The Beneficial Effects of Stingless Bee Honey from Heterotrigona itama against Metabolic Changes in Rats Fed with High-Carbohydrate and High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Kelulut Honey Preparation
2.2. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis of Kelulut Honey
2.3. Animals
2.4. Study Design
2.5. High-Carbohydrate and High-Fat Diet Preparation
2.6. Measurement of Body Weight
2.7. Physiological Parameters
2.8. Serum Fasting Lipid Profile Levels Measurement
2.9. Serum Insulin Level Measurement
2.10. Body Composition Measurements
2.11. Gross and Histomorphometry of the Adipose Tissue
2.12. Statistical Analysis
3. Results
3.1. Analysis of Kelulut Honey by LC-MS
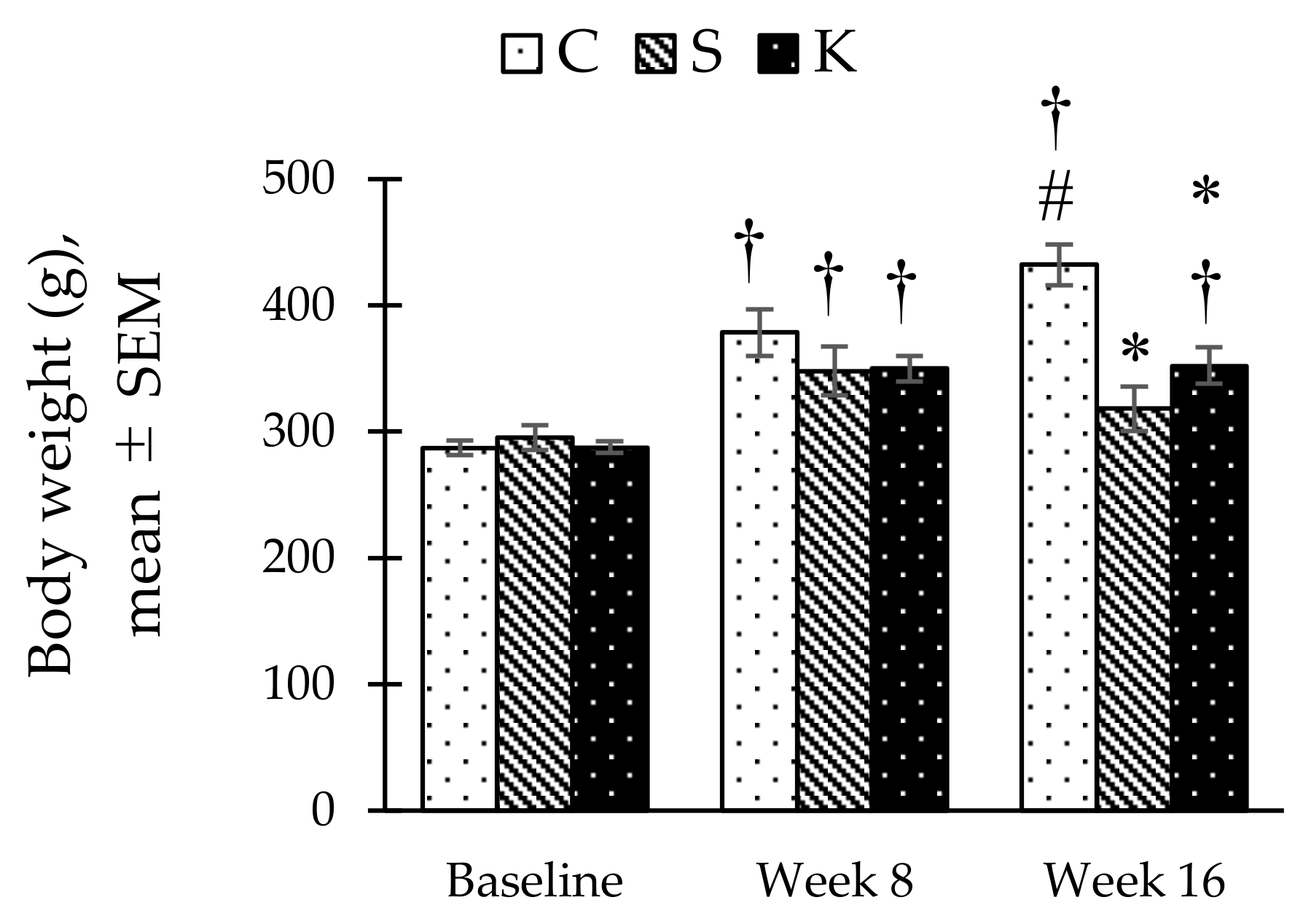
3.2. Changes in the Body Weight
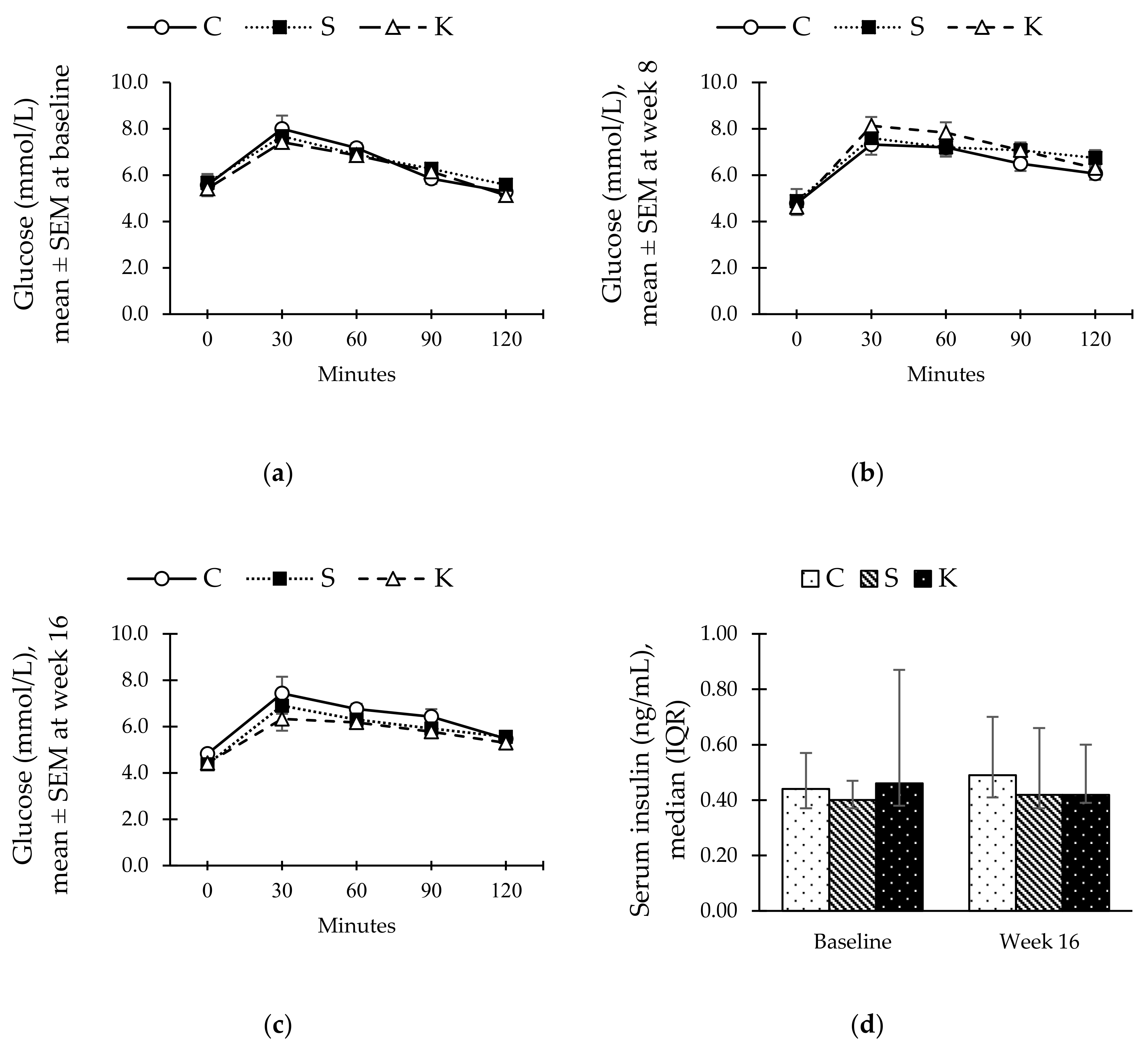
3.3. Changes in the Serum Insulin and Oral Glucose Tolerance Test
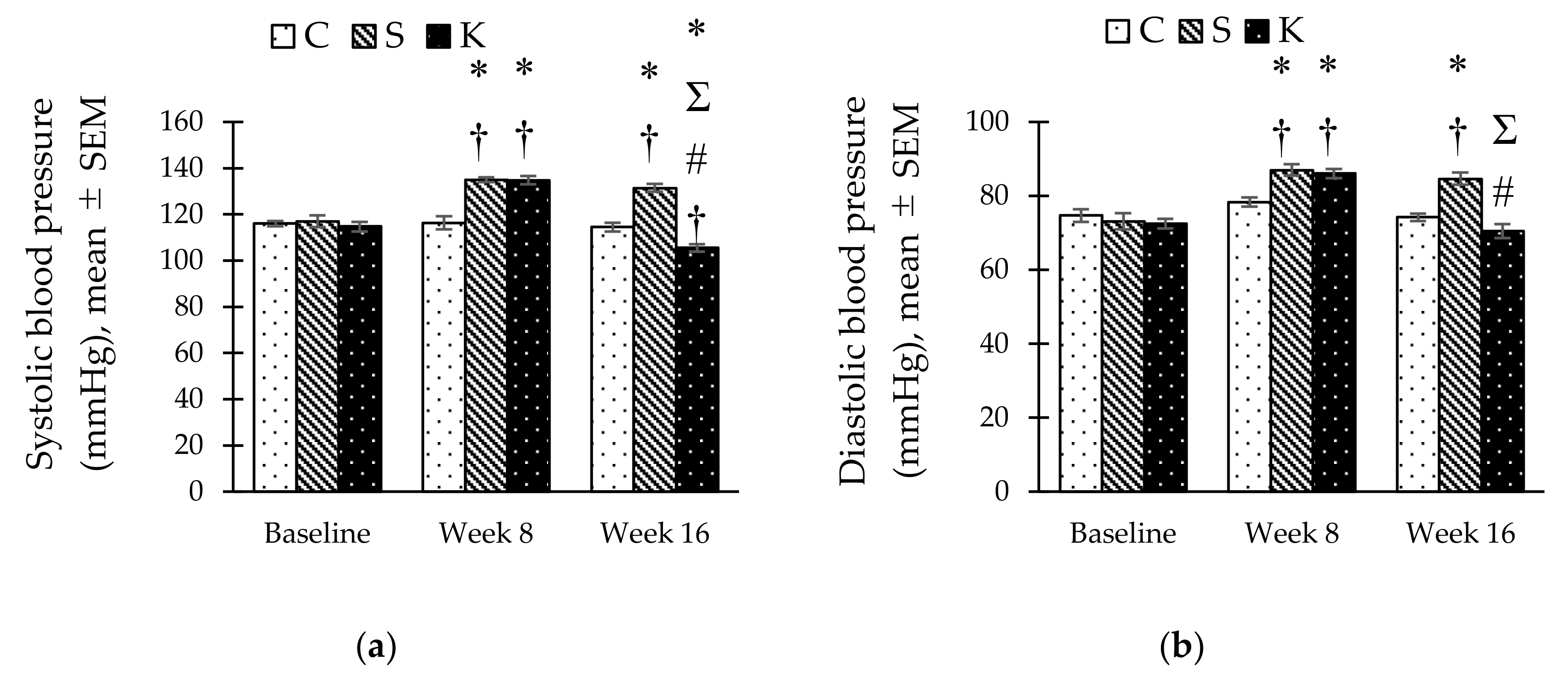
3.4. Changes in the Systolic and Diastolic Blood Pressure
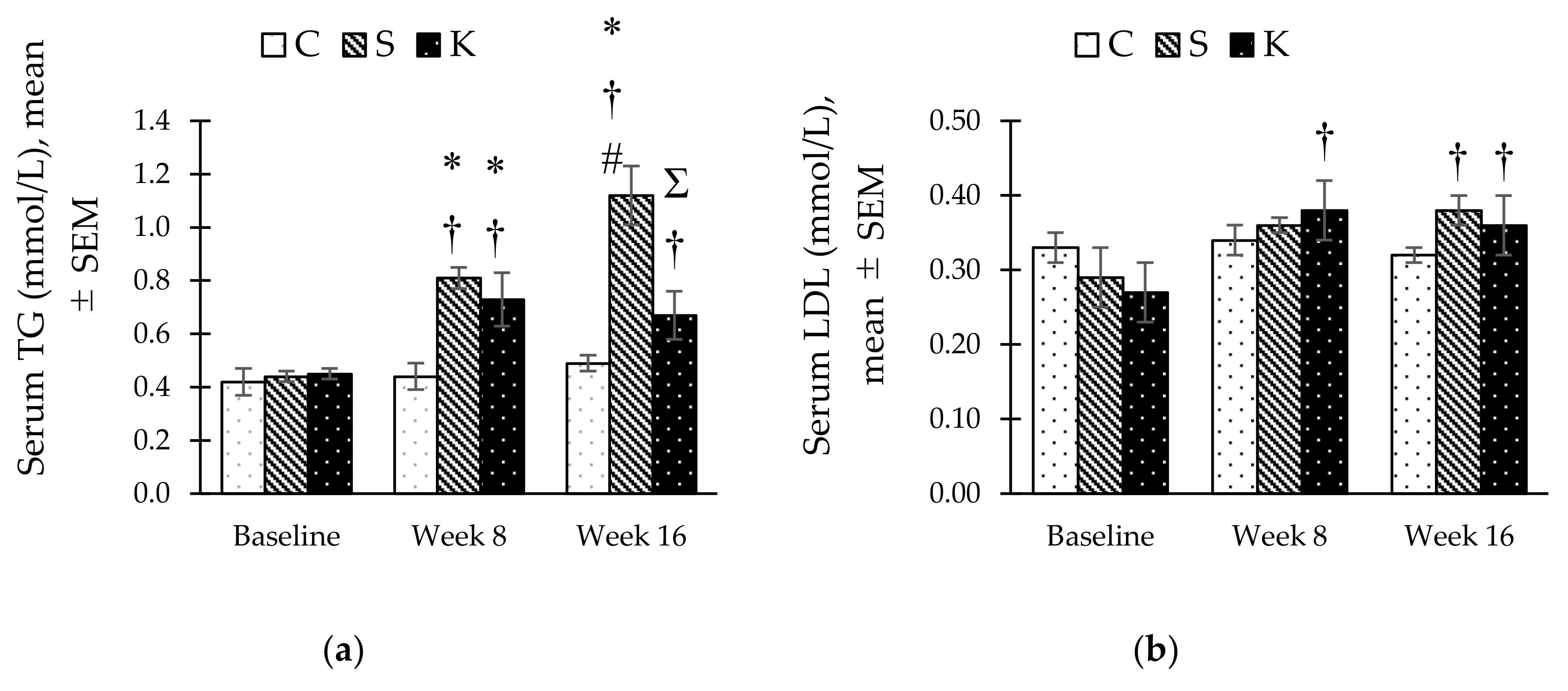
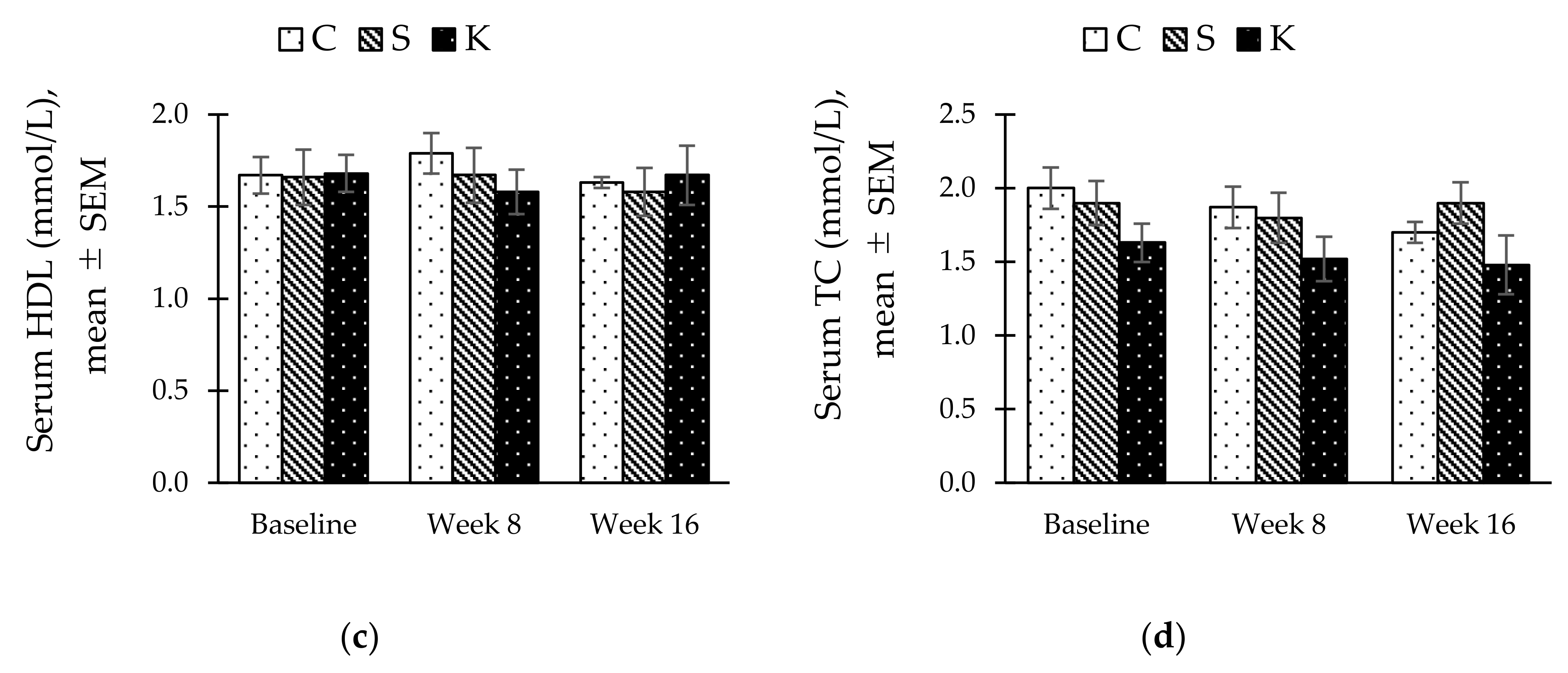
3.5. Changes in the Fasting Serum Lipid Profile
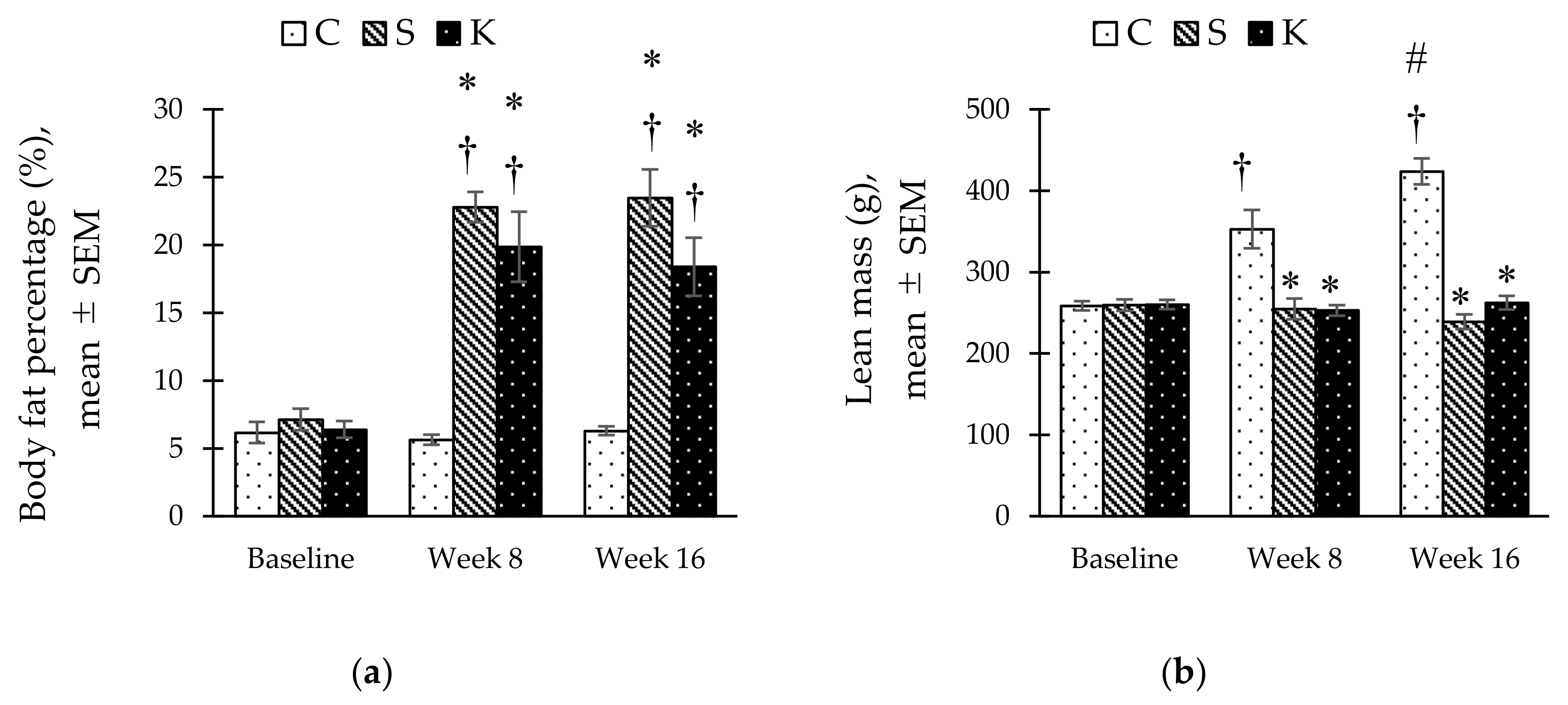
3.6. Changes in the Body Composition
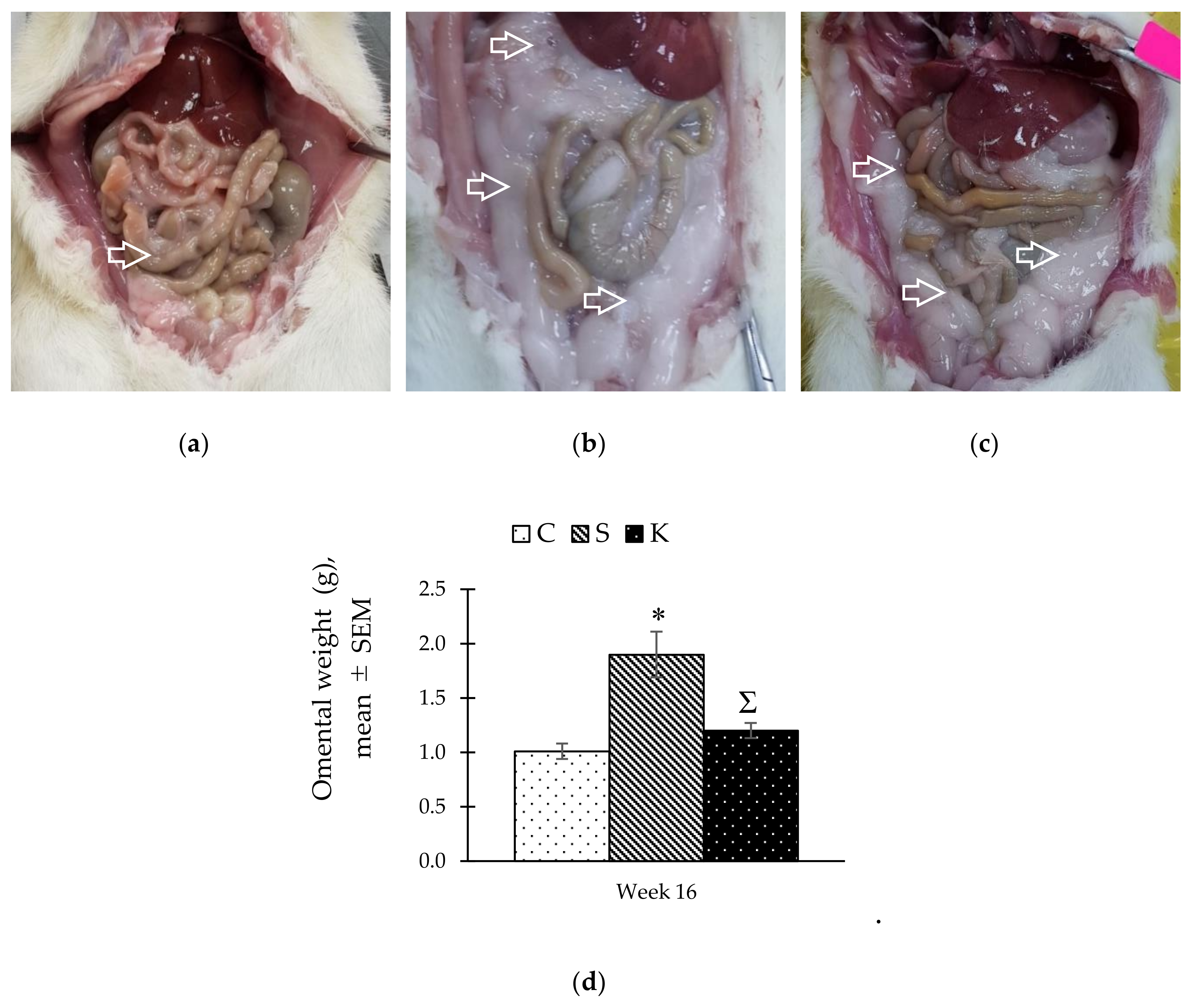
3.7. Changes in the Gross Adipose Tissue
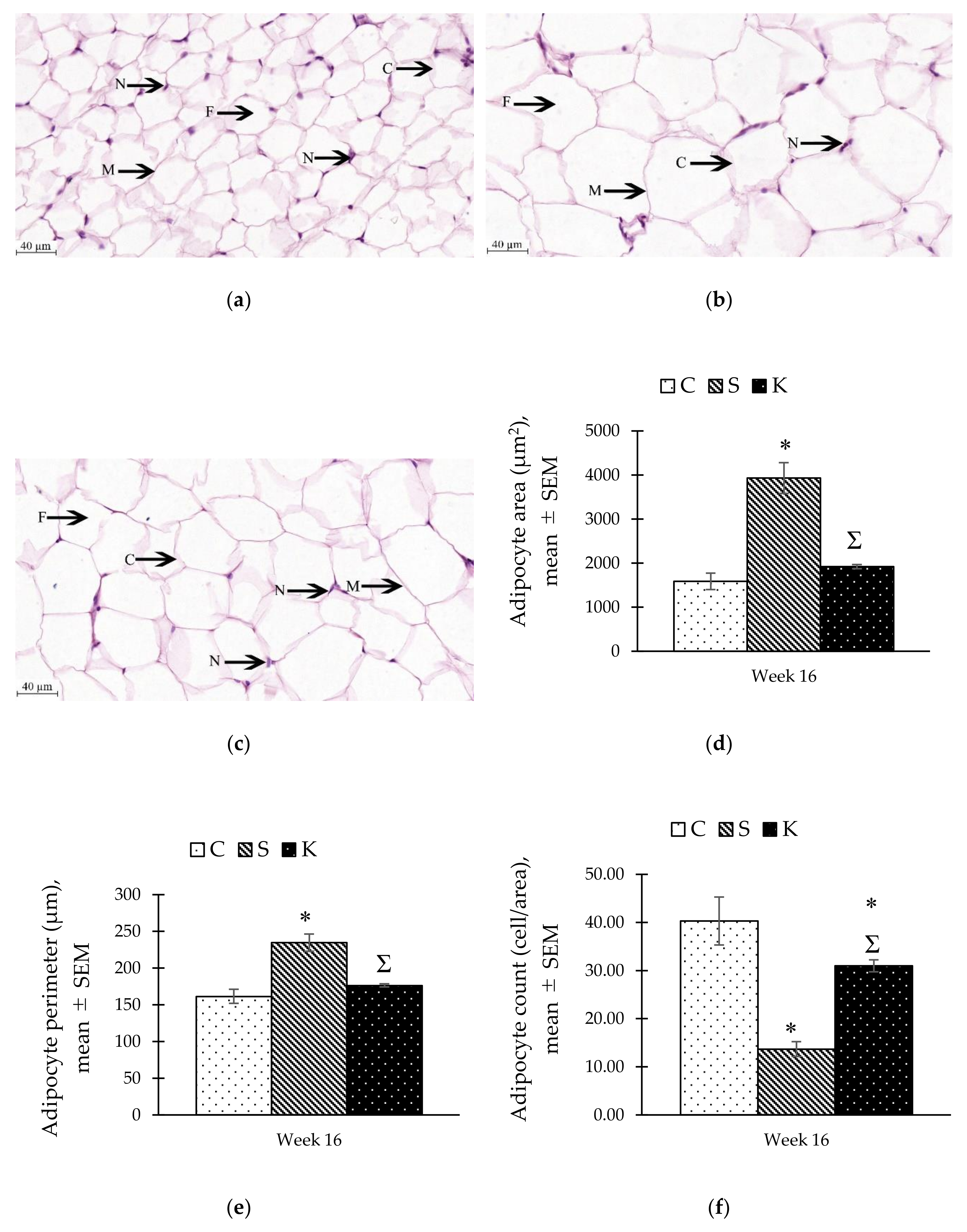
3.8. Changes in the Histomorphometry of the Visceral Adipose Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Mohamud, W.N.W.; Ismail, A.A.-S.; Sharifuddin, A.; Ismail, I.S.; Musa, K.I.; Kadir, K.A.; Kamaruddin, N.A.; Yaacob, N.A.; Mustafa, N.; Ali, O.; et al. Prevalence of metabolic syndrome and its risk factors in adult Malaysians: Results of a nationwide survey. Diabetes Res. Clin. Pract. 2011, 91, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the Asia-pacific region: A systematic review. BMC Public Health 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Misra, A.; Khurana, L. Obesity and the metabolic syndrome in developing countries. J. Clin. Endocrinol. Metab. 