Occurrence and Concentration of Chemical Additives in Consumer Products in Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Sample Collection
2.3. Sample Preparation
2.4. Liquid Chromatography—High-Resolution Mass Spectrometry
2.5. Quality Assurance and Quality Control
2.6. Data Processing
2.6.1. Target Screening
2.6.2. Suspect Screening
2.6.3. Non-Target Screening
3. Results and Discussion
3.1. Target Screening
3.1.1. Isothiazolinones
3.1.2. Phthalates
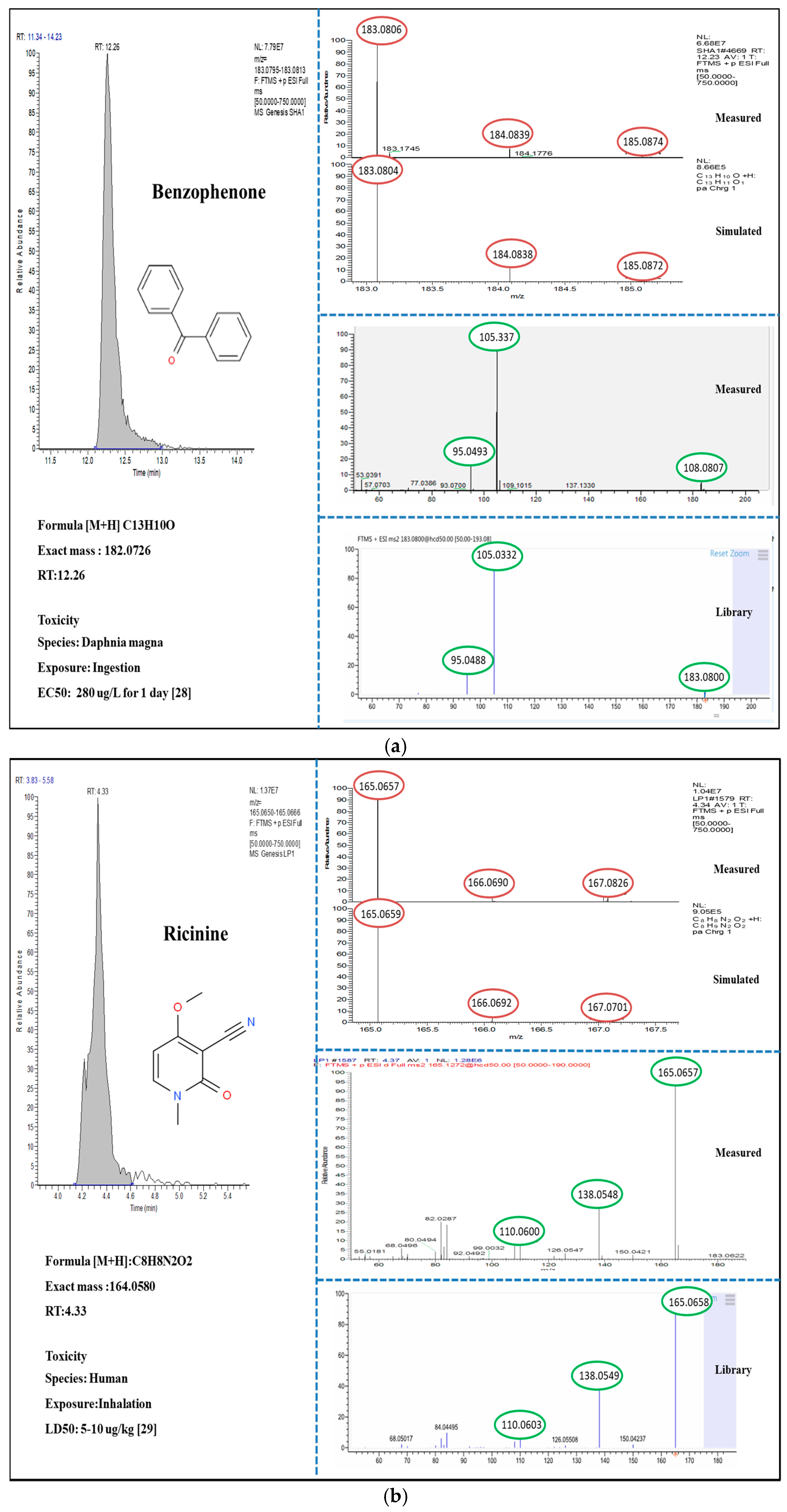
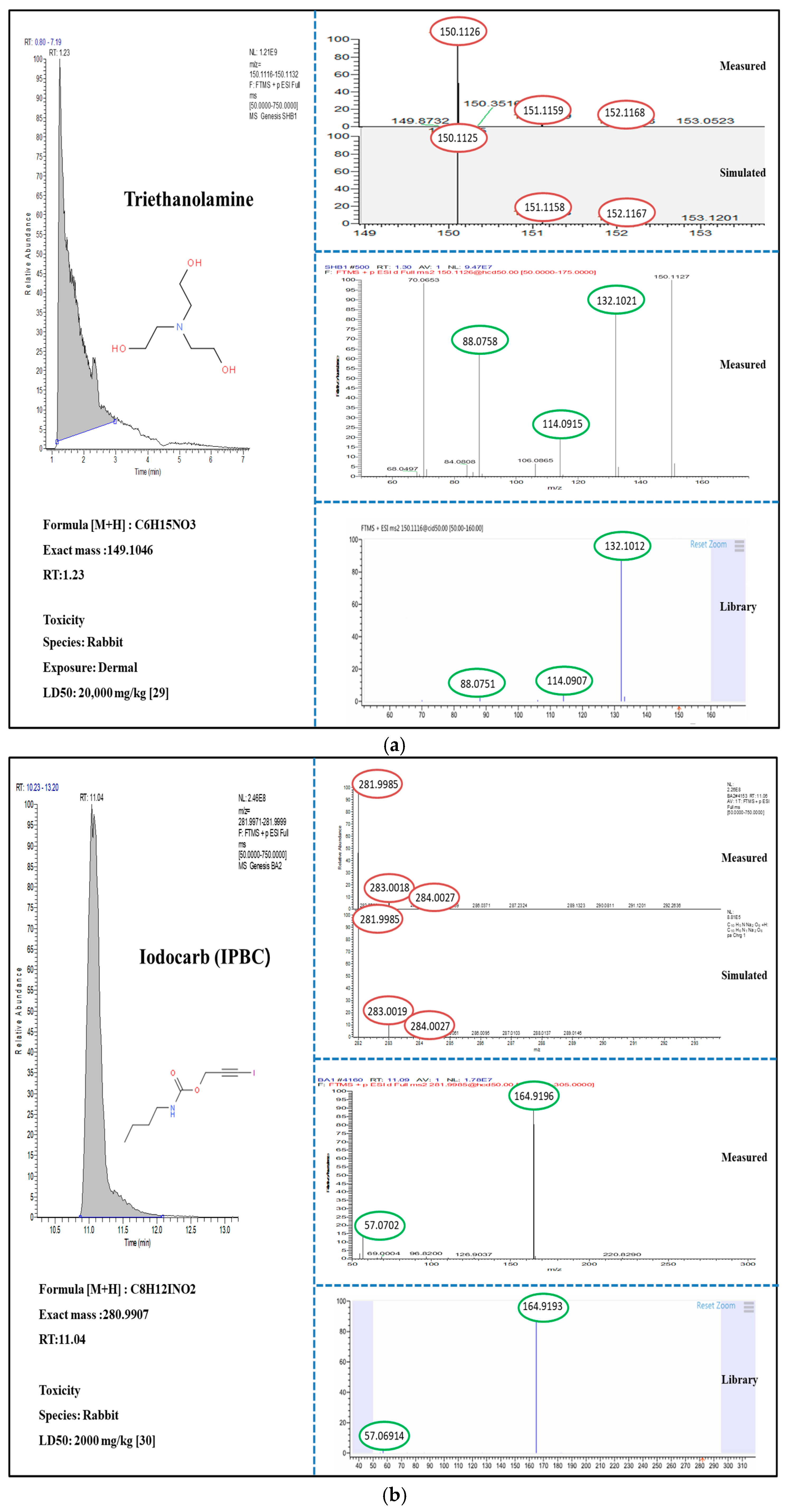
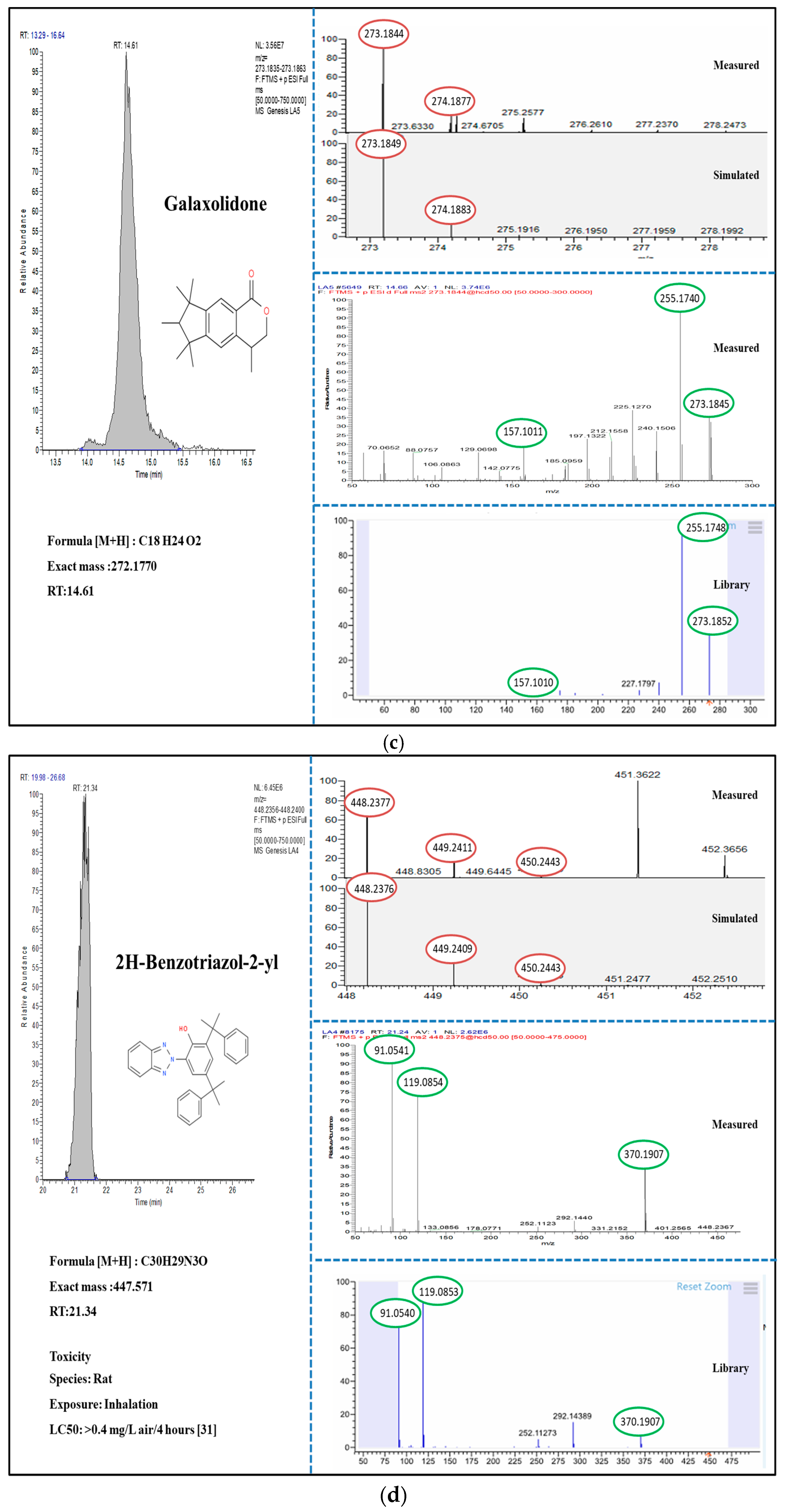
3.2. Suspect and Non-Target Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.H.; Kim, T.; Yoon, H.; Jo, A.; Lee, D.; Kim, P.; Seo, J. Health risk assessment of dermal and inhalation exposure to deodorants in Korea. Sci. Total Environ. 2018, 625, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.A.; Yau, A.; Favela, K.; Isaacs, K.; McEachran, A.; Grulke, C.; Richard, A.; Williams, A.; Sobus, J.; Thomas, R.; et al. Suspect Screening Analysis of Chemicals in Consumer Products. Environ. Sci. Technol. 2018, 52, 3125–3135. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, K.; Hwang, Y.; Kim, J.H. Determining the exposure factors of personal and home care products for exposure assessment. Food Chem. Toxicol. 2015, 77, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Rafoth, A.; Gabriel, S.; Sacher, F. Analysis of isothiazolinones in environmental waters by gas chromatography—Mass spectrometry. J. Chromatogr. A 2007, 1164, 74–81. [Google Scholar] [CrossRef]
