Pre-Pregnancy Weight and Symptoms of Attention Deficit Hyperactivity Disorder and Executive Functioning Behaviors in Preschool Children
Abstract
1. Introduction
2. Methods
2.1. Study Sample
2.2. Measures
2.2.1. Maternal Anthropometrics
2.2.2. Child ADHD Symptoms
2.2.3. Child Executive Behaviors
2.2.4. Covariates and Other Variables
2.3. Statistical Analyses
3. Results
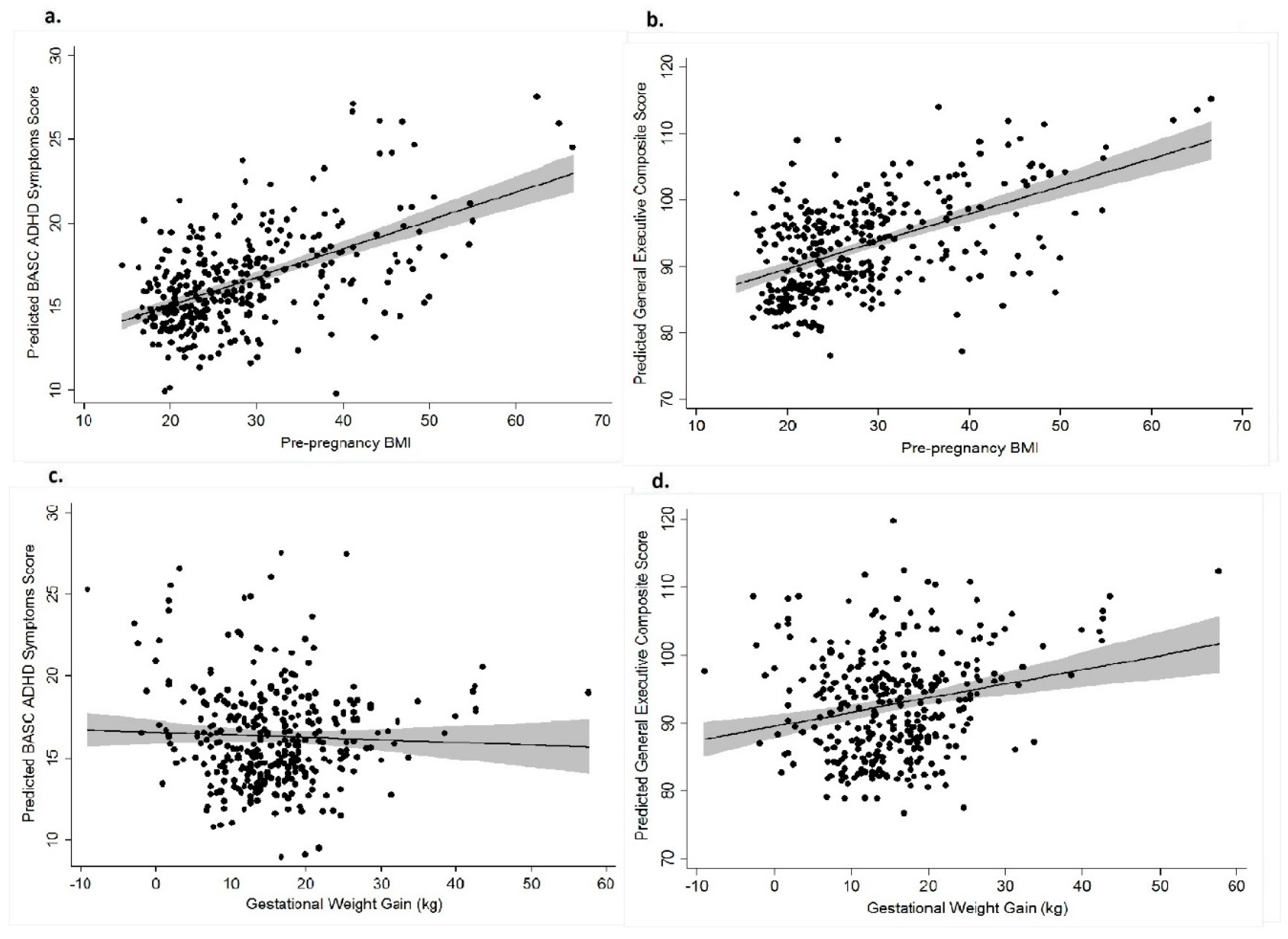
3.1. Pre-pregnancy BMI and BASC and BRIEF Scores
3.2. Gestational Weight Gain (GWG) and BASC and BRIEF Scores
3.3. Sensitivity Analyses
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Collaborators of GBD 2015 Obesity. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Branum, A.M.; Kirmeyer, S.E.; Gregory, E.C. Prepregnancy Body Mass Index by Maternal Characteristics and State: Data from the Birth Certificate, 2014. Natl. Vital Stat. Rep. 2016, 65, 1–11. [Google Scholar] [PubMed]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: A systematic review and meta-analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Tsang, V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Rivera, H.M.; Christiansen, K.J.; Sullivan, E.L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 2015, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Grissom, N.M.; Herdt, C.T.; Desilets, J.; Lidsky-Everson, J.; Reyes, T.M. Dissociable deficits of executive function caused by gestational adversity are linked to specific transcriptional changes in the prefrontal cortex. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, X.; Keim, S.; Li, L.; Zhang, L.; Zhang, J. Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project. Int. J. Epidemiol. 2014, 43, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Tanda, R.; Salsberry, P.J.; Reagan, P.B.; Fang, M.Z. The Impact of Prepregnancy Obesity on Children’s Cognitive Test Scores. Matern. Child Health J. 2012, 17, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J. Child Psychol. Psychiatryand Allied Discip. 2010, 51, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Bilder, D.A.; Bakian, A.V.; Viskochil, J.; Clark, E.A.; Botts, E.L.; Smith, K.R.; Pimentel, R.; McMahon, W.M.; Coon, H. Maternal prenatal weight gain and autism spectrum disorders. Pediatrics 2013, 132, e1276–e1283. [Google Scholar] [CrossRef] [PubMed]