2008, 11, S9–S30. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Grundy, S.M. Drug therapy of the metabolic syndrome: Minimizing the emerging crisis in polypharmacy. Nat. Rev. Drug Discov. 2006, 5, 295–309. [Google Scholar] [CrossRef]
- Maher, R.L.; Hanlon, J.; Hajjar, E.R. Clinical consequences of polypharmacy in elderly. Expert Opin. Drug Saf. 2014, 13, 57–65. [Google Scholar] [CrossRef]
- Potenza, M.V.; Mechanick, J.I. The metabolic syndrome: Definition, global impact, and pathophysiology. Nutr. Clin. Pract. 2009, 24, 560–577. [Google Scholar] [CrossRef]
- Saba, Z.; Suzana, M.; Anum, M.Y. Honey: Food or medicine. Med. Heal. 2013, 8, 3–18. [Google Scholar]
- Ramli, N.Z.; Chin, K.Y.; Zarkasi, K.A.; Ahmad, F.A. A review on the protective effects of honey against metabolic syndrome. Nutrients 2018, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.K.; Liana, S.; Razak, A.; Ismail, N.; Fai, N.C.; Asyraf, M.H.; Asgar, M.; Sharif, N.M.; Aan, G.J.; Jubri, Z. Malaysian gelam honey reduces oxidative damage and modulates antioxidant enzyme activities in young and middle aged rats. J. Med. Plant Res. 2011, 5, 5618–5625. [Google Scholar]
- Öztaşan, N.; Altinkaynak, K.; Akçay, F.; Göçer, F.; Dane, Ş. Effects of mad honey on blood glucose and lipid levels in rats with streptozocin-induced diabetes. Turk. J. Vet. Anim. Sci. 2005, 29, 1093–1096. [Google Scholar]
- Aziz, M.S.A.; Giribabu, N.; Rao, P.V.; Salleh, N. Pancreatoprotective effects of Geniotrigona thoracica stingless bee honey in streptozotocin-nicotinamide-induced male diabetic rats. Biomed. Pharmacother. 