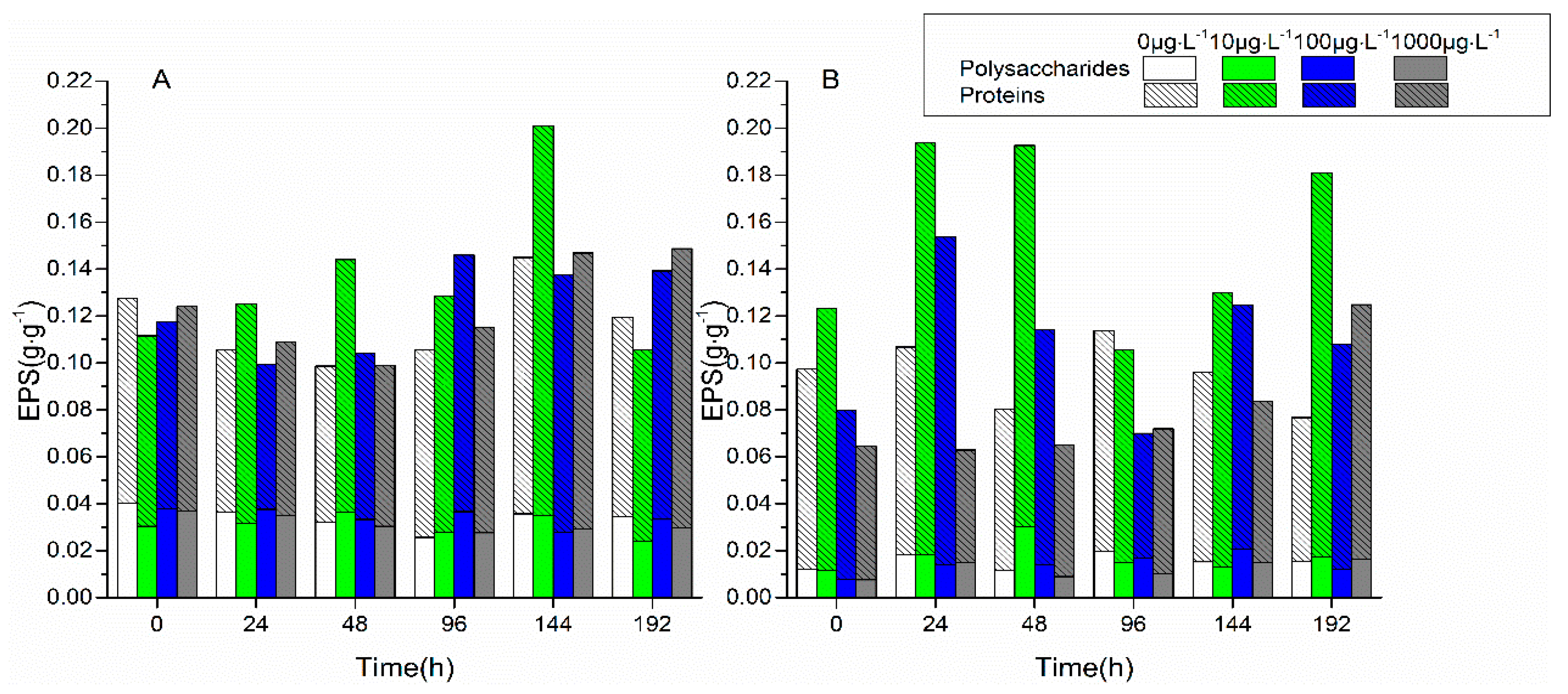
3.1. Changes in Biofilm Chlorophyll a Content, Enzyme Activities, and EPS Properties under FF and OFL Stress
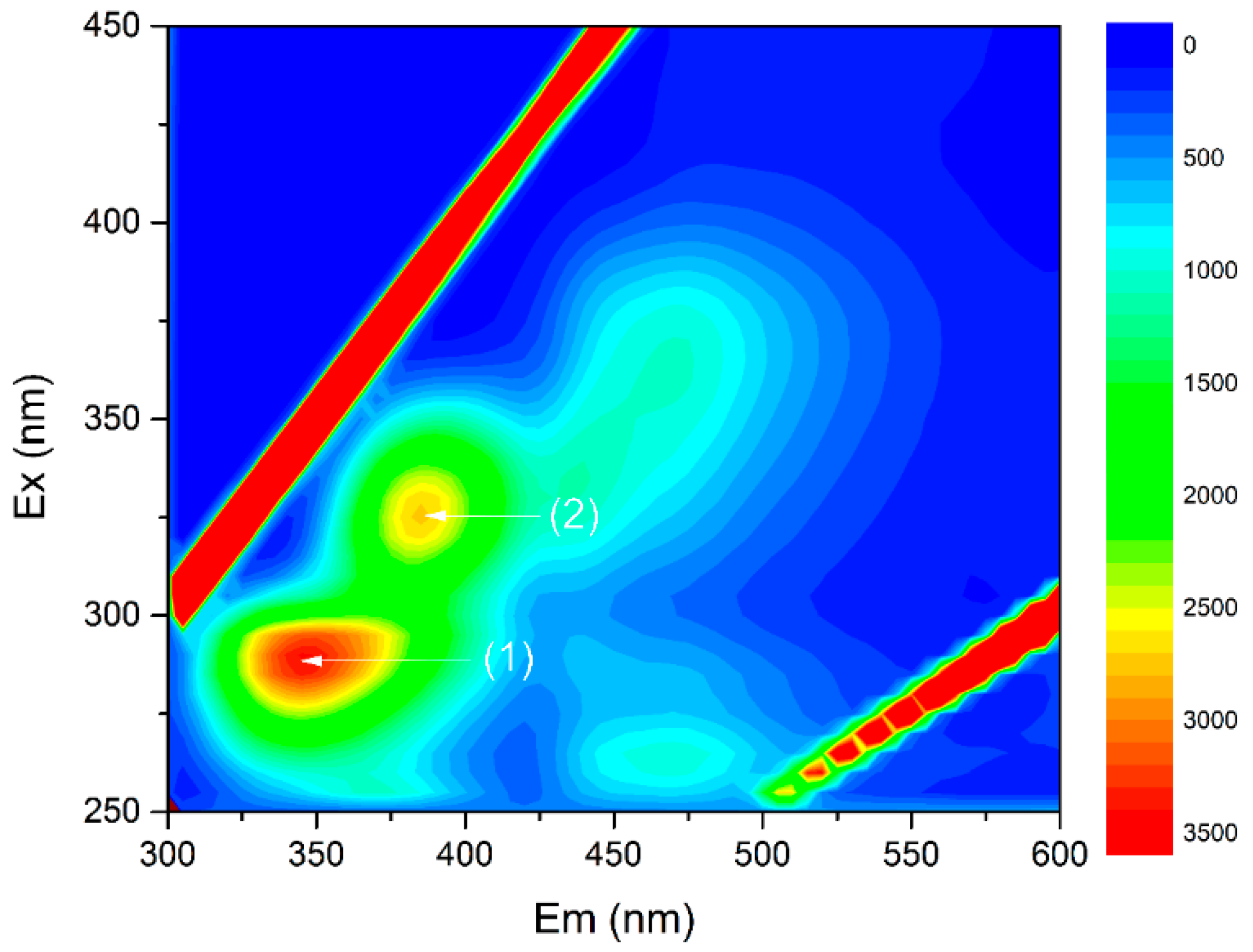
3.2. The Interactions of EPS with Antibiotics and the Role of EPS under the Antibiotic Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wright, G.D. Antibiotic resistance in the environment: A link to the clinic? Curr. Opin. Microbiol. 2010, 13, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Eze, E.A.; Mustapha, K.J.; Ndubuisi, I.A.; Nwodo, U.; Okoh, A. Studies on drug resistance among Klebsiella and Citrobacter spp isolated from two human groups and wild animals. Jundishapur J. Microbiol. 2018, 11, e58784. [Google Scholar]
- Higuera-Llantén, S.; Vásquez-Ponce, F.; Barrientos-Espinoza, B.; Mardones, F.O.; Marshall, S.H.; Olivares-Pacheco, J. Extended antibiotic treatment in salmon farms select multiresistant gut bacteria with a high prevalence of antibiotic resistance genes. PLoS ONE 2018, 13, e0203641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zhang, L.; Liu, S.; Guo, Z.; Hua, X. Antibiotics in water and sediments from Liao River in Jilin Province, China: Occurrence, distribution, and risk assessment. Environ. Earth Sci. 2016, 75, 1202. [Google Scholar] [CrossRef]
- He, S.; Dong, D.; Zhang, X.; Sun, C.; Wang, C.; Hua, X.; Zhang, L.; Guo, Z. Occurrence and ecological risk assessment of 22 emerging contaminants in the jilin songhua river (northeast china). Environ. Sci. Pollut. Res. 2018, 25, 24003–24012. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Huang, T.; Wang, S.; Wang, X.; Su, L.; Li, C.; Zhao, Y. Toxicity of 13 different antibiotics towards freshwater green algae Pseudokirchneriella subcapitata and their modes of action. Chemosphere 2017, 168, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zhang, L.; Guo, Z.; Hua, X. The role of extracellular polymeric substances on the sorption of pentachlorophenol onto natural biofilms in different incubation times: A fluorescence study. Chem. Ecol. 2017, 33, 131–142. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Sabater, S.; Guasch, H.; Ricart, M.; Romaní, A.; Vidal, G.; Klünder, C.; Schmitt-Jansen, M. Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal. Bioanal. Chem. 2007, 387, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, P.; Hou, J.; Yao, Y.; Liu, Z.; Liu, S.; Li, T. Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci. Total Environ. 2019, 650, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, C.; Hou, J.; Wang, P.; Ao, Y.; Li, Y.; Yao, Y.; Lv, B.; Yang, Y.; You, G.; et al. Response of wastewater biofilm to CuO nanoparticle exposure in terms of extracellular polymeric substances and microbial community structure. Sci. Total Environ. 2017, 579, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, C.; Hou, J.; Wang, P.; Ao, Y.; Li, Y.; Geng, N.; Yao, Y.; Lv, B.; Yang, Y.; et al. Aggregation and removal of copper oxide (CuO) nanoparticles in wastewater environment and their effects on the microbial activities of wastewater biofilms. Bioresour. Technol. 2016, 216, 537–544. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Wang, P.; Hou, J.; Wang, C.; Xu, Y.; Miao, L.; Lv, B.; Yang, Y.; Liu, Z.; Zhang, F. Insights into the short-term effects of CeO2 nanoparticles on sludge dewatering and related mechanism. Water Res. 2017, 118, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; You, G.; Xu, Y.; Wang, C.; Wang, P.; Miao, L.; Dai, S.; Lv, B.; Yang, Y. Antioxidant enzyme activities as biomarkers of fluvial biofilm to ZnO NPs ecotoxicity and the Integrated Biomarker Responses (IBR) assessment. Ecotox. Environ. Saf. 2016, 133, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rittmann, B.E. Biofilms, active substrata, and me. Water Res. 2018, 132, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, K.; Yu, D.; Zhang, J.; Wei, Y. Fouling characteristics and cleaning strategies of NF membranes for the advanced treatment of antibiotic production wastewater. Environ. Sci. Pollut. Res. 2017, 24, 8967. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Li, J.; Pan, X.; Sun, Z.; Ye, C.; Jin, G.; Fu, Z. Effects of streptomycin on growth of algae Chlorella vulgaris and Microcystis aeruginosa. Environ. Toxicol. 