The Oral NOAEL of Flurochloridone in Male Wistar Rats in Ninety-Day Subchronic Toxicity Test Was 3mg/kg/day
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Animals and Housing Conditions
2.3. Subchronic Oral Toxicity Study
2.3.1. Toxicokinetic Evaluation in Rats
2.3.2. Necropsy and Histopathology
2.4. Statistical Analysis
3. Results
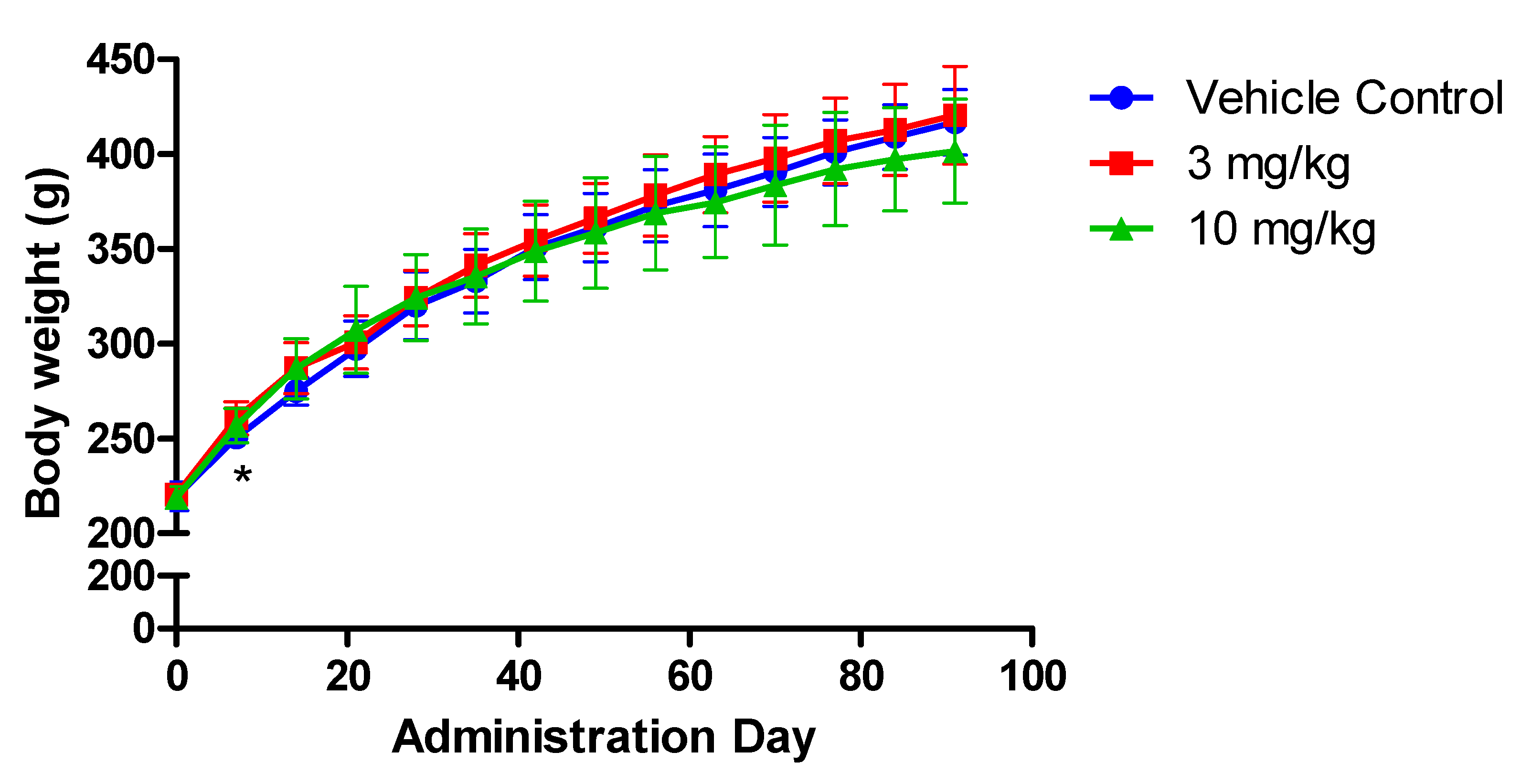
3.1. Clinical Observation
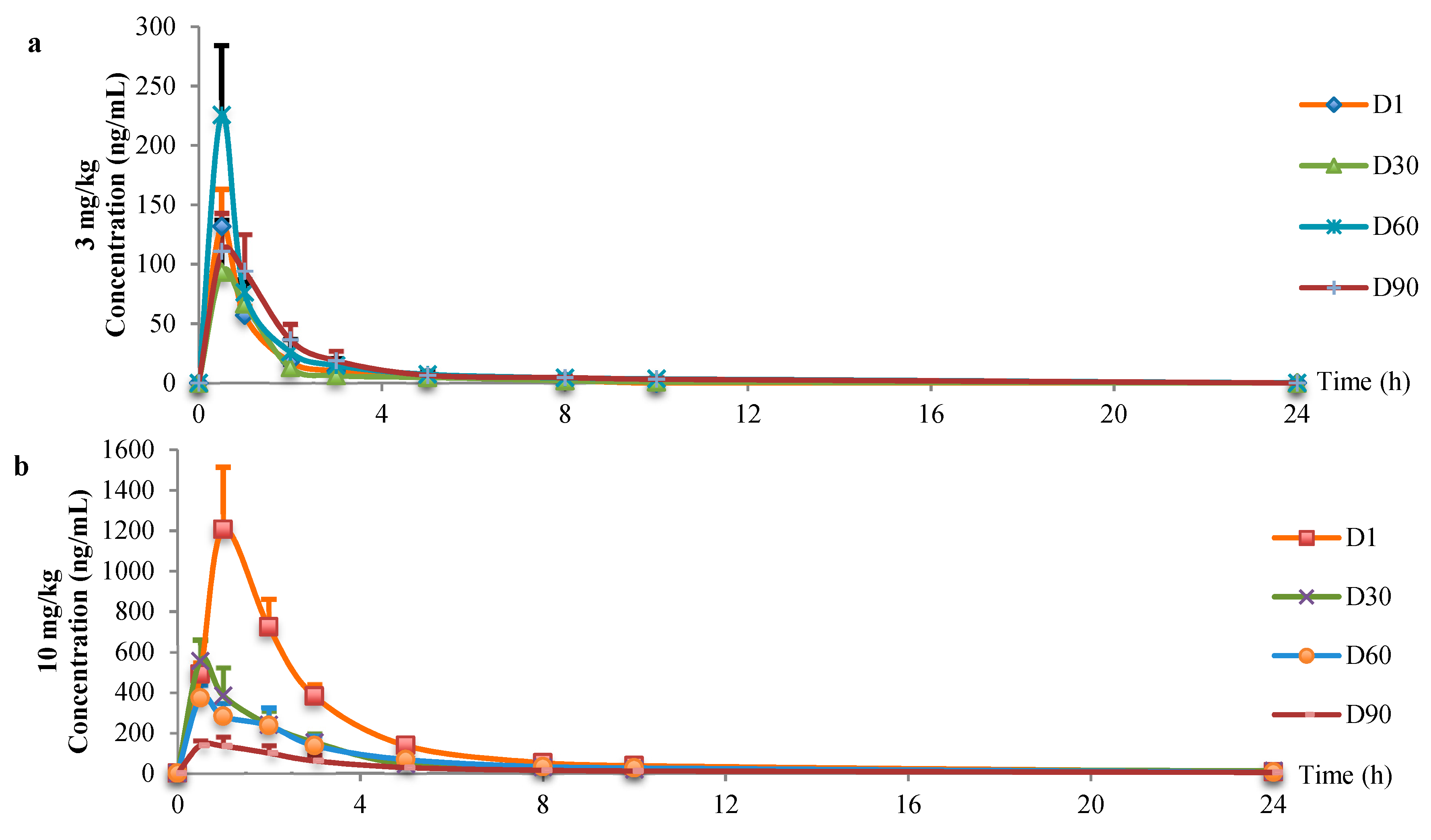
3.2. Toxicokinetic Evaluation in Rats
3.3. Endocrine Function
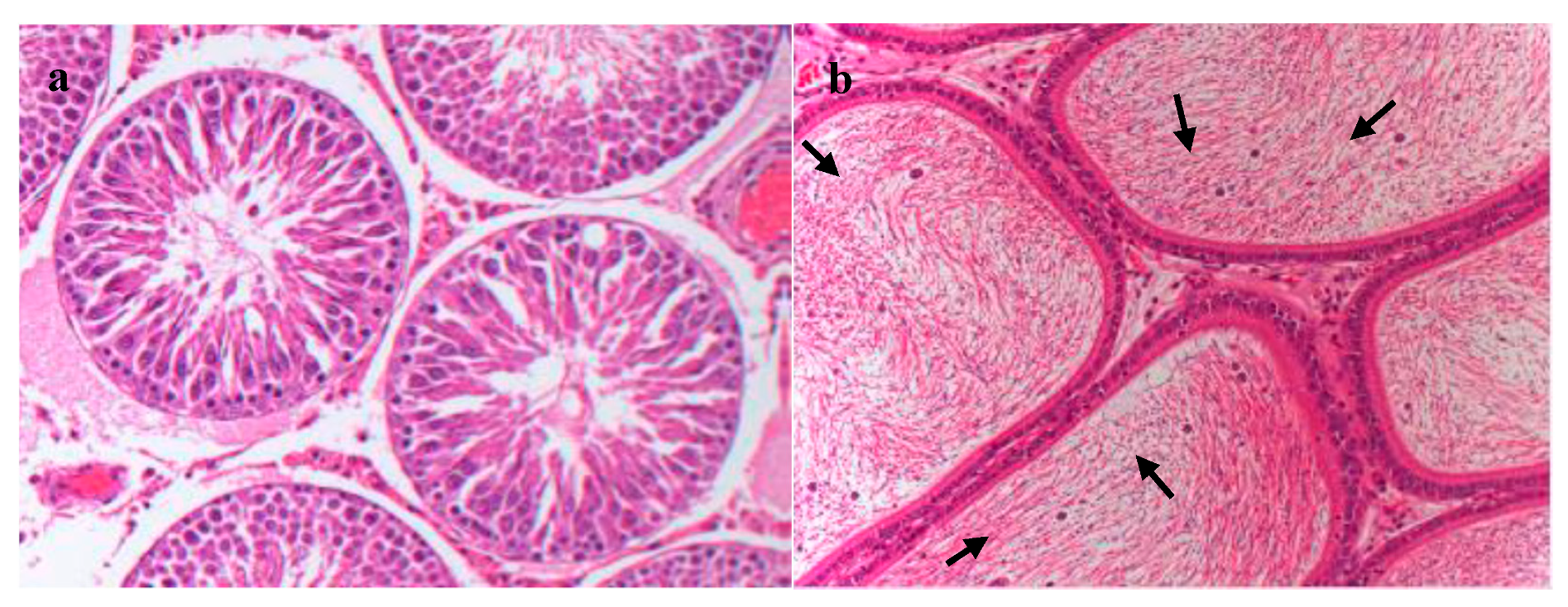
3.4. Necropsy and Histopathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marković, M.; Cupać, S.; Đurović, R.; Milinović, J.; Kljajić, P. Assessment of heavy metal and pesticide levels in soil and plant products from agricultural area of Belgrade, Serbia. Arch. Environ. Contam. Toxicol. 2010, 58, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Nikoloff, N.; Soloneski, S.; Larramendy, M.L. Genotoxic and cytotoxic evaluation of the herbicide flurochloridone on Chinese hamster ovary (CHO-K1) cells. Toxicol. In Vitro Int. J. Publ. Assoc. Bibra 2012, 26, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Yigit, E. The physiological and biochemical effects of salicylic acid on sunflowers (Helianthus annuus) exposed to flurochloridone. Ecotoxicol. Environ. Saf. 2014, 106, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Cong, C.; Wang, Z.; Li, R.; Li, L.; Bu, D.; Wang, J. Evaluation of Weed Efficacy and Crop Safety of Fluorochloridone in China. Weed Technol. 2014, 28, 721–728. [Google Scholar] [CrossRef]
- Conclusion on the peer review of the pesticide risk assessment of the active substance Flurochloridone (notified active substance). EFSA J. 2010, 8, 1869.