2017, 89, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Galal, R.M.; Zaki, H.F.; El-Nasr, M.M.S.; Agha, A.M. Potential protective effect of honey against paracetamol-induced hepatotoxicity. Arch. Iran. Med. 2012, 15, 674–680. [Google Scholar] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S.; Sirajudeen, K.N.S.; Salleh, S.; Gurtu, S. Honey supplementation in spontaneously hypertensive rats elicits antihypertensive effect via amelioration of renal oxidative stress. Oxid. Med. Cell. Longev. 2012, 2012. [Google Scholar] [CrossRef]
- Samat, S.; Salleh, M.A.M.; Adam, Z.; Wan Ismail, W.I. Pineapple honey inhibits adipocytes proliferation and reduces lipid droplet accumulation in 3T3-L1 adipocytes. Malays. Appl. Biol. 2019, 48, 21–26. [Google Scholar]
- Nemoseck, T.M.; Carmody, E.G.; Furchner-Evanson, A.; Gleason, M.; Li, A.; Potter, H.; Rezende, L.M.; Lane, K.J.; Kern, M. Honey promotes lower weight gain, adiposity, and triglycerides than sucrose in rats. Nutr. Res. 2011, 31, 55–60. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Total phenolic contents and colour intensity of Malaysian honeys from the Apis spp. and Trigona spp. bees. Agric. Agric. Sci. Procedia 2014, 2, 150–155. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Zarei, M.; Akim, A.M.; Hamid, H.A.; Khazaai, H. Malaysian stingless bee and Tualang honeys: A comparative characterization of total antioxidant capacity and phenolic profile using liquid chromatography-mass spectrometry. LWT-Food Sci. Technol. 2017, 89, 1–9. [Google Scholar] [CrossRef]
- Hamid, Z.; Mohamad, I.; Harun, A.; Salim, R.; Sulaiman, S.A. Antifungal effect of three local Malaysian honeys on selected pathogenic fungi of otomycosis: An in vitro evaluation. J. Young Pharm. 2018, 10, 414. [Google Scholar] [CrossRef]
