Cancer Mortality Trend in Central Italy: Focus on A “Low Rate of Land Use” Area from 1982 to 2011
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Processing and Statistical Elaboration
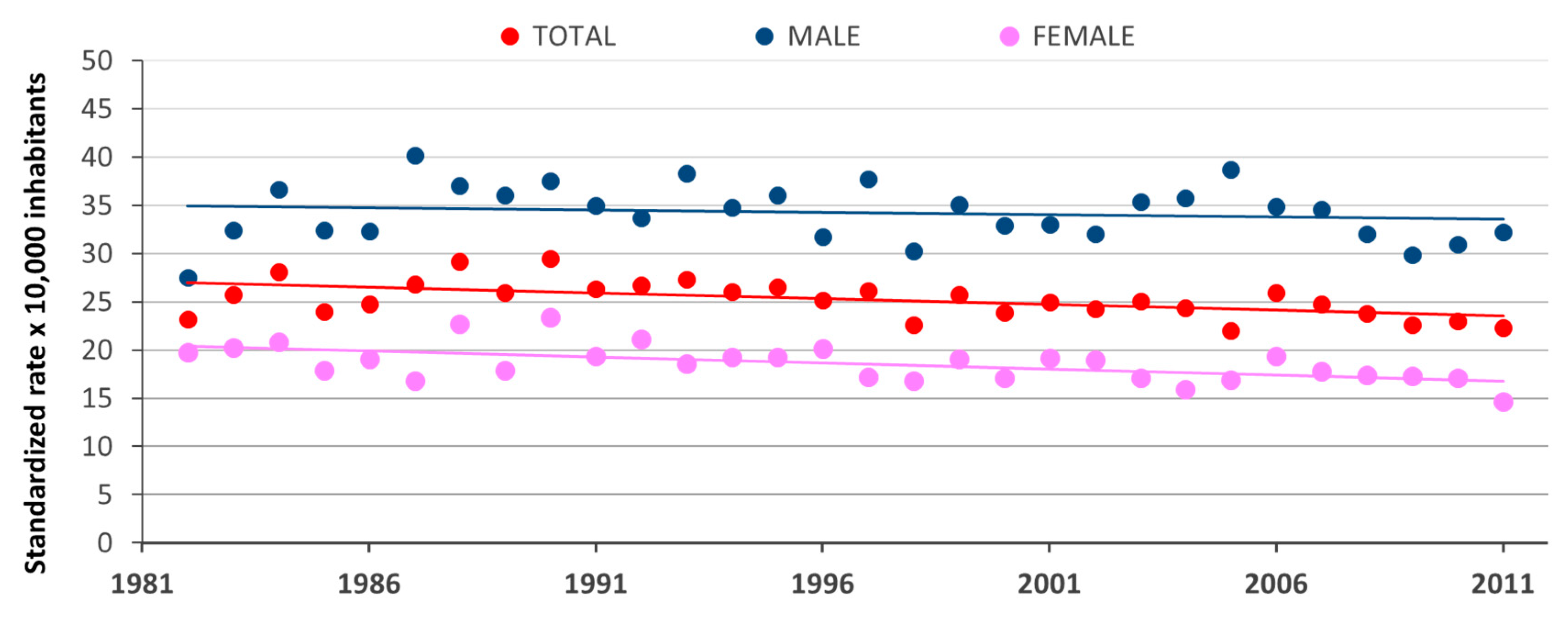
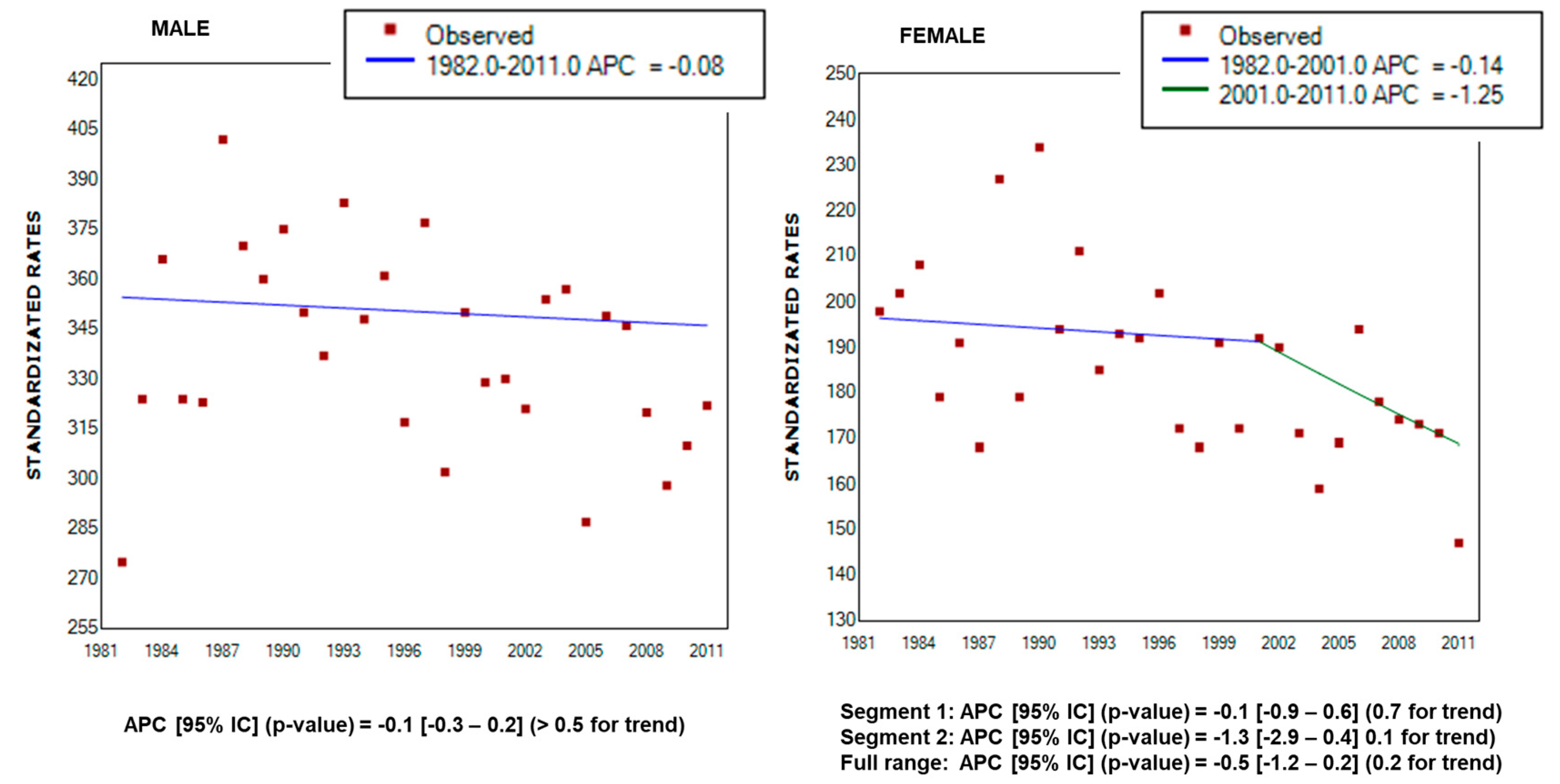