2012, 27, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Mariana, F.; Buchholz, F.; Lerchner, J.; Neu, T.R.; Harms, H.; Maskow, T. Chip-calorimetric monitoring of biofilm eradication with antibiotics provides mechanistic information. Int. J. Med. Microbiol. 2013, 303, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Shi, J.; Qin, L.; Feng, M.; Cheng, D.; Wang, T.; Zhang, X. Toxicity evaluation of 4,4′-di-CDPS and 4,4′-di-CDE on green algae Scenedesmus obliquus: Growth inhibition, change in pigment content, and oxidative stress. Environ. Sci. Pollut. Res. 2018, 25, 15630–15640. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tang, Z.; Zhou, F.; Zhang, W.; Song, L. Toxicity studies of tetracycline on Microcystis aeruginosa and Selenastrum capricornutum. Environ. Toxicol. Pharmacol. 2013, 35, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Kurade, M.B.; Abou-Shanab, R.A.; Ji, M.K.; Choi, J.; Kim, J.O.; Jeon, B.H. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, J.; Zhu, F.; Mai, D.; Xiang, Z.; Chen, J.; Guo, R. Comprehensive assessment of three typical antibiotics on cyanobacteria (Microcystis aeruginosa): The impact and recovery capability. Ecotox. Environ. Safe. 2018, 160, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, J.; Li, H.; Mao, S.; Wang, D.; Xiang, Z.; Guo, R.; Chen, J. The dual function of the algal treatment: Antibiotic elimination combined with CO2 fixation. Chemosphere 2018, 211, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Dukan, S.; Belkin, S.; Touati, D. Reactive oxygen species are partially involved in the bacteriocidal action of hypochlorous acid. Arch. Biochem. Biophys. 1999, 367, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Xie, W.; Chen, J. Assessing the combined effects from two kinds of cephalosporins on green alga (Chlorella pyrenoidosa) based on response surface methodology. Food Chem. Toxicol. 2015, 78, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wu, Y.; Ding, H.; Zhang, W. The combined and second exposure effect of copper (II) and chlortetracycline on fresh water algae, Chlorella pyrenoidosa and Microcystis aeruginosa. Environ. Toxicol. Pharmacol. 