Study on Analysis and Sedimentation of Alumina Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis of Nano-Al2O3 in Artificial Seawater
2.2.1. Study of Optimum Dispersion Conditions
2.2.2. Parameters of UV Spectrophotometry
2.2.3. Method Comparing between Gravimetry and UV Spectrophotometry
2.3. Nano-Al2O3 Settlement Experiments
3. Results and Discussion
3.1. Analysis Results of Nano-Al2O3 in Artificial Seawater
3.1.1. Optimal Dispersion Conditions
3.1.2. Parameters of UV Spectrophotometry
3.1.3. Difference between Gravimetry and UV Spectrophotometry
3.2. Results of Settlement Experiment of Nano-Al2O3
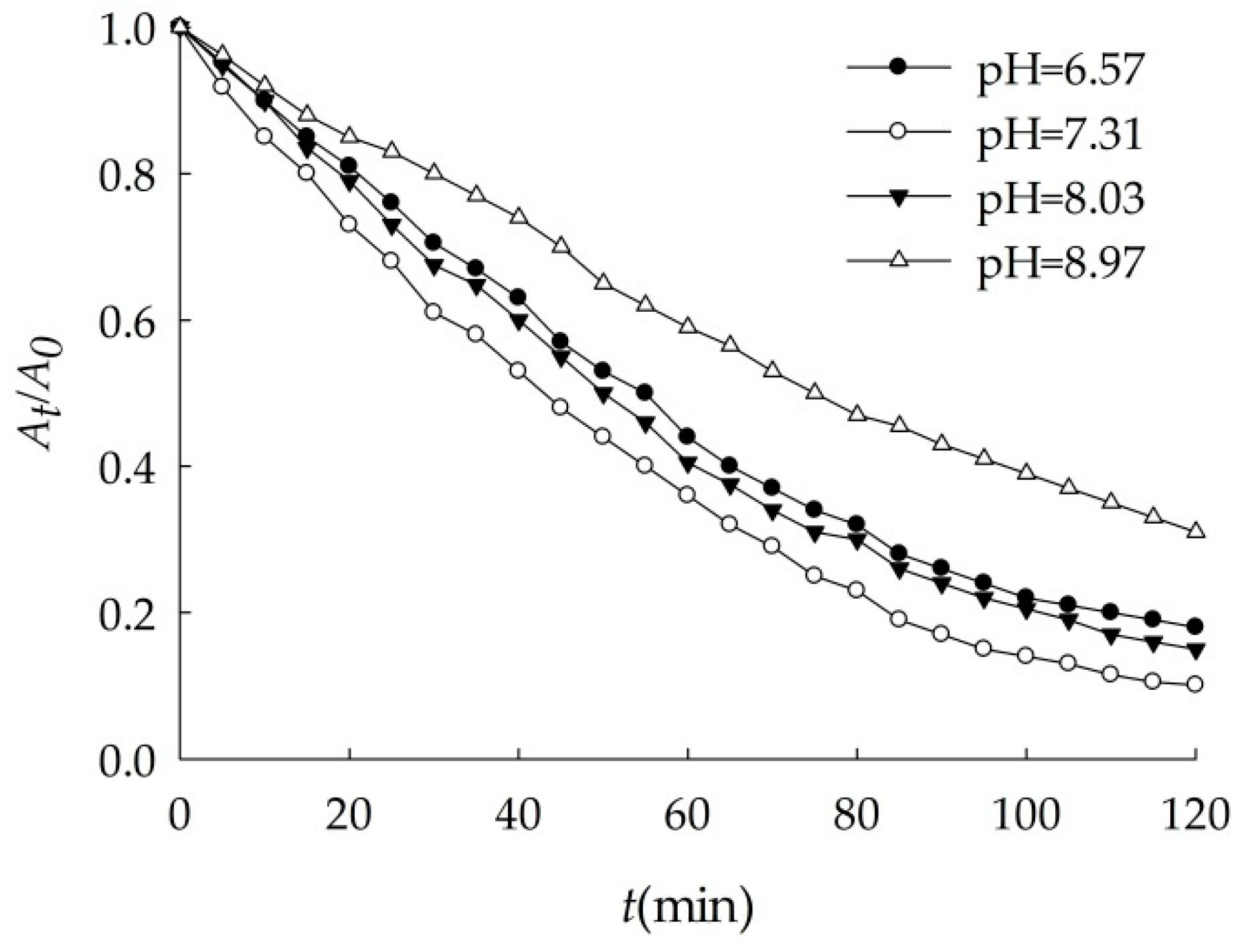
3.2.1. Effects of pH
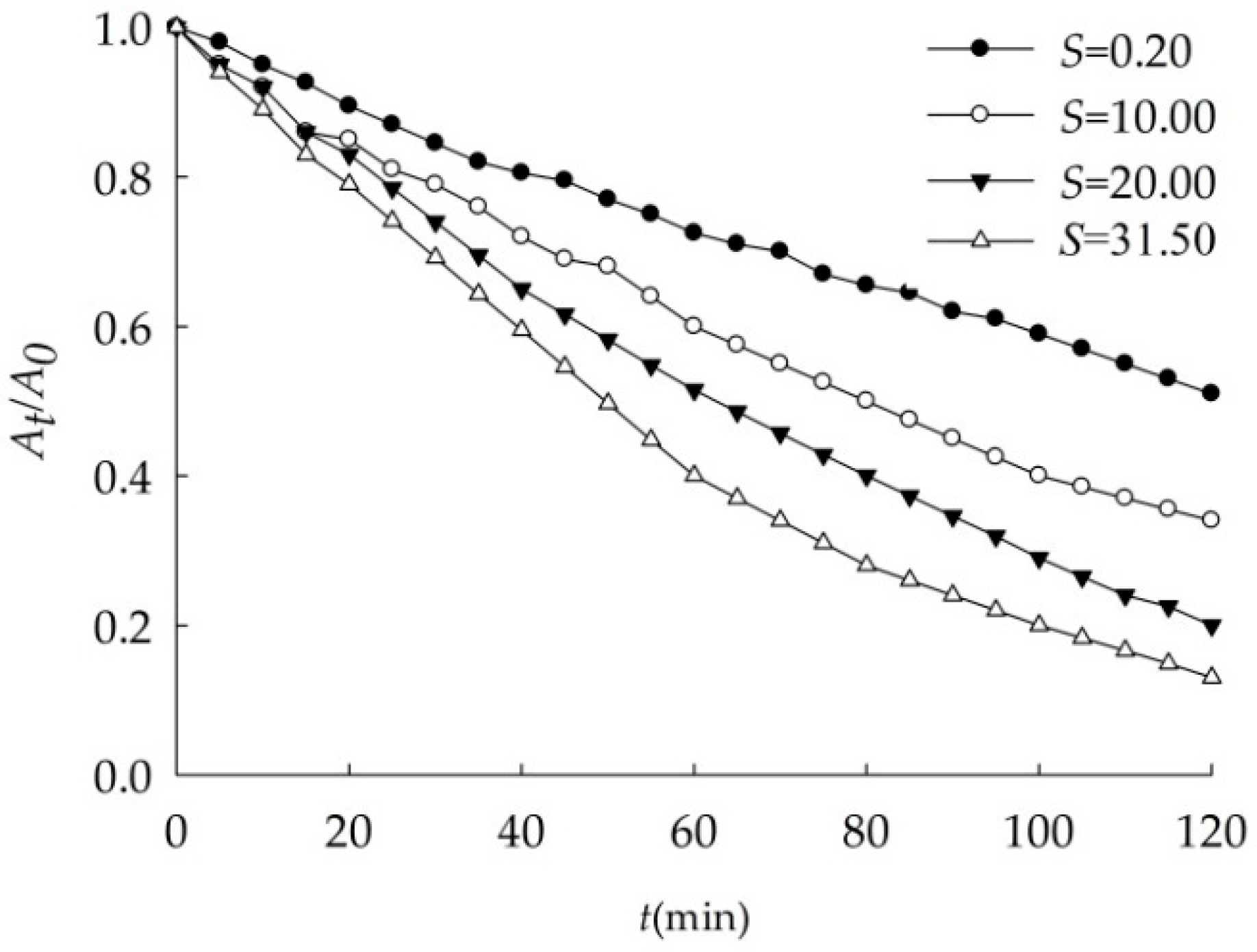
3.2.2. Effects of Salinity
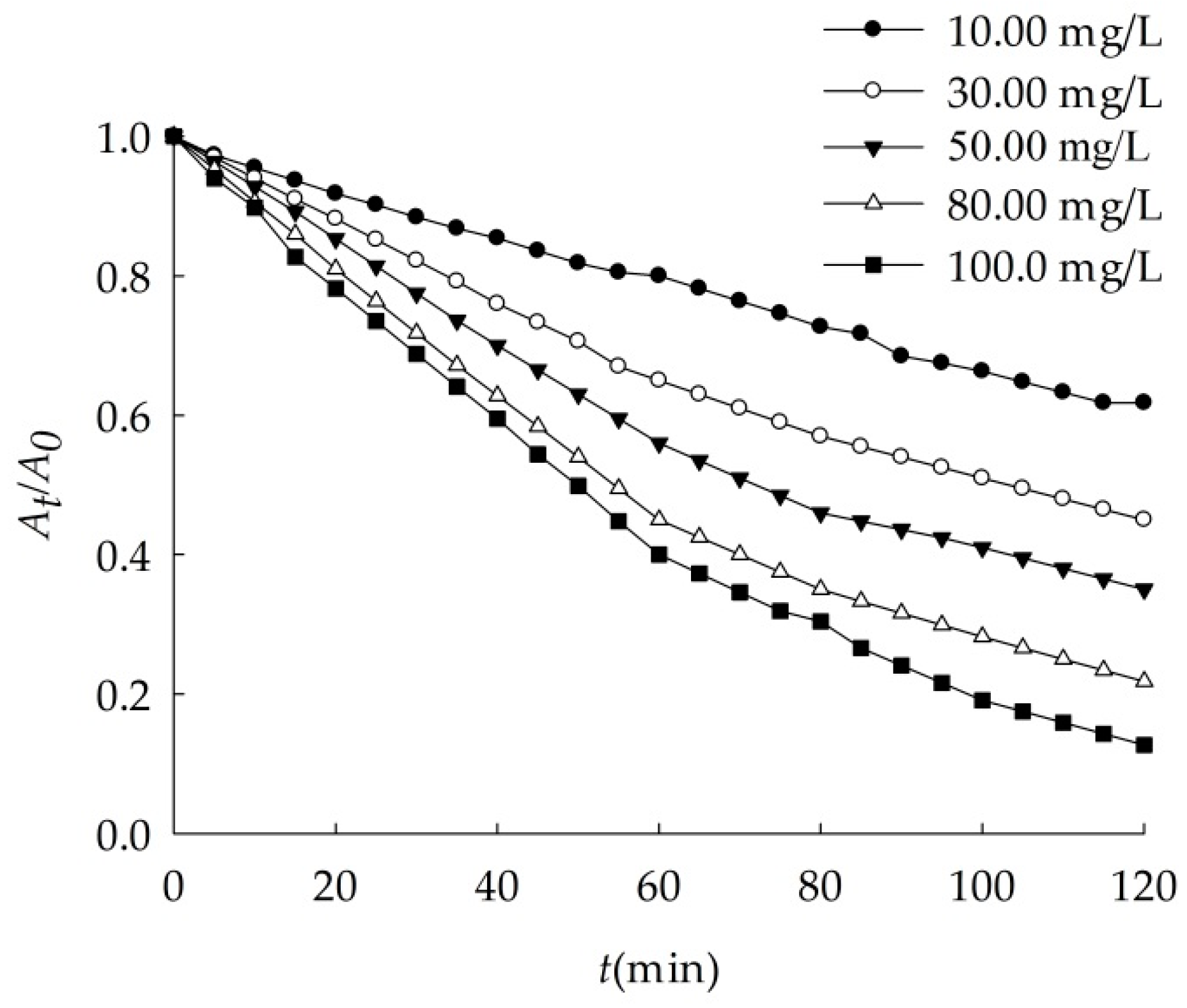
3.2.3. Effects of Nano-Al2O3 Concentration
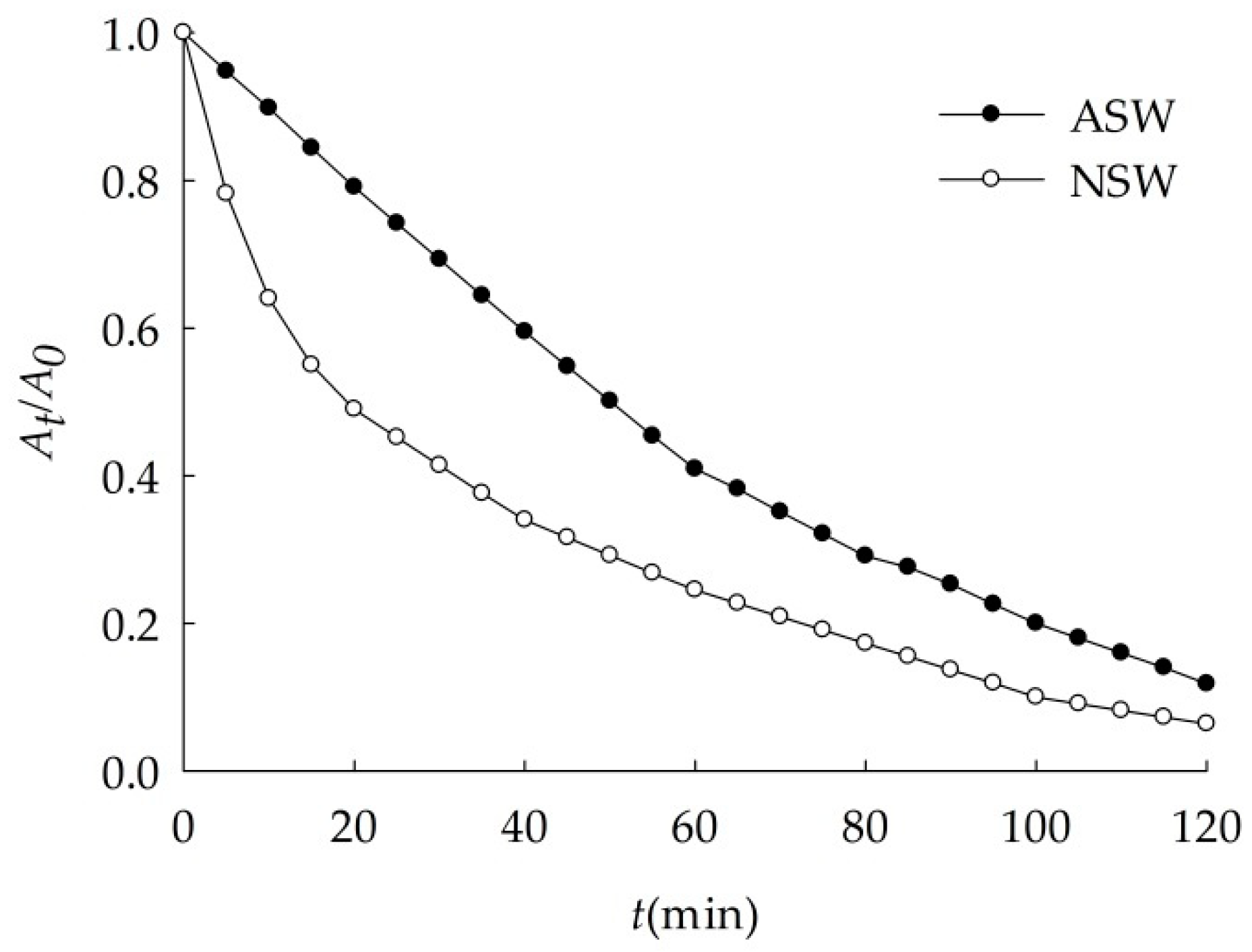
3.2.4. Sedimentation of Nano-Al2O3 in Natural Seawater
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdorster, G.; Philbert, M.A.; Ryan, J.; Seaton, A.; Stone, V.; et al. Safe handling of nanotechnology. Nature 2006, 444, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, M.R.; Lowry, G.V.; Alvarez, P.; Dionysiou, D.; Biswas, P. Assessing the risks of manufactured nanomaterials. Environ. Sci. Technol. 2006, 40, 4336–4345. [Google Scholar] [CrossRef]