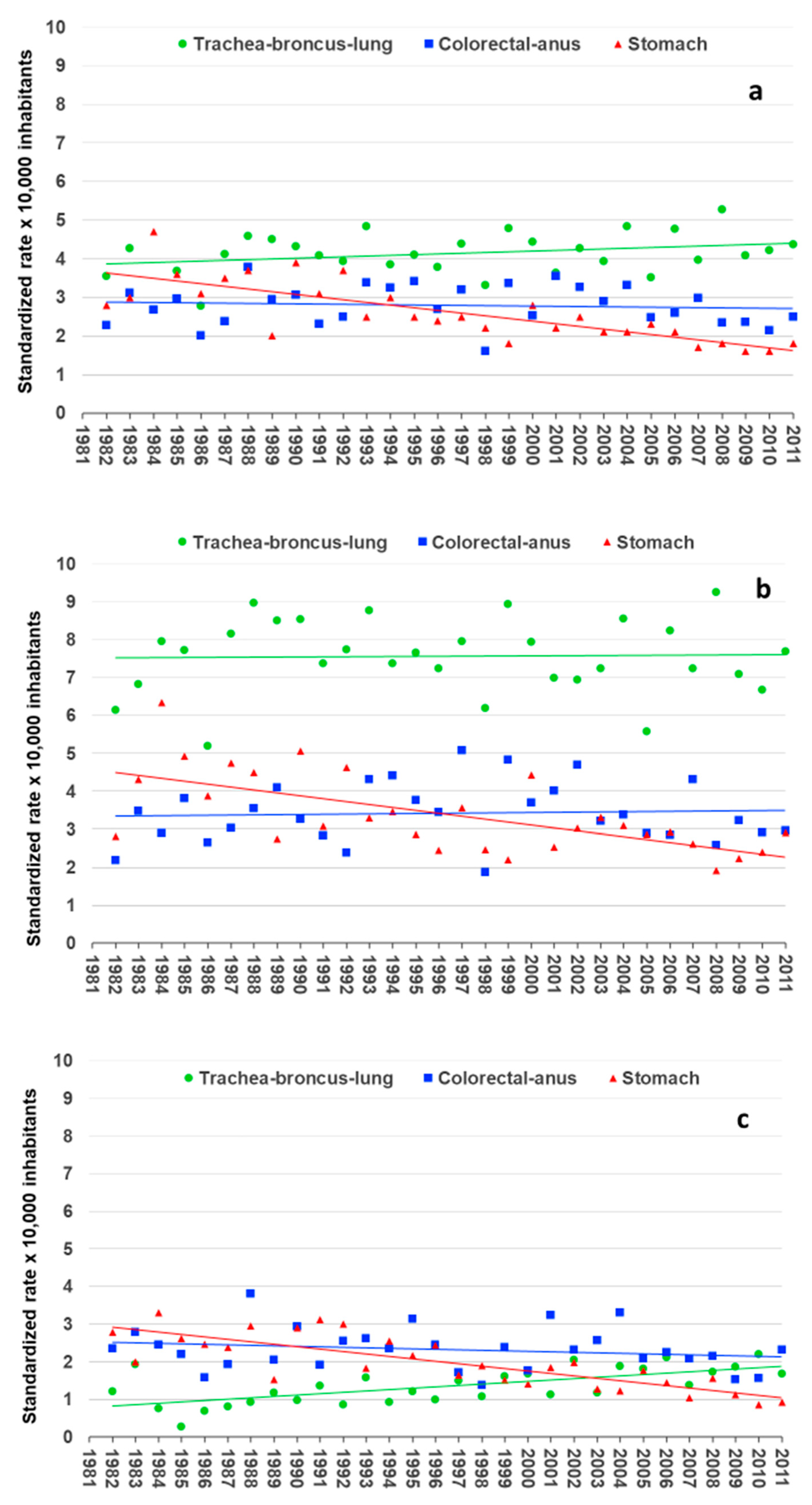
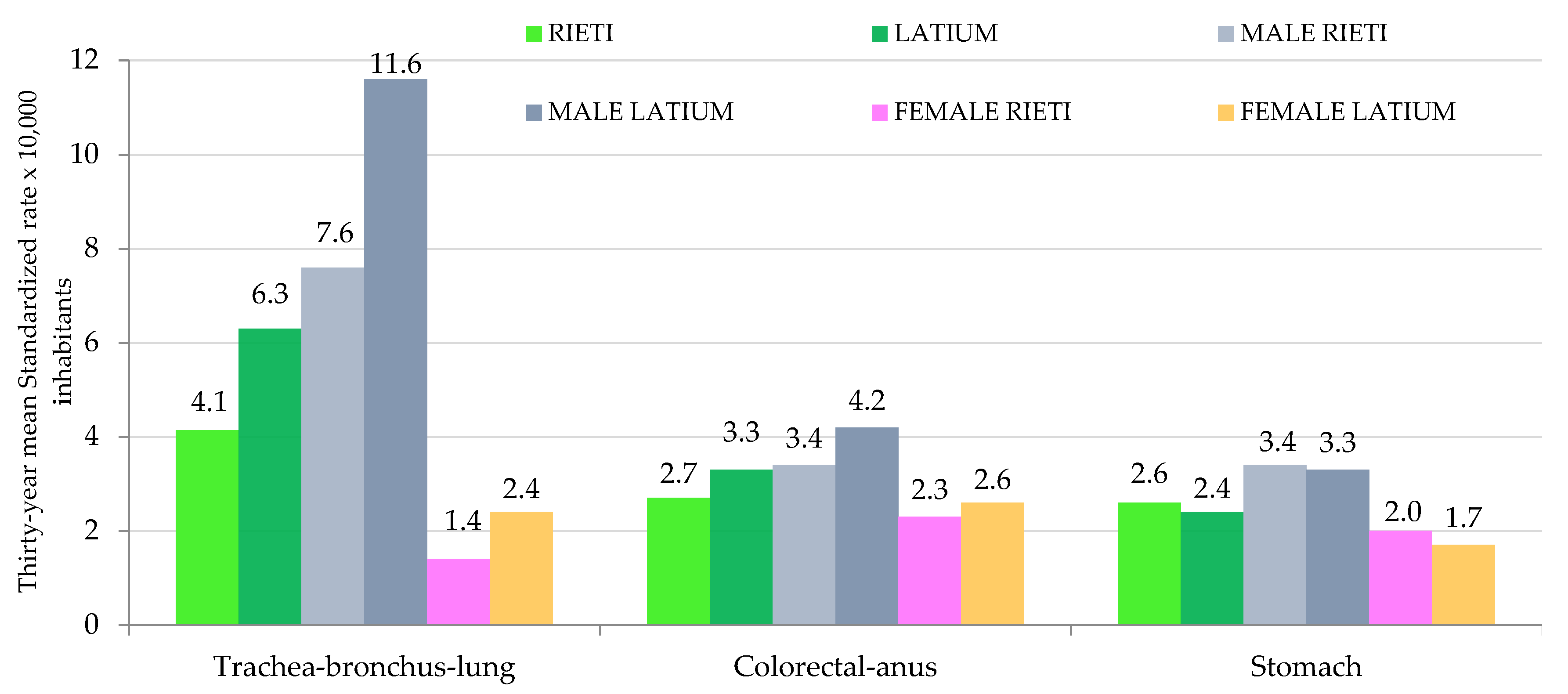
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Associazione Italiana Registro Tumori. Cancer Data. Available online: http://www.registri-tumori.it/PDF/AIOM2017/I_numeri_del_cancro_2017.pdf (accessed on 19 December 2018).