- Windham, G.C.; Anderson, M.; Lyall, K.; Daniels, J.L.; Kral, T.V.E.; Croen, L.A.; Levy, S.E.; Bradley, C.B.; Cordero, C.; Young, L.; et al. Maternal Pre-pregnancy Body Mass Index and Gestational Weight Gain in Relation to Autism Spectrum Disorder and other Developmental Disorders in Offspring. Autism Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Girchenko, P.; Tuovinen, S.; Lahti-Pulkkinen, M.; Lahti, J.; Savolainen, K.; Heinonen, K.; Pyhala, R.; Reynolds, R.M.; Hamalainen, E.; Villa, P.M.; et al. Maternal early pregnancy obesity and related pregnancy and pre-pregnancy disorders: Associations with child developmental milestones in the prospective PREDO Study. Int. J. Obes. 2018, 42, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.H.; Sundaram, R.; Ghassabian, A.; Xie, Y.; Buck Louis, G. Parental Obesity and Early Childhood Development. Pediatrics 2017, 139, e20161459. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018, 19, 464–484. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Lee, B.K.; Magnusson, C.; Rai, D.; Frisell, T.; Karlsson, H.; Idring, S.; Dalman, C. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: Results from a Swedish total population and discordant sibling study. Int. J. Epidemiol. 2015, 44, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.J.; Zeegers, M.; Jaddoe, V.; Verhulst, F.; Tiemeier, H.; Lawlor, D.A.; Smith, G.D. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics 2011, 127, e202–e211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sjolander, A.; Langstrom, N.; Rodriguez, A.; Serlachius, E.; D’Onofrio, B.M.; Lichtenstein, P.; Larsson, H. Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: A population-based cohort study using a sibling-comparison design. Int. J. Epidemiol. 2014, 43, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Musser, E.D.; Willoughby, M.T.; Wright, S.; Sullivan, E.L.; Stadler, D.D.; Olson, B.F.; Steiner, R.D.; Nigg, J.T. Maternal prepregnancy body mass index and offspring attention-deficit/hyperactivity disorder: A quasi-experimental sibling-comparison, population-based design. J. Child Psychol. Psychiatryand Allied Discip. 2017, 58, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Fuemmeler, B.F.; Kollins, S.H.; McClernon, F.J. Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. J. Pediatr. Psychol. 2007, 32, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Fuemmeler, B.F.; Ostbye, T.; Yang, C.; McClernon, F.J.; Kollins, S.H. Association between attention-deficit/hyperactivity disorder symptoms and obesity and hypertension in early adulthood: A population-based study. Int. J. Obes. 2011, 35, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Houts, R.M.; Belsky, D.W.; Harrington, H.; Hogan, S.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Childhood forecasting of a small segment of the population with large economic burden. Nat. Hum. Behav. 2016, 1, 0005. [Google Scholar] [CrossRef] [PubMed]
- Heckman, J.J. Schools, Skills, and Synapses. Econ. Inq. 2008, 46, 289–324. [Google Scholar] [CrossRef] [PubMed]
- Heckman, J.J. Role of income and family influence on child outcomes. Ann. N. Y. Acad. Sci. 2008, 1136, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef] [PubMed]
- Rinsky, J.L.; Richardson, D.B.; Wing, S.; Beard, J.D.; Alavanja, M.; Beane Freeman, L.E.; Chen, H.; Henneverger, P.K.; Kamel, F.; Sandler, D.P.; et al. Assessing the Potential for Bias from Nonresponse to a Study Follow-up Interview: An Example from the Agricultural Health Study. Am. J. Epidemiol. 2017, 186, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Gillman, M.W. Fetal origins of obesity. Obes. Res. 2003, 11, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.M.; Yaktine, A.L. Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Allison Bender, H.; Auciello, D.; Morrison, C.E.; MacAllister, W.S.; Zaroff, C.M. Comparing the convergent validity and clinical utility of the Behavior Assessment System for Children-Parent Rating Scales and Child Behavior Checklist in children with epilepsy. Epilepsy Behav. 2008, 13, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.R.; Vannest, K.J.; Reynolds, C.R. Behaviors that discriminate ADHD in children and adolescents: Primary symptoms, symptoms of comorbid conditions, or indicators of functional impairment? J. Atten. Disord. 2011, 15, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Fast Track Project. Available online: https://fasttrackproject.org/techrept/c/cbc/ (accessed on 22 February 2019).