- Murali, M.; Suganthi, P.; Athif, P.; Bukhari, A.S.; Mohamed, H.E.S.; Basu, H.; Singhal, R.K. Histological alterations in the hepatic tissues of Al2O3 nanoparticles exposed freshwater fish Oreochromis mossambicus. J. Trace Elem. Med. Biol. 2017, 44, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, X.; Chen, Q.; Yin, D. Ecotoxicology of nanomaterials on aquatic organisms. Environ. Chem. 2011, 30, 1993–2002. [Google Scholar]
- Matranga, V.; Corsi, I. Toxic effects of engineered nanoparticles in the marine environment: Model organisms and molecular approaches. Mar. Environ. Res. 2012, 76, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Corsi, I. Effects of nanomaterials on marine invertebrates. Sci. Total Environ. 2016, 565, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef]
- Brayner, R.; Dahaumane, S.A.; Yepremian, C.; Djediat, C.; Meyer, M.; Coute, A.; Fievet, F. ZnO nanoparticles: Synthesis, characterization, and ecotoxicological studies. Langmuir 2010, 26, 6522–6528. [Google Scholar] [CrossRef]
- Pakrashi, S.; Kumar, D.; Iswarya, V.; Bhuvaneshwari, M.; Chandrasekaran, N.; Mukherjee, A. A comparative ecotoxicity ananlysis of α- and γ- phase aluminium oxide nanoparticles towards a freshwater bacterial isolate Bacillus licheniformis. Bioprocess Biosyst. Eng. 2014, 37, 2415–2423. [Google Scholar] [CrossRef]
- Wang, D. Development and use of nano-alumina. Adv. Ceram. 2012, 164, 10–18. [Google Scholar]
- Doskocz, N.; Affek, K.; Zaleska-Radziwill, M. Effects of aluminium oxide nanoparticles on bacterial growth. In Proceedings of the E3S Web of Conferences, 9th Conference on Interdisciplinary Problems in Environmental Protection and Engineering (EKO-DOK), Boguszow Gorce, Poland, 23–25 April 2017. [Google Scholar]