2015, 40, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Aderemi, A.O.; Novais, S.C.; Lemos, M.F.; Alves, L.M.; Hunter, C.; Pahl, O. Oxidative stress responses and cellular energy allocation changes in microalgae following exposure to widely used human antibiotics. Aquat. Toxicol. 2018, 203, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Wang, L.; Zhu, M.; Zhu, X.; Qian, C.; Li, W. Responses of biofilm microorganisms from moving bed biofilm reactor to antibiotics exposure: Protective role of extracellular polymeric substances. Bioresour. Technol. 2018, 254, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; LiLow, W.; Gupta, A.; Cairul Iqbal Mohd Amin, M.; Radecka, I.; Britland, S.T.; Raj, P. Strategies for antimicrobial drug delivery to biofilm. Curr. Pharm. Design. 2015, 21, 43–66. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, S.; Wang, X.; Wang, S. Changes of the reactor performance and the properties of granular sludge under tetracycline (TC) stress. Bioresour. Technol. 2013, 139, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chang, Q.; Li, S.; Gao, M.; She, Z.; Guo, L.; Zhao, Y.; Jin, C.; Zheng, D.; Xu, Q. Impact of sulfadiazine on performance and microbial community of a sequencing batch biofilm reactor treating synthetic mariculture wastewater. Bioresour. Technol. 2017, 235, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, Z.; Chen, M.; Zhang, X.; Tang, C.Y.; Wu, Z. Acute responses of microorganisms from membrane bioreactors in the presence of NaOCl: Protective mechanisms of extracellular polymeric substances. Environ. Sci. Technol. 2017, 51, 3233–3241. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Nelson, Y.M.; Lion, L.W.; Shuler, M.L.; Ghiorse, W.C. Adsorption of Pb and Cd onto metal oxides and organic material in natural surface coatings as determined by selective extractions: New evidence for the importance of Mn and Fe oxides. Water Res. 2000, 34, 427–436. [Google Scholar] [CrossRef]