- Pogribny, I.P.; Rusyn, I. Environmental toxicants, epigenetics, and cancer. Adv. Exp. Med. Biol. 2013, 754, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Rahal, Z.; Abdulhai, F.; Kadara, H.; Saab, R. Genomics of adult and pediatric solid tumors. Am. J. Cancer Res. 2018, 8, 1356–1386. [Google Scholar] [PubMed]
- Katzke, V.A.; Kaaks, R.; Kühn, T. Lifestyle and cancer risk. Cancer J. 2015, 21, 104–110. [Google Scholar] [CrossRef] [PubMed]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and cancer risk: A potentially modifiable relationship. Am. J. Prev. Med. 2014, 46, S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Aunan, J.R.; Cho, W.C.; Søreide, K. The Biology of aging and cancer: A brief overview of shared and divergent molecular hallmarks. Aging Dis. 2017, 8, 628–662. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.-V.; Martin, M.; Lu, T. Aging: Cancer–an unlikely couple. Aging 2017, 9, 1949–1950. [Google Scholar] [CrossRef]
- Hashim, D.; Boffetta, P.; La Vecchia, C.; Rota, M.; Bertuccio, P.; Malvezzi, M.; Negri, E. The global decrease in cancer mortality: Trends and disparities. Ann. Oncol. 2016, 27, 926–933. [Google Scholar] [CrossRef]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Istituto Nazionale di Statistica. Demographic Projections. Available online: http://www.istat.it/it/files/2017/04/previsioni-demografiche.pdf (accessed on 19 December 2018).
- Arfè, A.; Malvezzi, M.; Bertuccio, P.; Decarli, A.; La Vecchia, C.; Negri, E. Cancer mortality trend analysis in Italy, 1970–2007. Eur. J. Cancer Prev. 2011, 20, 364–374. [Google Scholar] [CrossRef]
- Bosetti, C.; Bertuccio, P.; Malvezzi, M.; Levi, F.; Chatenoud, L.; Negri, E.; La Vecchia, C. Cancer mortality in Europe, 2005–2009, and an overview of trends since 1980. Ann. Oncol. 2013, 24, 2657–2671. [Google Scholar] [CrossRef] [PubMed]
- Chatenoud, L.; Bertuccio, P.; Bosetti, C.; Malvezzi, M.; Levi, F.; Negri, E.; La Vecchia, C. Trends in mortality from major cancers in the Americas: 1980–2010. Ann. Oncol. 2014, 25, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Associazione Italiana Registro Tumori. Cancer Data. Available online: http://www.registri-tumori.it/PDF/AIOM2016/I_numeri_del_cancro_2016.pdf (accessed on 19 December 2018).
- Mattei, V.; Santilli, F.; Martellucci, S.; Di Pasquale, J.; Liberati, F.; Protano, C.; Vitali, M.; Sorice, M. Cancer mortality in Rieti province (Latium region, Italy) for the years 2006–2010: Evaluation of temporal and spatial trends and comparison with the other Latium provinces. Ann. Ig. 2017, 29, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Mattei, V.; Tasciotti, V.; Santilli, F.; Martellucci, S.; Liberati, F.; Protano, C.; Vitali, M.; Sorice, M. Epidemiological profile of cancer mortality in a province of central Italy for the years 2008 and 2009: Preliminary analysis. Ann. Ig. 2015, 27, 613–622. [Google Scholar] [CrossRef]
- Istituto Nazionale di Statistica. Demographic Indicators. Aging Index. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCIS_INDDEMOG1 (accessed on 19 December 2018).
- Istituto Nazionale di Statistica. Number of Births 2015. Available online: http://demo.istat.it/altridati/IscrittiNascita/2016/T1.13.pdf (accessed on 19 December 2018).
- Agenzia Regionale Protezione Ambientale del Lazio. Environmental Indicators. Available online: http://www.arpalazio.gov.it/ambiente/indicatori/ (accessed on 19 December 2018).
- Agenzia Regionale Protezione Ambientale del Lazio. Waste Production. Available online: http://www.arpalazio.gov.it/ambiente/indicatori/doc/Indicatore_ambientale_Produzione_Rifiuti.pdf (accessed on 19 December 2018).
- World Health Organization (WHO). International Statistical Classification of Diseases, Injuries and Causes of Death; WHO: Geneva, Switzerland, 2008; Available online: http://www.who.int/classifications/icd/en/ (accessed on 19 December 2018).