- Wiesner, M.R.; Lowry, G.V.; Jones, K.L.; Hochella, M.F.; Di Giulio, R.T.; Casman, E.; Bernhardt, E.S. Decreasing uncertainties in assessing environmental exposure, risk, and ecological implications of nanomaterials. Environ. Sci. Technol. 2009, 43, 6458–6462. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Guo, L.; Chen, M. Adsorption and fractionation of thorium and protactinium on nanoparticles in seawater. Mar. Chem. 2014, 162, 50–59. [Google Scholar] [CrossRef]
- Van Hoecke, K.; De Schamphelaere, K.A.C.; Van der Meeren, P.; Smagghe, G.; Janssen, C.R. Aggregation and ecotoxicity of CeO2 nanoparticles in synthetic and natural waters with variable pH, organic matter concentration and ionic strength. Environ. Pollut. 2011, 159, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Omar, F.M.; Aziz, H.A.; Stoll, S. Aggregation and disaggregation of ZnO nanoparticles: Influence of Ph and adsorption of Suwannee River humic acid. Sci. Total Environ. 2014, 468–469, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, W.; Guo, X.; Lv, X. Research on the stability of nanoTiO2 in the system of water dispersion. Appl. Chem. Ind. 2009, 28, 267–269. [Google Scholar]
- Zhou, X.; Li, W.; He, L. Dispersion stability of nanoparticles and their assessment methods. Mater. Prot. 2005, 38, 72–74. [Google Scholar]
- Aureli, F.; D’Amato, M.; De Berardis, B.; Raggi, A.; Turco, A.C.; Cubadda, F. Investigating agglomeration and dissolution of silica nanoparticles in aqueous suspensions by dynamic reaction cell inductively coupled plasma-mass spectrometry in time resolved mode. J. Anal. At. Spectrom. 2012, 27, 1540–1548. [Google Scholar] [CrossRef]