- Lu, S.; Yu, Y.; Ren, L.; Zhang, X.; Liu, G.; Yu, Y. Estimation of intake and uptake of bisphenols and triclosan from personal care products by dermal contact. Sci. Total Environ. 2017, 621, 1389–1396. [Google Scholar] [CrossRef]
- Huang, L.; Ernstoff, A.; Fantke, P.; Csiszar, S.A.; Jolliet, O. A review of models for near- fi eld exposure pathways of chemicals in consumer products. Sci. Total Environ. 2017, 574, 1182–1208. [Google Scholar] [CrossRef]
- Garcia-hidalgo, E.; Von Goetz, N.; Siegrist, M.; Hungerbühler, K. Use-patterns of personal care and household cleaning products in Switzerland. Food Chem. Toxicol. 2017, 99, 24–39. [Google Scholar] [CrossRef]
- Maier, A.; Vincent, M.J.; Gadagbui, B.; Patterson, J.; Beckett, W.; Dalton, P.; Kimber, I.; Selgrade, M.J.K. Integrating asthma hazard characterization methods for consumer products. Regul. Toxicol. Pharmacol. 2014, 70, 37–45. [Google Scholar] [CrossRef]
- Wong, K.H.; Durrani, T.S. Exposures to Endocrine Disrupting Chemicals in Consumer Products—A Guide for Pediatricians. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 107–118. [Google Scholar] [CrossRef]
- Liu, W.; Zho, J.L.; Liu, Y.S.; Chen, Z.-F.; Yang, Y.-Y.; Zhang, Q.-Q.; Ying, G.-G. Biocides in the Yangtze River of China: Spatiotemporal distribution, mass load and risk assessment. Environ. Pollut. 2015, 200, 53–63. [Google Scholar] [CrossRef]