- Jeffrey, S.T.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, D.; Hua, X.; Guo, Z. Inhibitory effects of extracellular polymeric substances on ofloxacin sorption by natural biofilms. Sci. Total Environ. 2018, 625, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lu, D.; Zhang, P.; Diao, J.; Zhou, Z. Enantioselective toxic effects of hexaconazole enantiomers against Scenedesmus obliquus. Chirality 2012, 24, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.T.; Tam, N.F.Y. Growth, photosynthesis and antioxidant responses of two microalgal species, Chlorella vulgaris and Selenastrum capricornutum, to nonylphenol stress. Chemosphere 2011, 82, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wei, Y.; Qiu, Y.; Qi, Y.; Li, Y.; Kitamura, Y. Biodegradability and mechanism of florfenicol via Chlorella sp. UTEX1602 and L38: Experimental study. Bioresour. Technol. 2019, 272, 529–534. [Google Scholar] [PubMed]
- Liu, Y.; Guan, Y.; Gao, B.; Yue, Q. Antioxidant responses and degradation of two antibiotic contaminants in Microcystis aeruginosa. Ecotox. Environ. Saf. 2012, 86, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, A.W.; Krieger-Liszkay, A. Herbicide-induced oxidative stress in photosystem II. Trends Biochem. Sci. 2001, 26, 648–653. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, G.P.; Ma, Y.; Wang, L.F.; Yu, H.Q. Roles of extracellular poly-meric substances (EPS) in the migration and removal of sulfamethazine in activated sludge system. Water Res. 2013, 47, 5298–5306. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Wang, L.; Zhang, H.; Zhu, M.; Zhang, P.; Zhu, X. Extracellular polymeric substances affect the responses of multi-species biofilms in the presence of sulfamethizole. Environ. Pollut. 2017, 235, 283. [Google Scholar] [CrossRef] [PubMed]
| Freundlich Model | Distribution Coefficient | |||
|---|---|---|---|---|
| Samples | Qe = KFCen | Kd = Qe/Ce | ||
| KF/(mg·g−1)·(mg·L−1)−n | 1·n−1 | r2adj | Kd/L·g−1 | |
| FF biofilms | 0.48 ± 0.03 | 1.07 ± 0.15 | 0.946 | 0.44 ± 0.07 |
| FF–EPS-free biofilms | 1.44 ± 0.26 | 1.01 ± 0.21 | 0.890 | 3.15 ± 0.24 |
| OFL biofilms | 0.81 ± 0.03 | 0.70 ± 0.05 | 0.982 | 1.06 ± 0.17 |