- Gioia, G.; Espy, K.; Isquith, P. (BRIEF-P) Behavior Rating Inventory of Executive Function—Preschool Version; Psychological Assessment Resources: Lutz, FL, USA, 2003. [Google Scholar]
- Kessler, R.C.; Adler, L.; Ames, M.; Demler, O.; Faraone, S.; Hiripi, E.; Howes, M.J.; Jin, R.; Secnik, K.; Spencer, T.; et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): A short screening scale for use in the general population. Psychol. Med. 2005, 35, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Krasner, A.J.; Turner, J.B.; Feldman, J.F.; Silberman, A.E.; Fisher, P.W.; Workman, C.C.; Posner, J.E.; Greenhill, L.L.; Lorenz, J.M.; Shaffer, D.; et al. ADHD Symptoms in a Non-Referred Low Birthweight/Preterm Cohort: Longitudinal Profiles, Outcomes, and Associated Features. J. Atten. Disord. 2015, 22, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Schieve, L.A.; Sharma, A.J.; Hinkle, S.N.; Li, R.; Lind, J.N. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics 2015, 135, e1198–e1209. [Google Scholar] [CrossRef] [PubMed]
- Buss, C.; Entringer, S.; Davis, E.P.; Hobel, C.J.; Swanson, J.M.; Wadhwa, P.D.; Sandman, C.A. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS ONE 2012, 7, e37758. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.J.; Hutcheon, J.A.; Richardson, G.A.; Brooks, M.M.; Himes, K.P.; Day, N.L.; Bodnar, L.M. Gestational weight gain, prepregnancy body mass index and offspring attention-deficit hyperactivity disorder symptoms and behaviour at age 10. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Smith, M.S.; Grove, K.L. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology 2011, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.M.; Freeman, D.J.; Ramsay, J.E.; Greer, I.A.; Caslake, M.; Ferrell, W.R. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J. Clin. Endocrinol. Metab. 2007, 92, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Jonakait, G.M. The effects of maternal inflammation on neuronal development: Possible mechanisms. Int. J. Dev. Neurosci. 2007, 25, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.H.; Goldstein, B.I. Inflammation in children and adolescents with neuropsychiatric disorders: A systematic review. J. Am. Acad. Child. Adolesc. Psychiatry 2014, 53, 274–296. [Google Scholar] [CrossRef] [PubMed]
- Zerbo, O.; Yoshida, C.; Grether, J.K.; Van de Water, J.; Ashwood, P.; Delorenze, G.N.; Hansen, R.L.; Kharrazi, M.; Croen, L.A. Neonatal cytokines and chemokines and risk of Autism Spectrum Disorder: The Early Markers for Autism (EMA) study: A case-control study. J. Neuroinflamm. 2014, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Dozmorov, M.G.; Bilbo, S.D.; Kollins, S.H.; Zucker, N.; Do, E.K.; Schechter, J.C.; Zhang, J.J.; Murphy, S.K.; Hoyo, C.; Fuemmeler, B.F. Associations between maternal cytokine levels during gestation and measures of child cognitive abilities and executive functioning. Brainbehav. Immun. 2018, 70, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Connor Gorber, S.; Tremblay, M.S. The bias in self-reported obesity from 1976 to 2005: A Canada-US comparison. Obesity (Silver Spring) 2010, 18, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Langley, K.; Rice, F.; van den Bree, M.B.; Thapar, A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatr. 2005, 57, 359–371. [Google Scholar] [PubMed]