- Yazan, L.S.; Muhamad Zali, M.F.S.; Ali, R.M.; Zainal, N.A.; Esa, N.; Sapuan, S.; Ong, Y.S.; Tor, Y.S.; Gopalsamy, B.; Voon, F.L.; et al. Chemopreventive properties and toxicity of Kelulut honey in Sprague Dawley rats induced with azoxymethane. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Zainol, M.I.; Mohd Yusoff, K.; Mohd Yusof, M.Y. Antibacterial activity of selected Malaysian honey. BMC Complement. Altern. Med. 2013, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0—The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Ulrich, E.L.; Akutsu, H.; Doreleijers, J.F.; Harano, Y.; Ioannidis, Y.E.; Lin, J.; Livny, M.; Mading, S.; Maziuk, D.; Miller, Z.; et al. BioMagResBank. Nucleic Acids Res. 2008, 36, D402–D408. [Google Scholar] [CrossRef]
- Mistrik, R.; Huang, Y.; Lutisan, J.; Suchy, M.; Wang, J.; Raab, M. mzCloud: A key conceptual shift to understand “Who’s Who” in untargeted metabolomics. In Proceedings of the 9th Annual Conference of the Metabolomics Society, Glasgow, Scotland, 1–4 July 2013; pp. 9–55. [Google Scholar]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The effects of a modified high-carbohydrate high-fat diet on metabolic syndrome parameters in male rats. Exp. Clin. Endocrinol. Diabetes 2018, 126, 205–212. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Mohd Norowi, H.; Sajap, A.S.; Rosliza, J.; Mohd Fahimie, J.; Suri, R. Conservation and sustainable utilization of stingless bees for pollination services in agricultural ecosystems in Malaysia. In Proceedings of the International Seminar on Enhancement of Functional Biodiversity Relevant to Sustainable Food Production in ASPAC, Tsukuba, Japan, 9–11 November 2010. [Google Scholar]
- Takahama, U.; Hirota, S.; Nishioka, T.; Oniki, T. Human salivary peroxidase-catalyzed oxidation of nitrite and nitration of salivary components 4-hydroxyphenylacetic acid and proteins. Arch. Oral Biol. 2003, 48, 679–690. [Google Scholar] [CrossRef]
- Biskup, I.; Golonka, I.; Gamian, A.; Sroka, Z. Antioxidant activity of selected phenols estimated by ABTS and FRAP methods. Postepy Hig. Med. Dosw. 2013, 67, 958–963. [Google Scholar] [CrossRef]