- Zhang, S.; Cheng, X.; Wang, Y.; Fan, J.; Li, R.; Zhou, S.; Liu, S.; Shi, J.; Sun, J.; Hu, Y.; et al. Ninety Day Toxicity and Toxicokinetics of Fluorochloridone after Oral Administration in Rats. Int. J. Environ. Res. Public Health 2015, 12, 4942–4966. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shi, J.; Fan, J.; Sun, J.; Zhang, S.; Hu, Y.; Wei, L.; Wu, C.; Chang, X.; Tang, L.; et al. A quantitative determination of fluorochloridone in rat plasma by UPLC-MS/MS method: Application to a pharmacokinetic study. BMC Biomed. Chromatogr. 2016, 30, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Sireeratawong, S.; Jaijoy, K.; Khonsung, P.; Lertprasertsuk, N.; Ingkaninan, K. Acute and chronic toxicities of Bacopa monnieri extract in Sprague-Dawley rats. BMC Complementary Altern. Med. 2016, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Yuet Ping, K.; Darah, I.; Chen, Y.; Sreeramanan, S.; Sasidharan, S. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. Biomed. Res. Int. 2013, 182064. [Google Scholar]
- Katoh, C.; Kitajima, S.; Saga, Y.; Kanno, J.; Horii, I.; Inoue, T. Assessment of quantitative dual-parameter flow cytometric analysis for the evaluation of testicular toxicity using cyclophosphamide- and ethinylestradiol-treated rats. J. Toxicol. Sci. 2002, 27, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Jenardhanan, P.; Panneerselvam, M.; Mathur, P.P. Effect of environmental contaminants on spermatogenesis. Semin. Cell Dev. Biol. 2016, 59, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Le Moal, J.; Rolland, M.; Goria, S.; Wagner, V.; De Crouy-Chanel, P.; Rigou, A.; De Mouzon, J.; Royère, D. Semen quality trends in French regions are consistent with a global change in environmental exposure. Reproduction 2014, 147, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Su, Z.J.; Ge, R.S. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules 2011, 16, 9983–10001. [Google Scholar] [CrossRef] [PubMed]
- Huhtaniemi, I. A short evolutionary history of FSH-stimulated spermatogenesis. Hormones 2015, 14, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Yeung, B.H.; Wan, H.T.; Law, A.Y.; Wong, C.K. Endocrine disrupting chemicals: Multiple effects on testicular signaling and spermatogenesis. Spermatogenesis 2011, 1, 231. [Google Scholar] [CrossRef] [PubMed]
- De Gendt, K.; Swinnen, J.V.; Saunders, P.T.; Schoonjans, L.; Dewerchin, M.; Devos, A.; Tan, K.; Atanassova, N.; Claessens, F.; Lécureuil, C.; et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl. Acad. Sci. USA 2004, 101, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Pragya, P.; Ram, K.R.; Chowdhuri, D.K. Environmental chemical mediated male reproductive toxicity: Drosophila melanogaster as an alternate animal model. Theriogenology 2011, 76, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, G.; Li, J.; Zhao, H.; Wang, Y.; Chen, J.; Zhao, H. Association of polybrominated diphenylethers (PBDEs) and hydroxylated metabolites (OH-PBDEs) serum levels with thyroid function in thyroid cancer patients. Environ. Res. 2017, 159, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.O.N.G.; Jia, Z.C.; Chen, J.Y.; Hu, J.X.; Zhang, L.S. Toxic effects of atrazine on reproductive system of male rats. Biomed. Environ. Sci. 2014, 27, 281–288. [Google Scholar]