- Joinpoint Regression Program. Statistical Methodology and applications Branch. In Surveillance Research Program; National Cancer Institute: Bethesda, MD, USA, 2017. [Google Scholar]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-Based Report; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute: Atlanta, GA, USA, 2017. Available online: https://www.cdc.gov/cancer/npcr/pdf/uscs_factsheet.pdf (accessed on 19 December 2018).
- Kachuri, L.; De, P.; Ellison, L.F.; Semenciw, R.; Advisory Committee on Canadian Cancer Statistics. Cancer incidence, mortality and survival trends in Canada, 1970–2007. Chronic Dis. Inj. Can. 2013, 33, 69–80. [Google Scholar]
- Katanoda, K.; Matsuda, T.; Matsuda, A.; Shibata, A.; Nishino, Y.; Fujita, M.; Soda, M.; Ioka, A.; Sobue, T.; Nishimoto, H. An updated report of the trends in cancer incidence and mortality in Japan. Jpn. J. Clin. Oncol. 2013, 43, 492–507. [Google Scholar] [CrossRef]
- Malvezzi, M.; Bertuccio, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2013. Ann. Oncol. 2013, 24, 792–800. [Google Scholar] [CrossRef]
- Malvezzi, M.; Bertuccio, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2014. Ann. Oncol. 2014, 25, 1650–1656. [Google Scholar] [CrossRef]
- Malvezzi, M.; Bertuccio, P.; Rosso, T.; Rota, F.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2015: Does lung cancer have the highest death rate in EU women? Ann. Oncol. 2015, 26, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Malvezzi, M.; Rosso, T.; Bertuccio, P.; Gallus, S.; Chatenoud, L.; Levi, F.; La Vecchia, C.; Negri, E. Lung cancer mortality in European women: Trends and predictions. Lung Cancer 2012, 78, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Ahn, C.; Symanski, E.; Lai, D.; Du, X.L. Relative impact of earlier diagnosis and improved treatment on survival for colorectal cancer: A US database study among elderly patients. Cancer Epidemiol. 2014, 38, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Ciombor, K.K.; Wu, C.; Goldberg, R.M. Recent therapeutic advances in the treatment of colorectal cancer. Annu. Rev. Med. 2015, 66, 83–95. [Google Scholar] [CrossRef]
- Elmunzer, B.J.; Hayward, R.A.; Schoenfeld, P.S.; Saini, S.D.; Deshpande, A.; Waljee, A.K. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012, 9, e1001352. [Google Scholar] [CrossRef]
- Hewitson, P.; Glasziou, P.; Watson, E.; Towler, B.; Irwig, L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): An update. Am. J. Gastroenterol. 2008, 103, 1541–1549. [Google Scholar] [CrossRef]
- La Vecchia, C.; Franceschi, S. Nutrition and gastric cancer with a focus on Europe. Eur. J. Cancer Prev. 2000, 9, 291–295. [Google Scholar]
- Plummer, M.; van Doorn, L.J.; Franceschi, S.; Kleter, B.; Canzian, F.; Vivas, J.; Lopez, G.; Colin, D.; Muñoz, N.; Kato, I. Helicobacter pylori cytotoxin-associated genotype and gastric precancerous lesions. J. Natl. Cancer Inst. 2007, 99, 1328–1334. [Google Scholar] [CrossRef]
- Pritchard, D.M.; Crabtree, J.E. Helicobacter pylori and gastric cancer. Curr. Opin. Gastroenterol. 2006, 22, 620–625. [Google Scholar] [CrossRef]