- Tao, M.; Li, D.; Song, R.; Suh, S.; Keller, A.A. OrganoRelease e A framework for modeling the release of organic chemicals from the use and post-use of consumer products. Environ. Pollut. 2018, 234, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, Y.; Liu, Y.; Zhao, J.; Zhang, Q.; Yao, L. Biocides in the river system of a highly urbanized region: A systematic investigation involving runoff input. Sci. Total Environ. 2018, 624, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.E.; Nishioka, M.; Standley, L.J.; Perovich, L.J.; Brody, J.G. Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products. Environ Health Perspect. 2012, 120, 935–943. [Google Scholar] [CrossRef]
- Krauss, M.; Singer, H.; Hollender, J. LC—high resolution MS in environmental analysis: From target screening to the identification of unknowns. Anal. Bioanal. Chem. 2010, 397, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Boix, C.; Ibanez, M.; Bagnati, R.; Zuccato, E.; Hernandez, F.; Castiglioni, S. High resolution mass spectrometry to investigate omeprazole and venlafaxine metabolites in wastewater. J. Hazard. Mater. 2016, 302, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, L.; Emke, E.; Hernández, F.; de Voogt, P. Investigation of drugs of abuse and relevant metabolites in Dutch sewage water by liquid chromatography coupled to high resolution mass spectrometry. Chemosphere 2012, 89, 1399–1406. [Google Scholar] [CrossRef]