| Group | Parameters | Day 1 | Day 30 | Day 60 | Day 90 |
|---|---|---|---|---|---|
| 3 mg/kg | AUC(0-t) (μg·L−1·h) | 254.11 ± 63.93 | 174.95 ± 46.28 | 456.18 ± 118.38 | 278.57 ± 56.14 |
| Tmax (h) | 0.50 ± 0.00 | 0.60 ± 0.22 | 0.50 ± 0.00 | 0.60 ± 0.22 | |
| CLz (L·h−1·kg−1) | 11.97 ± 2.88 | 17.93 ± 3.75 | 6.34 ± 1.25 | 11.25 ± 3.00 | |
| Cmax (μg/L) | 131.80 ± 31.14 | 99.19 ± 36.01 | 225.60 ± 58.58 | 113.94 ± 32.19 | |
| 10 mg/kg | AUC(0-t) (μg·L−1·h) | 3019.81 ± 488.96 | 1469.59 ± 410.57 | 1453.90 ± 283.36 | 609.05 ± 201.43 |
| Tmax (h) | 1.00 ± 0.00 | 0.50 ± 0.00 | 0.50 ± 0.00 | 0.70 ± 0.27 | |
| CLz (L·h−1·kg−1) | 3.33 ± 0.63 | 7.14 ± 2.03 | 6.91 ± 1.08 | 16.71 ± 4.24 | |
| Cmax (μg/L) | 1206.20 ± 307.84 | 579.00 ± 102.67 | 372.80 ± 64.32 | 156.20 ± 33.96 |
| Parameters | Control | 3 mg/kg | 10 mg/kg |
|---|---|---|---|
| FSH (mIU/ml) | 3.09 ± 1.19 | 3.77 ± 0.82 | 3.16 ± 0.21 |
| LH (mIU/ml) | 4.75 ± 0.39 | 5.01 ± 1.24 | 4.47 ± 0.82 |
| Testosterone (ng/ml) | 1.01 ± 0.31 | 0.56 ± 0.34 | 0.17 ± 0.07 * |
| Parameters | Control | 3 mg/kg | 10 mg/kg |
|---|---|---|---|
| Epididymis ratio | 0.2892 ± 0.0661 | 0.2626 ± 0.0195 | 0.2313 ± 0.0196 * |
| Testis ratio | 0.9176 ± 0.1526 | 0.8275 ± 0.0066 | 0.7642 ± 0.0600 * |
| Sperm abnormality rate (%) | 0.47 ± 0.19 | 0.51 ± 0.26 | 1.14 ± 0.38 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Li, R.; Zhou, S.; Zhang, S.; Wang, Y.; Liu, S.; Song, Q.; Chang, X.; Zhang, Y.; Liu, L.; et al. The Oral NOAEL of Flurochloridone in Male Wistar Rats in Ninety-Day Subchronic Toxicity Test Was 3mg/kg/day. Int. J. Environ. Res. Public Health 2019, 16, 553. https://doi.org/10.3390/ijerph16040553
Zhu H, Li R, Zhou S, Zhang S, Wang Y, Liu S, Song Q, Chang X, Zhang Y, Liu L, et al. The Oral NOAEL of Flurochloridone in Male Wistar Rats in Ninety-Day Subchronic Toxicity Test Was 3mg/kg/day. International Journal of Environmental Research and Public Health. 2019; 16(4):553. https://doi.org/10.3390/ijerph16040553
Chicago/Turabian StyleZhu, Hongyan, Rui Li, Su Zhou, Suhui Zhang, Yu Wang, Shihong Liu, Qingwen Song, Xiuli Chang, Yubin Zhang, Luqing Liu, and et al. 2019. "The Oral NOAEL of Flurochloridone in Male Wistar Rats in Ninety-Day Subchronic Toxicity Test Was 3mg/kg/day" International Journal of Environmental Research and Public Health 16, no. 4: 553. https://doi.org/10.3390/ijerph16040553
APA StyleZhu, H., Li, R., Zhou, S., Zhang, S., Wang, Y., Liu, S., Song, Q., Chang, X., Zhang, Y., Liu, L., Tang, L., & Zhou, Z. (2019). The Oral NOAEL of Flurochloridone in Male Wistar Rats in Ninety-Day Subchronic Toxicity Test Was 3mg/kg/day. International Journal of Environmental Research and Public Health, 16(4), 553. https://doi.org/10.3390/ijerph16040553