- Chinnapongse, S.L.; MacCuspie, R.I.; Hackley, V.A. Persistence of singly dispersed silver nanoparticles in natural freshwaters, synthetic seawater, and simulated estuarine waters. Sci. Total Environ. 2011, 409, 2443–2450. [Google Scholar] [CrossRef]
- Sikder, M.; Lead, J.R.; Chandler, G.T.; Baalousha, M. A rapid approach for measuring silver nanoparticle concentration and dissolution in seawater by UV-Vis. Sci. Total Environ. 2018, 618, 597–607. [Google Scholar] [CrossRef]
- Yegin, B.A.; Lamprecht, A. Lipid nanocapsule size analysis by hydrodynamic chromatography and photon correlation spectroscopy. Int. J. Pharm. 2006, 320, 165–170. [Google Scholar] [CrossRef]
- Bidwell, J.P.; Spotte, S. Artificial Seawaters: Formulas and Method; Jones and Bartlett: Boston, MA, USA, 1985; Volume 349. [Google Scholar]
- Li, H. Comparison of several calculation methods of detection Limit. Chin. J. Spectrosc. Lab. 2010, 27, 2465–2469. [Google Scholar]
- GB 17378.4-2007. The Specification for Marine Monitoring: Part 4: Seawater Analysis; China Standards Press: Shenzhen, China, 2007; pp. 88–91. [Google Scholar]
- Abel, J.S.; Stangle, G.C.; Schilling, C.H.; Aksay, I.A. Sedimentation in flocculating colloidal suspensions. J. Mater. Res. 1994, 9, 451–461. [Google Scholar] [CrossRef]