- Hug, C.; Ulrich, N.; Chulze, T.; Brack, S.W.; Krauss, M. Identification of novel micropollutants in wastewater by a combination of suspect and nontarget screening. Environ. Pollut. 2014, 184, 25–32. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. A Survey of Phthalates and Parabens in Personal Care Products from the United States and Its Implications for Human Exposure. Environ. Sci. Technol. 2013, 4, 14442–14449. [Google Scholar] [CrossRef]
- Bletsou, A.A.; Jeon, J.; Hollender, J.; Archontaki, E.; Thomaidis, N.S. Targeted and non-targeted liquid chromatography-mass spectrometric workflows for identification of transformation products of emerging pollutants in the aquatic environment. Trends Anal. Chem. 2015, 66, 32–44. [Google Scholar] [CrossRef]
- Avagyan, R.; Åberg, M.; Westerholm, R. Suspect screening of OH-PAHs and non-target screening of other organic compounds in wood smoke particles using HR-Orbitrap-MS. Chemosphere 2016, 163, 313–321. [Google Scholar] [CrossRef]
- Chemical Watch. Available online: https://chemicalwatch.com/register?o=76687&productID=1&layout=main (accessed on 12 December 2019).
- Garcia-Hidalgo, E.; Sottas, V.; Von Goetz, N.; Hauri, U.; Bogdal, C.; Hungerbühler, K. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermat. 