| OFL–EPS-free biofilms | 10.92 ± 0.81 | 1.11 ± 0.03 | 0.997 | 8.20 ± 0.56 |
| Components | Forfenicol | Ofloxacin | ||
|---|---|---|---|---|
| Kq/L·mol−1·s−1 | r2 | Kq/L·mol−1·s−1 | r2 | |
| Protein-like matter | 1.17 × 1012 | 0.924 | 4.11 × 1012 | 0.958 |
| Humic-like matter | 3.83 × 1011 | 0.980 | 5.76 × 1011 | 0.985 |
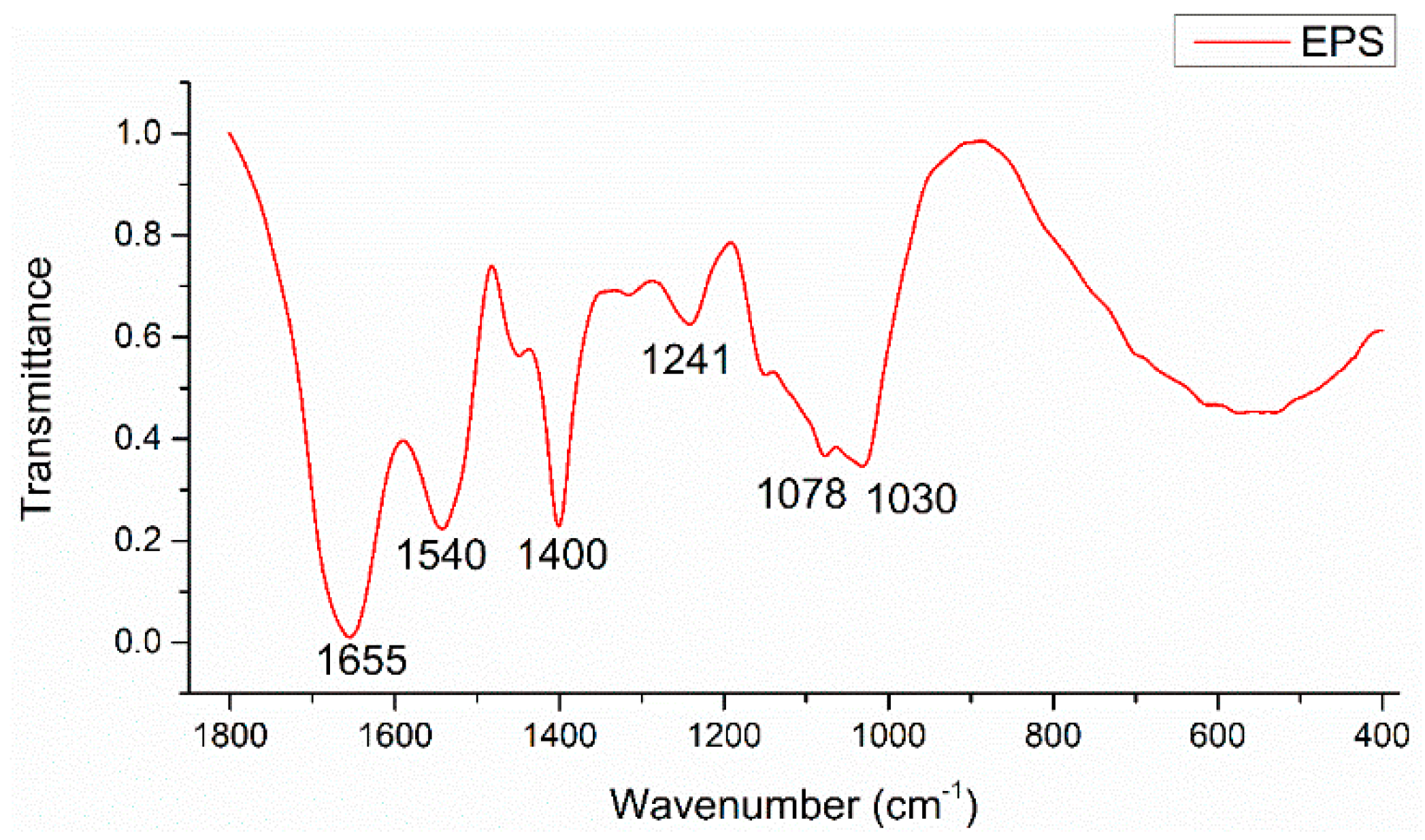
| Band Position/cm−1 | Band Assignments |
|---|---|
| 1655 | νsC=O stretch (amide I) associated with proteins; NH2 scissors of primary amines |
| 1540 | δN–H and νsC–N stretches (amide II) associated with proteins |
| 1400 | νsCOO− stretches associated with amino acids |
| 1241 | νsC-N stretch associated with secondary amides of proteins (amide III) |
| 1078, 1030 | νC-O-C ring vibrations and νC-OH stretches derived from polysaccharides |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Dong, D.; Zhang, L.; Song, Z.; Hua, X.; Guo, Z. Response of Freshwater Biofilms to Antibiotic Florfenicol and Ofloxacin Stress: Role of Extracellular Polymeric Substances. Int. J. Environ. Res. Public Health 2019, 16, 715. https://doi.org/10.3390/ijerph16050715
Wang C, Dong D, Zhang L, Song Z, Hua X, Guo Z. Response of Freshwater Biofilms to Antibiotic Florfenicol and Ofloxacin Stress: Role of Extracellular Polymeric Substances. International Journal of Environmental Research and Public Health. 2019; 16(5):715. https://doi.org/10.3390/ijerph16050715
Chicago/Turabian StyleWang, Chaoqian, Deming Dong, Liwen Zhang, Ziwei Song, Xiuyi Hua, and Zhiyong Guo. 2019. "Response of Freshwater Biofilms to Antibiotic Florfenicol and Ofloxacin Stress: Role of Extracellular Polymeric Substances" International Journal of Environmental Research and Public Health 16, no. 5: 715. https://doi.org/10.3390/ijerph16050715
APA StyleWang, C., Dong, D., Zhang, L., Song, Z., Hua, X., & Guo, Z. (2019). Response of Freshwater Biofilms to Antibiotic Florfenicol and Ofloxacin Stress: Role of Extracellular Polymeric Substances. International Journal of Environmental Research and Public Health, 16(5), 715. https://doi.org/10.3390/ijerph16050715