- Singh, B.P.; Menchavez, R.; Takai, C.; Fuji, M.; Takahashi, M. Stability of dispersions of colloidal alumina particles in aqueous suspensions. J. Colloid Interface Sci. 2005, 291, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, N.; Fang, J. Dispersion and deposition of the suspensions of TiO2 nanoparticles in the presence of surfactant. J. Zhejiang Univ. Technol. 2012, 40, 595–598. [Google Scholar]
- Chen, Q.; Wang, J.; Li, W.; Yin, Z. Influence of dispersants on dispersion stability of super-fine alumina particles suspension. China Powder Sci. Technol. 2008, 14, 33–37. [Google Scholar]
- Wu, Q.; Yang, C.; Hu, X.; Dang, Z.; LI, Y. Influences of environmental factors on aggregation of titanium dioxide nanoparticles. Acta Sci. Circumst. 2012, 32, 1596–1603. [Google Scholar]
- Mudunkotuwa, I.A.; Grassian, V.H. Citric acid adsorption on TiO2 nanoparticles in aqueous suspensions at acidic and circumneutral pH: Surface coverage, surface speciation, and its impact on nanoparticle-nanoparticle interactions. J. Am. Chem. Soc. 2010, 132, 14986–14994. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kanno, Y.; Xie, Z.P. Fabrication of alumina green body through gelcasting process using alginate. Mater. Lett. 2003, 57, 2530–2534. [Google Scholar] [CrossRef]
- Akhondi, H.; Taheri-Nassaj, E.; Sarpoolaky, H.; Taavoni-Gilan, A. Gelcasting of alumina nanopowders based on gelation of sodium alginate. Ceram. Int. 2009, 35, 1033–1037. [Google Scholar] [CrossRef]