2016, 76, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Aerts, O.; Meert, H.; Goossens, A.; Janssens, S.; Lambert, J.; Apers, S. Methylisothiazolinone in selected consumer products in Belgium: Adding fuel to the fire? Contact Dermat. 2015, 73, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.M.; Nelson, M.L.; Unice, K.M.; Keenan, J.J.; Paustenbach, D.J. Estimation of the safe use concentrations of the preservative 1, 2-benzisothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem. Toxicol. 2013, 56, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Koniecki, D.R.; Wang, R.; Moody, R.P.; Zhu, J. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef]
- LIompart, M.; Celeiro, M.; Lamas, P.J.; Sanchez-prado, L.; Lores, M.; Garcia-jares, C. Analysis of plasticizers and synthetic musks in cosmetic and personal care products by matrix solid-phase dispersion gas chromatography—Mass spectrometry. J. Chromatogr. A. 2013, 1293, 10–19. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Benzophenone (accessed on 12 December 2019).
- Hazordious Substance Data Base (HDBS). Available online: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm (accessed on 12 December 2019).
- Cosmetics Ingreients Review. Available online: https://www.cirsafety.org/sites/default/files/butylcarbamate_rr.pdf (accessed on 12 December 2019).
- European Chemicals Agency (ECHA). Available online: https://echa.europa.eu/documents/10162/0a105049-c60d-c800-5ecd-a6eeb1f529d9 (accessed on 12 December 2019).