- Godos, J.; Sinatra, D.; Blanco, I.; Mulè, S.; La Verde, M.; Marranzano, M. Association between dietary phenolic acids and hypertension in a mediterranean cohort. Nutrients 2017, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Huang, S.L.; Yen, G.C. Inhibitory effect of phenolic acids on the proliferation of 3T3-L1 preadipocytes in relation to their antioxidant activity. J. Agric. Food Chem. 2006, 54, 4191–4197. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Monforte, M.; Sánchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia. Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Bopp, M.J.; Houston, D.K.; Lenchik, L.; Easter, L.; Kritchevsky, S.B.; Nicklas, B.J. Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J. Am. Diet. Assoc. 2008, 108, 1216–1220. [Google Scholar] [CrossRef]
- Mohamed, N.; Gwee Sian Khee, S.; Shuid, A.N.; Muhammad, N.; Suhaimi, F.; Othman, F.; Babji, A.S.; Soelaiman, I.N. The Effects of Cosmos caudatus on structural bone histomorphometry in ovariectomized rats. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Ribeiro, S.M.L.; Rogero, M.M.; Bacurau, R.F.P.; de Campos, P.L.; Luz, S.d.S.; Lancha, A.H.; Tirapegui, J. Effects of different levels of protein intake and physical training on growth and nutritional status of young rats. J. Nutr. Sci. Vitaminol. 2010, 56, 177–184. [Google Scholar] [CrossRef]
- Uzunlulu, M.; Oguz, A. Is metabolic syndrome a condition independent of prediabetes and type 2 diabetes mellitus? A report from Turkey. Endocr. J. 2007, 54, 745–750. [Google Scholar] [CrossRef]
- Park, O.J.; Cesar, D.; Faix, D.; Wu, K.; Shackleton, C.H.L.; Hellerstein, M.K. Mechanisms of fructose-induced hypertriglyceridaemia in the rat. Activation of hepatic pyruvate dehydrogenase through inhibition of pyruvate dehydrogenase kinase. Biochem. J. 1992, 282, 753–757. [Google Scholar] [CrossRef]