- Drake, P.; Driscoll, A.K.; Mathews, T.J. Cigarette Smoking During Pregnancy: United States, 2016. In NCHS Data Brief; Centers for Disease Control and Prevention: Atlanta, CA, USA, 2018; pp. 1–8. [Google Scholar]
| Characteristics | n | (%) | BASC ADHD | BRIEF GEC | ||
|---|---|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | |||
| Overall | 331 | 100.0 | 16.32 | (15.55–17.09) | 92.90 | (90.66–95.14) |
| Mother age at delivery, years | ||||||
| 18–24 | 80 | 24.2 | 17.63 | (15.87–19.38) | 98.23 | (93.52–102.95) |
| 25–29 | 91 | 27.5 | 16.68 | (14.94–18.42) | 92.58 | (87.51–97.65) |
| 30–34 | 109 | 32.9 | 15.33 | (14.25–16.42) | 90.05 | (86.57–93.52) |
| 35+ | 51 | 15.4 | 15.75 | (13.98–17.51) | 91.92 | (86.57–97.27) |
| Maternal education level | ||||||
| College graduate | 170 | 51.4 | 14.80 | (13.91–15.69) | 87.23 | (84.46–89.969) |
| Some college | 72 | 21.8 | 17.96 | (15.90–20.02) | 97.68 | (92.43–102.93) |
| High school graduate/GED | 54 | 16.3 | 17.41 | (15.42–19.39) | 99.64 | (93.42–105.86) |
| Less than high school | 35 | 10.6 | 18.66 | (16.05–21.27) ** | 101.76 | (94.54–108.98) ** |
| Race | ||||||
| White | 151 | 45.6 | 15.99 | (14.94–17.04) | 89.93 | (86.99–92.87) |
| Black | 155 | 46.8 | 16.65 | (15.37–17.93) | 95.82 | (92.03–99.62) |
| Hispanic | 10 | 3.0 | 18.10 | (15.75–20.45) | 97.50 | (87.90–107.10) |
| Other | 15 | 4.5 | 15.07 | (12.41–17.73) | 92.47 | (81.96–102.97) |
| Parity | ||||||
| Primiparous | 125 | 37.8 | 16.02 | (14.86–17.18) | 92.16 | (88.79–95.53) |
| 1–3 | 112 | 33.8 | 17.08 | (15.60–18.56) | 93.62 | (89.41–97.82) |
| ≥4 | 94 | 28.4 | 15.81 | (14.37–17.25) | 93.37 | (88.86–97.87) |
| Maternal ADHD Symptoms | ||||||
| Negative screen | 292 | 88.2 | 15.91 | (15.11–16.71) | 92.04 | (89.64–94.44) |
| Positive screen | 39 | 11.8 | 19.41 | (16.77–22.05) * | 100.35 | (93.42–107.29) * |
| Maternal smoking | ||||||
| No | 262 | 79.2 | 15.93 | (15.12–16.74) | 90.97 | (88.63 - 93.31) |
| Yes | 69 | 20.8 | 17.80 | (15.71–19.89) | 100.95 | (94.77–107.14) ** |
| Prepregnancy BMI (Body Mass Index) | ||||||
| Underweight | 21 | 6.3 | 13.90 | (11.04–16.77) | 79.90 | (73.47–86.33) |
| Normal weight | 137 | 41.4 | 15.64 | (14.53–16.75) | 91.58 | (88.27–94.88) |
| Overweight | 68 | 20.5 | 15.93 | (14.15–17.70) | 94.59 | (89.35–99.83) |
| Obese class I | 38 | 11.5 | 15.50 | (13.83–17.17) | 91.27 | (86.28–96.260 |
| Obese class II and greater | 67 | 20.2 | 19.33 | (17.23–21.43) * | 99.41 | (93.11–105.70) * |
| Weight gain during pregnancy, IOM (Institute of Medicine) category | ||||||