- Politis, M.; Higuera, G.; Chang, L.R.; Gomez, B.; Bares, J.; Motta, J. Trend analysis of cancer mortality and incidence in panama, using Joinpoint regression analysis. Medicine 2015, 94, e970. [Google Scholar] [CrossRef]
- Rosso, T.; Bertuccio, P.; La Vecchia, C.; Negri, E.; Malvezzi, M. Cancer mortality trend analysis in Italy, 1980–2010, and predictions for 2015. Tumori 2015, 101, 664–675. [Google Scholar] [CrossRef] [PubMed]
| Year | Population Density (People per sq km of Land Area) | Ageing Index (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rieti | Rome | Latina | Frosinone | Viterbo | Italy | Rieti | Rome | Latina | Frosinone | Viterbo | Italy | |
| 1981 | 51.9 | 689.1 | 192.4 | 141.8 | 74.3 | 187.2 | 97.4 | 53.2 | 38.9 | 58.8 | 75.6 | 61.7 |
| 1991 | 52.7 | 701.3 | 211.5 | 148.1 | 77.6 | 187.9 | 139.7 | 94.9 | 62.9 | 82.5 | 114.4 | 92.5 |
| 2001 | 53.4 | 690.8 | 217.9 | 149.2 | 79.8 | 188.7 | 172.3 | 130.9 | 99.6 | 125.5 | 163.6 | 129.3 |
| 2011 | 56.4 | 743.4 | 240.8 | 151.8 | 86.4 | 196.7 | 188.9 | 141.5 | 124.9 | 152.5 | 172.8 | 145.7 |
| Pollutants | Rieti | Rome | Latina | Frosinone | Viterbo |
|---|---|---|---|---|---|
| Airborne benzene (mg m−3) 1 | 2.2 | 3.3 | 2.6 | 4.2 | 2.2 |
| Airborne PM10 (mg m−3) 2 | 21.8 | 32.2 | 28.3 | 36.4 | 24.1 |
| Airborne PM2.5 (mg m−3) 3 | 12.5 | 17.2 | 16.8 | 21.2 | 11.6 |
| Arsenic in drinking water (mg L−1) 4 | 0.6 | 5.0 | 7.6 | 7.2 | 13.7 |
| Radon (Bq m−3) 5 | 104 | 96 | 127 | 142 | 144 |
| Geographical Area | 1982–1986 | 1987–1991 | 1992–1996 | 1997–2001 | 2002–2006 | 2007–2011 | Mean 1982–2011 |
|---|---|---|---|---|---|---|---|
| Rieti province | 25.3 | 27.6 | 26.4 | 24.7 | 24.4 | 23.3 | 25.3 |
| Latium region | 31.4 | 32.3 | 30.7 | 29.2 | 28.2 | 27.5 | 29.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santilli, F.; Martellucci, S.; Di Pasquale, J.; Mei, C.; Liberati, F.; Protano, C.; Vitali, M.; Sorice, M.; Mattei, V. Cancer Mortality Trend in Central Italy: Focus on A “Low Rate of Land Use” Area from 1982 to 2011. Int. J. Environ. Res. Public Health 2019, 16, 628. https://doi.org/10.3390/ijerph16040628
Santilli F, Martellucci S, Di Pasquale J, Mei C, Liberati F, Protano C, Vitali M, Sorice M, Mattei V. Cancer Mortality Trend in Central Italy: Focus on A “Low Rate of Land Use” Area from 1982 to 2011. International Journal of Environmental Research and Public Health. 2019; 16(4):628. https://doi.org/10.3390/ijerph16040628
Chicago/Turabian StyleSantilli, Francesca, Stefano Martellucci, Jennifer Di Pasquale, Cecilia Mei, Fabrizio Liberati, Carmela Protano, Matteo Vitali, Maurizio Sorice, and Vincenzo Mattei. 2019. "Cancer Mortality Trend in Central Italy: Focus on A “Low Rate of Land Use” Area from 1982 to 2011" International Journal of Environmental Research and Public Health 16, no. 4: 628. https://doi.org/10.3390/ijerph16040628
APA StyleSantilli, F., Martellucci, S., Di Pasquale, J., Mei, C., Liberati, F., Protano, C., Vitali, M., Sorice, M., & Mattei, V. (2019). Cancer Mortality Trend in Central Italy: Focus on A “Low Rate of Land Use” Area from 1982 to 2011. International Journal of Environmental Research and Public Health, 16(4), 628. https://doi.org/10.3390/ijerph16040628