- Shapiro, A.; Mu, W.; Roncal, C.; Cheng, K.-Y.; Johnson, R.J.; Scarpace, P.J. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R1370–R1375. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Q.; Pang, Z.-R.; Pan, M.-R.; Zhang, W. Flavonoid-enriched extract from Hippophae rhamnoides seed reduces high fat diet induced obesity, hypertriglyceridemia, and hepatic triglyceride accumulation in C57BL/6 mice. Pharm. Biol. 2017, 55, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Toop, R.C.; Gentili, S. Fructose beverage consumption induces a metabolic syndrome phenotype in the rat: A systematic review and meta-analysis. Nutrients 2016, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Fine, E.J. Fructose in perspective. Nutr. Metab. 2013, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T. Fructose induced lipogenesis: From sugar to fat to insulin resistance. Trends Endocrinol. Metab. 2011, 22, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Harchaoui, K.; Visser, M.; Kastelein, J.; Stroes, E.; Dallinga-Thie, G. Triglycerides and cardiovascular Risk. Curr. Cardiol. Rev. 2009, 5, 216–222. [Google Scholar] [CrossRef]
- Busserolles, J.; Gueux, E.; Rock, E.; Mazur, A.; Rayssiguier, Y. Substituting honey for refined carbohydrates protects rats from hypertriglyceridemic and prooxidative effects of fructose. J. Nutr. 2002, 132, 3379–3382. [Google Scholar] [CrossRef]
- Jan Mei, S.; Mohd Nordin, M.S.; Norrakiah, A.S. Fructooligosaccharides in honey and effects of honey on growth of Bifidobacterium longum BB 536. Int. Food Res. J. 2010, 17, 557–561. [Google Scholar]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Nakamura, Y.; Natsume, M.; Yasuda, A.; Ishizaka, M.; Kawahata, K.; Koga, J. Fructooligosaccharides suppress high-fat diet-induced fat accumulation in C57BL/6J mice. Biofactors 2011, 43, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ness, G.C.; Gertz, K.R. Hepatic HMG-CoA reductase expression and resistance to dietary cholesterol. Exp. Biol. Med. 2004, 229, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Machi, J.F.; Bernardes, N.; Mostarda, C.; Moraes-Silva, I.C.; Irigoyen, M.C.; De Angelis, K.; Wichi, R.B. Walking promotes metabolic and baroreflex sensitivity improvement in fructose-fed male rats. Eur. J. Appl. Physiol. 2013, 113, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.J.; Liu, C.A.; Huang, B.; Tseng, A.H.; Wang, D.L. Shear-induced endothelial mechanotransduction: The interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 2014, 21, 3. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Arumugam, T.V.; Brown, L. Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J. Nutr. 2011, 141, 1062–1069. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef]
- Chepulis, L.; Starkey, N. The long-term effects of feeding honey compared with sucrose and a sugar-free diet on weight gain, lipid profiles, and DEXA measurements in rats. J. Food Sci. 2008, 73, H1–H7. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S1–S78. [Google Scholar] [CrossRef]
- Cook, D.G.; Mendall, M.A.; Whincup, P.H.; Carey, I.M.; Ballam, L.; Morris, J.E.; Miller, G.J.; Strachan, D.P. C-reactive protein concentration in children: Relationship to adiposity and other cardiovascular risk factors. Atherosclerosis 2000, 149, 139–150. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Yannakoulia, M.; Chrysohoou, C.; Stefanadis, C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis 2005, 183, 308–315. [Google Scholar] [CrossRef]