| Less than recommended | 52 | 15.7 | 18.58 | (16.40–20.75) | 95.33 | (89.80–100.87) |
| Recommended | 79 | 23.9 | 14.42 | (13.05–15.78) | 88.07 | (83.62–92.53) |
| More than recommended | 200 | 60.4 | 16.49 | (15.48–17.49) * | 94.18 | (91.18–97.18) |
| Maternal Diabetes Status | ||||||
| Normal | 281 | 84.9 | 15.96 | (15.14–16.78) | 92.09 | (89.73–94.45) |
| Gestational Diabetes | 23 | 7.0 | 21.57 | (17.53–25.60) | 105.65 | (92.51–118.79) |
| Type I Diabetes | 10 | 3.0 | 17.50 | (14.30–20.70) | 99.50 | (88.14–110.86) |
| Type II Diabetes | 17 | 5.1 | 14.53 | (11.68–17.38) * | 85.80 | (77.62–93.98) * |
| Gestational age, weeks | ||||||
| 34–36 | 17 | 5.1 | 16.06 | (13.49–18.63) | 92.13 | (84.03–100.22) |
| 37–40 | 237 | 71.6 | 16.86 | (15.93–17.79) | 94.48 | (91.75–97.21) |
| >41 | 77 | 23.3 | 14.71 | (13.10–16.33) | 88.53 | (83.83–93.24) |
| Birthweight (g) | ||||||
| < 2500 | 27 | 8.2 | 16.30 | (13.87–18.72) | 94.73 | (86.94–102.52) |
| 2500–4200 | 289 | 87.3 | 16.51 | (15.66–17.35) | 93.38 | (90.90–95.87) |
| >4200 | 15 | 4.5 | 12.80 | (9.97–15.63) | 83.00 | (76.08–89.92) |
| Child gender | ||||||
| Male | 171 | 51.7 | 16.83 | (15.70–17.96) | 92.86 | (89.54–96.17) |
| Female | 160 | 48.3 | 15.78 | (14.72–16.83) | 93.16 | (90.02–96.31) |
| Model | ADHD | Hyperactivity | Attention Problem | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | se | β | p | B | se | β | p | B | se | β | p | |
| Model 1 | ||||||||||||
| BMI (continuous) | 0.17 | 0.05 | 0.20 | 0.001 | 0.09 | 0.04 | 0.16 | 0.02 | 0.08 | 0.02 | 0.21 | <0.001 |
| Model 2 | ||||||||||||
| BMI (categorical) | ||||||||||||
| 0–24.9 (referent) | ||||||||||||
| 25–29.9 | 0.04 | 1.05 | 0.002 | 0.97 | −0.39 | 0.82 | −0.03 | 0.64 | 0.47 | 0.41 | 0.06 | 0.25 |
| 30–34.9 | −0.44 | 0.91 | -0.02 | 0.63 | −0.64 | 0.64 | −0.04 | 0.32 | 0.22 | 0.52 | 0.02 | 0.67 |
| >35 | 4.22 | 1.34 | 0.21 | 0.002 | 2.43 | 1.05 | 0.18 | 0.02 | 1.79 | 0.50 | 0.20 | <0.001 |
| Model 3 | ||||||||||||
| Gestational weight gain (kg) (continuous) | 0.01 | 0.05 | 0.02 | 0.80 | −0.02 | 0.04 | −0.02 | 0.69 | 0.03 | 0.02 | 0.07 | 0.26 |
| Model 4 | ||||||||||||
| Gestational weight gain (categorical) | ||||||||||||
| Less than adequate | 2.74 | 1.25 | 0.15 | 0.03 | 2.04 | 0.89 | 0.16 | 0.02 | 0.70 | 0.56 | 0.09 | 0.22 |
| Adequate (referent) | ||||||||||||
| More than adequate | 1.16 | 0.88 | 0.08 | 0.19 | 0.52 | 0.61 | 0.05 | 0.39 | 0.62 | 0.37 | 0.10 | 0.09 |
| Model | GEC Score | Inhibit | Shift | Emotional Control | Working Memory | Plan/Organize | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | se | β | p | B | se | β | p | B | se | β | p | B | se | β | p | B | se | β | p | B | se | β | p | |
| Model 1 | ||||||||||||||||||||||||
| BMI (continuous) | 0.38 | 0.16 | 0.16 | 0.02 | 0.09 | 0.04 | 0.13 | 0.04 | 0.06 | 0.03 | 0.16 | 0.02 | 0.04 | 0.03 | 0.08 | 0.23 | 0.12 | 0.05 | 0.17 | 0.02 | 0.07 | 0.02 | 0.18 | 0.004 |
| Model 2 | ||||||||||||||||||||||||
| BMI (categorical) | ||||||||||||||||||||||||