- Liao, C.; Kannan, K. Widespread Occurrence of Benzophenone-Type UV Light Filters in Personal Care Products from China and the United States: An Assessment of Human Exposure. Environ. Sci Technol. 2014, 48, 4103–4109. [Google Scholar] [CrossRef]
- Suzuki, T.; Kitamura, S.; Khota, R.; Sugihara, K.; Fujimoto, N.; Ohta, S. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicol. Appl. Pharmacol. 2005, 203, 9–17. [Google Scholar] [CrossRef]
- Gonzalez, H.; Farbrot, A.; Larkö, O.; Wennberg, A.M. Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation. Br. J. Dermatol. 2006, 154, 337–340. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.D.; Wei, D.; Du, Y. Acute toxicity formation potential of benzophenone-type UV filters in chlorination disinfection process. J. Environ. Sci. (China) 2014, 26, 440–447. [Google Scholar] [CrossRef]
- Johnson, W. Final report on the safety assessment of ricinus communis (castor) seed oil, hydrogenated castor oil, glyceryl ricinoleate, glyceryl ricinoleate SE, ricinoleic acid, potassium ricinoleate, sodium ricinoleate, zinc ricinoleate, cetyl ricinoleate, ethyl ric. Int. J. Toxicol. 2007, 26, 31–77. [Google Scholar]
- Isenberg, S.L.; Carter, M.D.; Miller, M.A.; Noras, A.I.; Carlsen, S.T.; Bulathsinghala, C.P.; Thomas, J.D.; Jhonsan, R.C. Quantification of Ricinine and Abrine in Human Plasma by HPLC-MS-MS: Biomarkers of Exposure to Ricin and Abrin. J. Anal. Toxicol. 2018, 42, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Gamer, A.O.; Rossbacher, R.; Kaufmann, W.; van Ravenzwaay, B. The Inhalation toxicity of di- and triethanolamine upon repeated exposure. Food Chem. Toxicol. 2008, 46, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Frauen, M.; Steinhart, H.; Rapp, C.; Hintze, U. Rapid quantification of iodopropynyl butylcarbamate as the preservative in cosmetic formulations using high-performance liquid chromatography-electrospray mass spectrometry. J. Pharm. Biomed. Anal. 2001, 25, 965–970. [Google Scholar] [CrossRef]
- Bryld, L.E.; Agner, T.S.; Rastogi, C.; Menne, T. Iodopropynyl butylcarbamate: A new contact allergen. Contact Dermat. 1997, 36, 156–158. [Google Scholar] [CrossRef]
- Busetti, F.; Ruff, M.; Linge, K.L. Target screening of chemicals of concern in recycled water. Environ. Sci. Water Res. Technol. 2015, 1, 659–667. [Google Scholar] [CrossRef]
- Kim, J.W.; Isobe, T.; Malarvannan, G.; Sudaryanto, A.; Chang, K.H.; Prudente, M.; Tanabe, S. Contamination of benzotriazole ultraviolet stabilizers in house dust from the Philippines: Implications on human exposure. Sci. Total Environ. 2012, 424, 174–181. [Google Scholar] [CrossRef]
- Park, N.; Choi, Y.; Kim, D.; Kim, K.; Jeon, J. Science of the Total Environment Prioritization of highly exposable pharmaceuticals via a suspect/non- target screening approach: A case study for Yeongsan River, Korea. Sci. Total Environ. 2018, 639, 570–579. [Google Scholar] [CrossRef]
| Products | MI | CMI | BIT | DEP | DMP |
|---|---|---|---|---|---|
| Shampoos (n = 12) | |||||
| SA1 | - a | - | - | <LOQ b | <LOQ |
| SA2 | - | - | - | - | 0.26 |
| SA3 | - | - | - | 0.4 | - |