- Domingos, R.F.; Tufenkji, N.; Wilkinson, K.J. Aggregation of titanium dioxide nanoparticles: Role of a fulvic acid. Environ. Sci. Technol. 2009, 43, 1282–1286. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.S.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Martin, J.M.; Cauwet, G. The significant role of colloids in the transport and transformation of organic carbon and associated trace metals (Cd, Cu and Ni) in the Rhône delta (France). Mar. Chem. 1995, 51, 159–175. [Google Scholar] [CrossRef]
- Hua, J.; Yuan, J.; Sheng, G. Aggregation and sedimentation of metal oxides nanoparticles in aquatic environment. Environ. Sci. Technol. 2016, 39, 17–22. [Google Scholar]
- Van Koetsem, F.; Verstraete, S.; Vander Meeren, P.; Du Laing, G. Stability of engineered nanomaterials in complex aqueous matrices: Settling behavior of CeO2 nanoparticles in natural surface waters. Environ. Res. 2015, 142, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Johnson, G.O.; Nurmi, J.T.; Tratnyek, P.G. Natural organic matter enhanced mobility of nano zerovalent iron. Environ. Sci. Technol. 2009, 43, 5455–5460. [Google Scholar] [CrossRef] [PubMed]
- Pelley, A.J.; Tufenkji, N. Effect of particle size and natural organic matter on the migration of nano-and microscale latex particles in saturated porous media. J. Colloid Interface Sci. 2008, 321, 74–83. [Google Scholar] [CrossRef]
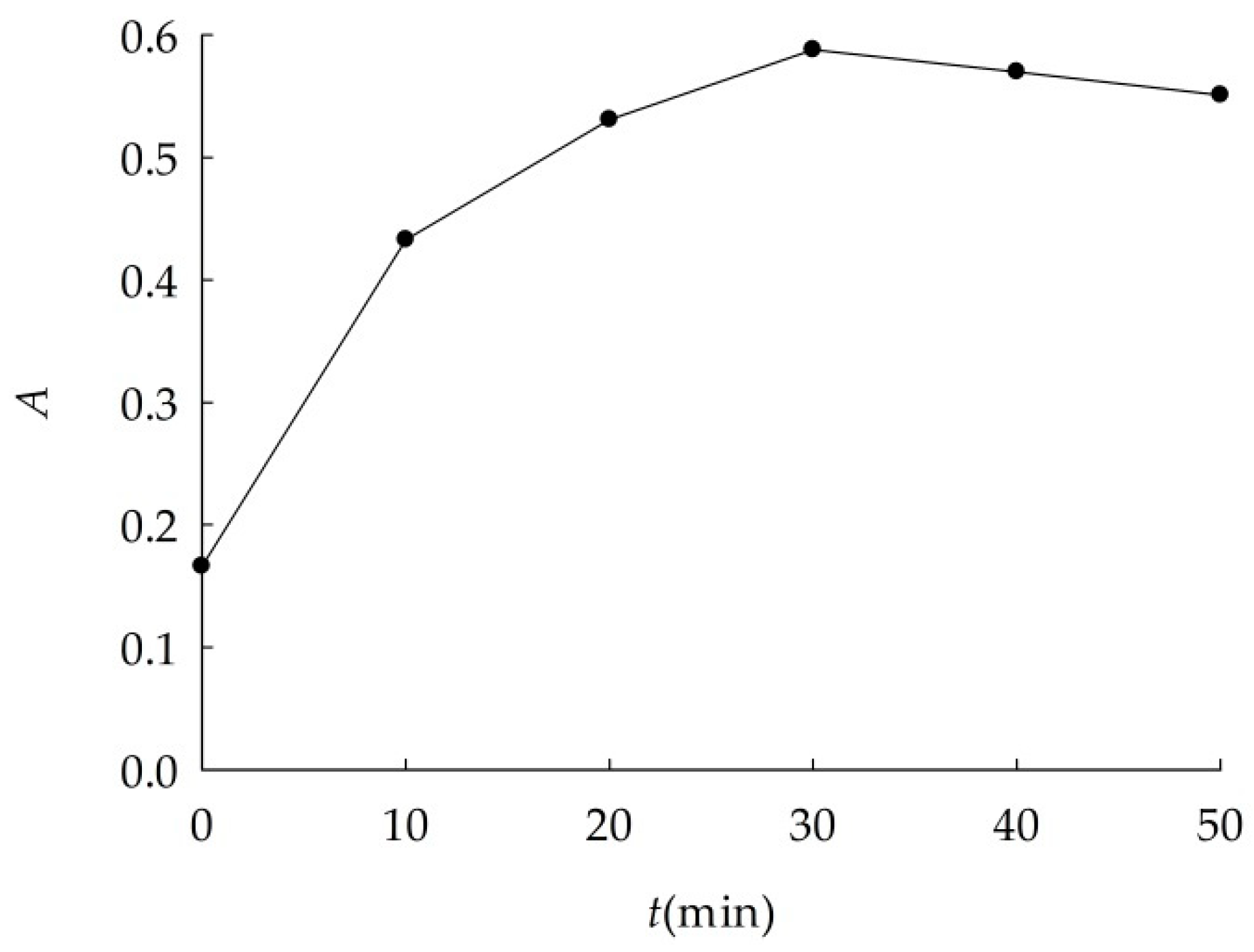
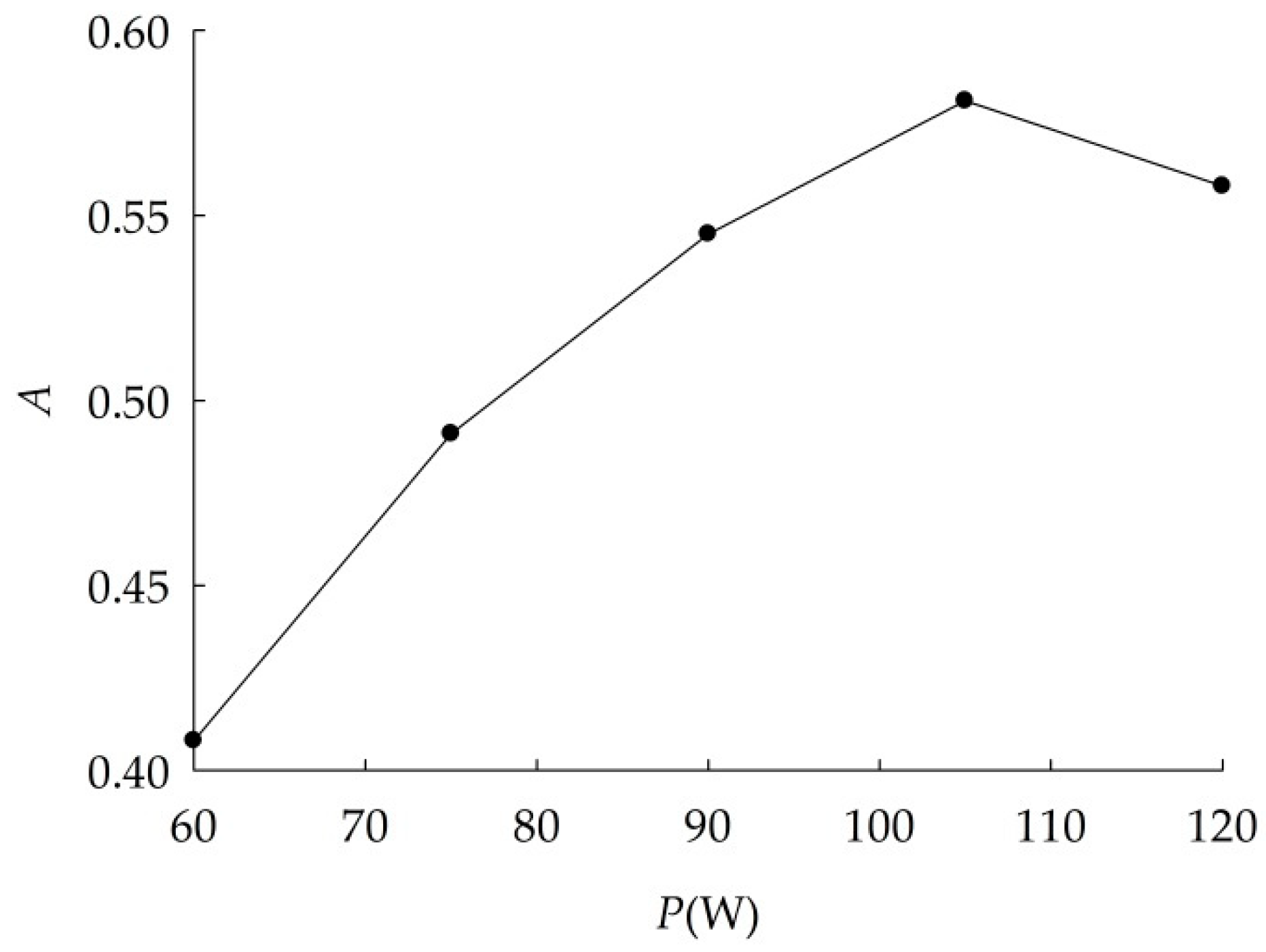
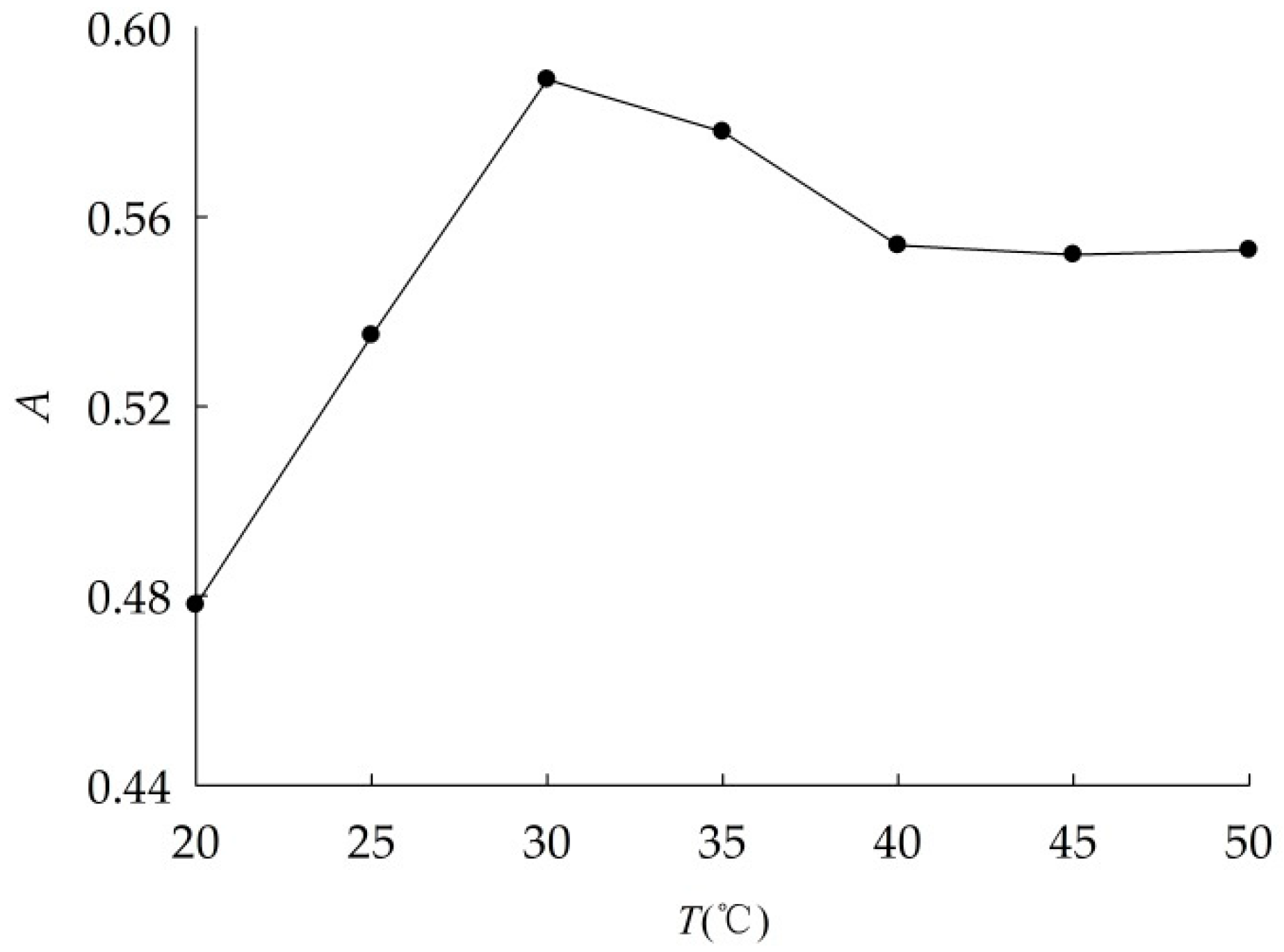
| No. | Constant Conditions | Varied Conditions |
|---|---|---|
| 1 | P = 105 W, T = 30 °C | t (min) = 0, 10, 20, 30, 40, 50 |
| 2 | t = 30 min, T = 30 °C | P (W) = 60, 75, 90, 105, 120 |