| 0–24.9 (referent) | ||||||||||||||||||||||||
| 25–29.9 | 1.02 | 2.81 | 0.02 | 0.72 | 0.13 | 0.92 | 0.01 | 0.89 | 0.16 | 0.44 | 0.02 | 0.72 | −0.41 | 0.49 | −0.04 | 0.41 | 0.93 | 0.94 | 0.06 | 0.33 | 0.20 | 0.50 | 0.02 | 0.69 |
| 30–34.9 | −2.50 | 3.35 | −0.04 | 0.46 | −1.04 | 0.99 | −0.05 | 0.29 | −0.79 | 0.61 | −0.07 | 0.20 | −0.90 | 0.79 | −0.07 | 0.25 | −0.09 | 0.97 | −0.005 | 0.92 | 0.20 | 0.59 | 0.02 | 0.73 |
| >35 | 9.31 | 4.30 | 0.16 | 0.03 | 2.23 | 1.14 | 0.13 | 0.05 | 1.41 | 0.70 | 0.14 | 0.05 | 1.04 | 0.76 | 0.09 | 0.17 | 3.21 | 1.35 | 0.19 | 0.02 | 1.42 | 0.71 | 0.14 | 0.05 |
| Model 3 | ||||||||||||||||||||||||
| Gestational weight gain (kg) (continuous) | 0.27 | 0.14 | 0.11 | 0.06 | 0.05 | 0.04 | 0.07 | 0.23 | 0.03 | 0.02 | 0.07 | 0.20 | 0.03 | 0.03 | 0.06 | 0.29 | 0.08 | 0.04 | 0.10 | 0.05 | 0.07 | 0.02 | 0.15 | 0.01 |
| Model 4 | ||||||||||||||||||||||||
| Gestational weight gain (kg) (categorical) | ||||||||||||||||||||||||
| Less than adequate | 1.66 | 3.83 | 0.03 | 0.67 | 0.80 | 1.07 | 0.06 | 0.45 | 0.38 | 0.62 | 0.04 | 0.54 | 0.70 | 0.73 | 0.07 | 0.34 | −0.11 | 1.13 | −0.01 | 0.92 | −0.79 | 0.68 | −0.09 | 0.24 |
| Adequate (referent) | ||||||||||||||||||||||||
| More than adequate | 2.86 | 2.85 | 0.07 | 0.32 | 0.66 | 0.79 | 0.06 | 0.41 | 0.39 | 0.47 | 0.06 | 0.41 | 0.40 | 0.53 | 0.05 | 0.45 | 0.41 | 0.85 | 0.03 | 0.63 | 0.26 | 0.48 | 0.04 | 0.59 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuemmeler, B.F.; Zucker, N.; Sheng, Y.; Sanchez, C.E.; Maguire, R.; Murphy, S.K.; Kollins, S.H.; Hoyo, C. Pre-Pregnancy Weight and Symptoms of Attention Deficit Hyperactivity Disorder and Executive Functioning Behaviors in Preschool Children. Int. J. Environ. Res. Public Health 2019, 16, 667. https://doi.org/10.3390/ijerph16040667
Fuemmeler BF, Zucker N, Sheng Y, Sanchez CE, Maguire R, Murphy SK, Kollins SH, Hoyo C. Pre-Pregnancy Weight and Symptoms of Attention Deficit Hyperactivity Disorder and Executive Functioning Behaviors in Preschool Children. International Journal of Environmental Research and Public Health. 2019; 16(4):667. https://doi.org/10.3390/ijerph16040667
Chicago/Turabian StyleFuemmeler, Bernard F., Nancy Zucker, Yaou Sheng, Carmen E. Sanchez, Rachel Maguire, Susan K. Murphy, Scott H. Kollins, and Cathrine Hoyo. 2019. "Pre-Pregnancy Weight and Symptoms of Attention Deficit Hyperactivity Disorder and Executive Functioning Behaviors in Preschool Children" International Journal of Environmental Research and Public Health 16, no. 4: 667. https://doi.org/10.3390/ijerph16040667
APA StyleFuemmeler, B. F., Zucker, N., Sheng, Y., Sanchez, C. E., Maguire, R., Murphy, S. K., Kollins, S. H., & Hoyo, C. (2019). Pre-Pregnancy Weight and Symptoms of Attention Deficit Hyperactivity Disorder and Executive Functioning Behaviors in Preschool Children. International Journal of Environmental Research and Public Health, 16(4), 667. https://doi.org/10.3390/ijerph16040667