| SC1 | - | - | 0.165 | - | - |
| D.F | 0% | 0% | 8% | 17% | 17% |
| Body Washer (n = 6) | |||||
| BWA1 | <LOQ | - | - | - | - |
| BWA2 | <LOQ | - | - | - | - |
| BWA3 | <LOQ | - | - | - | - |
| BWA4 | <LOQ | - | - | - | - |
| BWA5 | <LOQ | - | - | - | - |
| BWB1 | <LOQ | - | - | - | - |
| D.F | 100% | 0% | 0% | 0% | 0% |
| Face Cleansers (n = 12) | |||||
| FCA2 | - | - | - | - | 5.1 |
| FCA3 | - | - | - | - | 7.9 |
| FCA4 | - | - | - | - | 4.0 |
| FCA5 | - | - | - | - | 4.1 |
| FCC2 | - | - | <LOQ | - | - |
| D.F | 0% | 0% | 8% | 0% | 33% |
| Lipstick (n = 5) | |||||
| LP1 | - | - | - | 1.1 | 10 |
| LP2 | - | - | - | <LOQ | 12 |
| LP4 | - | - | - | - | - |
| LP5 | - | - | - | - | 21 |
| D.F | 0% | 0% | 0% | 40% | 80% |
| Hair Dyes (n = 5) | |||||
| D.F | 0% | 0% | 0% | 0% | 0% |
| Dish Washer (n = 15) | |||||
| DWB2 | - | - | - | 2.0 | - |
| D.F | 0% | 0% | 0% | 7% | 0% |
| Laundry detergent (n = 15) | |||||
| LDA1 | - | - | 103 | - | - |
| LDA2 | - | - | 92 | - | - |
| LDA3 | - | - | 103 | - | - |
| LDA4 | - | - | 7.6 | - | - |
| LDA5 | - | - | 49 | - | - |
| LDB1 | - | - | 390 | - | - |
| LDB2 | - | - | 509 | - | - |
| LDB3 | - | - | 518 | - | - |
| LDB4 | - | - | 419 | - | - |
| LDB5 | - | - | 509 | - | - |
| LDC1 | - | - | 125 | - | - |
| LDC2 | - | - | 121 | - | - |
| LDC3 | - | - | 78 | - | - |
| LDC4 | - | - | 68 | - | - |
| LDC5 | - | - | 1.2 | - | - |
| D.F | 0% | 0% | 100% | 0% | 0% |
| Fabric Softener (n = 15) | |||||
| FSA1 | 1.1 | 1.47 | 49 | 1.1 | - |
| FSA2 | - | 1.22 | 41 | - | <LOQ |
| FSA3 | - | 1.37 | 41 | - | <LOQ |
| FSA4 | - | - | 38 | - | <LOQ |
| FSA5 | - | - | 39 | - | <LOQ |
| FSB1 | - | <LOQ | 46 | - | - |
| FSB2 | - | <LOQ | 44 | - | - |
| FSB3 | - | <LOQ | 43 | - | - |
| FSB4 | - | <LOQ | 48 | - | - |
| FSB5 | - | 8.0 | 0.62 | - | - |
| D.F | 53% | 0% | 67% | 7% | 27% |
| All CPs | |||||
| Median | 1.4 | - | 49 | 1.0 | 5.1 |
| Average | 3 | - | 134 | 1.1 | 7.3 |
| Maximum | 7.1 | - | 518 | 2.0 | 21.2 |
| Minimum | 1.2 | - | 0.2 | 0.4 | 0.26 |
| D.F | 15% | 0% | 32% | 7% | 16% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardar, S.W.; Choi, Y.; Park, N.; Jeon, J. Occurrence and Concentration of Chemical Additives in Consumer Products in Korea. Int. J. Environ. Res. Public Health 2019, 16, 5075. https://doi.org/10.3390/ijerph16245075
Sardar SW, Choi Y, Park N, Jeon J. Occurrence and Concentration of Chemical Additives in Consumer Products in Korea. International Journal of Environmental Research and Public Health. 2019; 16(24):5075. https://doi.org/10.3390/ijerph16245075
Chicago/Turabian StyleSardar, Syed Wasim, Younghun Choi, Naree Park, and Junho Jeon. 2019. "Occurrence and Concentration of Chemical Additives in Consumer Products in Korea" International Journal of Environmental Research and Public Health 16, no. 24: 5075. https://doi.org/10.3390/ijerph16245075
APA StyleSardar, S. W., Choi, Y., Park, N., & Jeon, J. (2019). Occurrence and Concentration of Chemical Additives in Consumer Products in Korea. International Journal of Environmental Research and Public Health, 16(24), 5075. https://doi.org/10.3390/ijerph16245075