| 3 | P = 105 W, t = 30 min | T (°C) = 20, 25, 30, 35, 40, 45, 50 |
| No. | Constant Conditions. | Varied Conditions |
|---|---|---|
| 1 | S = 31.5, C (Al2O3, mg/L) = 100.0 | pH = 6.57, 7.31, 8.03, 8.97 |
| 2 | pH = 8.00, C (Al2O3, mg/L) = 100.0 | S = 0.2, 10.0, 20.0, 31.5 |
| 3 | S = 31.5, pH = 8.00 | C (Al2O3, mg/L) = 10.00, 30.00, 50.00, 80.00, 100.0 |
| Initial Concentration (mg/L) | Additive (mg/L) | Result (mg/L) | Recovery (%) |
|---|---|---|---|
| 5.00 | 14.90 | 106.4 | |
| 9.00 | 10.00 | 19.82 | 104.3 |
| 15.00 | 25.13 | 104.7 | |
| 10.00 | 28.44 | 97.4 | |
| 19.21 | 20.00 | 39.93 | 101.8 |
| 30.00 | 50.13 | 101.9 | |
| 20.00 | 59.90 | 99.0 | |
| 40.52 | 30.00 | 70.91 | 100.6 |
| 40.00 | 81.20 | 100.8 |
| Actual Concentration (mg/L) | Testing Concentration (mg/L) | RSD (%) | Relative Deviation (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| 10.00 | 8.49 | 8.25 | 8.73 | 8.83 | 8.97 | 8.49 | 3.1 | 13.7 |
| 50.00 | 49.50 | 51.90 | 48.05 | 54.08 | 51.91 | 46.60 | 5.5 | 0.7 |
| 100.0 | 100.2 | 105.7 | 100.9 | 104.0 | 102.6 | 100.4 | 2.2 | 2.3 |
| Actual Concentration (mg/L) | Gravimetry | UV Spectrophotometry | ||
|---|---|---|---|---|
| Testing Concentration (mg/L) | Relative Deviation (%) | Testing Concentration (mg/L) | Relative Deviation (%) | |
| 10.00 | 7.12 | 26.0 | 8.97 | 13.2 |
| 6.57 | 8.25 | |||
| 8.51 | 8.83 | |||
| 50.00 | 45.12 | 13.0 | 49.50 | 2.2 |
| 41.06 | 51.91 | |||
| 44.32 | 51.90 | |||
| 100.0 | 88.96 | 9.3 | 100.2 | 0.5 |
| 90.68 | 100.9 | |||
| 92.34 | 100.4 | |||
| pH | k (min−1) | r |
|---|---|---|
| 6.57 | 0.016 | 0.9970 |
| 7.31 | 0.021 | 0.9955 |
| 8.03 | 0.019 | 0.9954 |
| 8.97 | 0.010 | 0.9979 |
| S | k (min−1) | r |
|---|---|---|
| 0.2 | 0.006 | 0.9970 |
| 10.0 | 0.010 | 0.9972 |
| 20.0 | 0.013 | 0.9970 |
| 31.5 | 0.019 | 0.9954 |
| C (Al2O3, mg/L) | k (min−1) | r |
|---|---|---|
| 10.00 | 0.007 | 0.9943 |
| 30.00 | 0.008 | 0.9980 |
| 50.00 | 0.011 | 0.9961 |
| 80.00 | 0.015 | 0.9974 |
| 100.0 | 0.019 | 0.9954 |
| Medium | k (min−1) | r |
|---|---|---|
| NSW | 0.021 | 0.9957 |
| ASW | 0.019 | 0.9954 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Li, Y.; Chen, D.; Zheng, A.; Que, Q. Study on Analysis and Sedimentation of Alumina Nanoparticles. Int. J. Environ. Res. Public Health 2019, 16, 510. https://doi.org/10.3390/ijerph16030510
Zheng X, Li Y, Chen D, Zheng A, Que Q. Study on Analysis and Sedimentation of Alumina Nanoparticles. International Journal of Environmental Research and Public Health. 2019; 16(3):510. https://doi.org/10.3390/ijerph16030510
Chicago/Turabian StyleZheng, Xuehong, Yuehan Li, Ding Chen, Airong Zheng, and Qikang Que. 2019. "Study on Analysis and Sedimentation of Alumina Nanoparticles" International Journal of Environmental Research and Public Health 16, no. 3: 510. https://doi.org/10.3390/ijerph16030510
APA StyleZheng, X., Li, Y., Chen, D., Zheng, A., & Que, Q. (2019). Study on Analysis and Sedimentation of Alumina Nanoparticles. International Journal of Environmental Research and Public Health, 16(3), 510. https://doi.org/10.3390/ijerph16030510