- Jayarathne, S.; Koboziev, I.; Park, O.-H.; Oldewage-Theron, W.; Shen, C.-L.; Moustaid-Moussa, N. Anti-inflammatory and anti-obesity properties of food bioactive components: Effects on adipose tissue. Prev. Nutr. Food Sci. 2017, 22, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Jayarathne, S.; Stull, A.; Miranda, A.; Scoggin, S.; Claycombe-Larson, K.; Kim, J.; Moustaid-Moussa, N. Tart cherry reduces inflammation in adipose tissue of zucker fatty rats and cultured 3T3-L1 adipocytes. Nutrients 2018, 10, 1576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-H.; Zhao, C.-Y.; Gao, L.-F.; Feng, F.-F.; Gao, W.; Chen, Z.-L.; Zhang, F.; Cao, L.-G.; Bi, X.-Y.; Chen, Y.; et al. The novel inhibitory effect of Pangdahai on fatty acid synthase. IUBMB Life 2008, 60, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Muir, L.A.; Baker, N.A.; Washabaugh, A.R.; Neeley, C.K.; Flesher, C.G.; DelProposto, J.B.; Geletka, L.M.; Ghaferi, A.A.; Finks, J.F.; Singer, K.; et al. Adipocyte hypertrophy-hyperplasia balance contributes to weight loss after bariatric surgery. Adipocyte 2017, 6, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Mamikutty, N.; Thent, Z.C.; Sapri, S.R.; Sahruddin, N.N.; Mohd Yusof, M.R.; Haji Suhaimi, F. The establishment of metabolic syndrome model by induction of fructose drinking water in male Wistar rats. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Frühbeck, G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E691–E714. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Romero-Silva, S.; Angel Martinez, R.M.; Romero-Romero, L.P.; Rodriguez, O.; Gerardo Salas, C.G.; Morel, N.; Javier Lopez-Munoz, F.; Angel Lima-Mendoza, L.; Bravo, G. Effects of honey against the accumulation of adipose tissue and the increased blood pressure on carbohydrate-induced obesity in rat. Lett. Drug Des. Discov. 2011, 8, 69–75. [Google Scholar] [CrossRef]
| No. | Mass (Da) | Retention Time (min.) | Compound Name | Molecular Formula |
|---|---|---|---|---|
| 1. | 151.3016 | 2.997 | 4-Hydroxyphenyl Acetic Acid | C8H7O3 |
| 2. | 474.3035 | 3.278 | Caffeic Acid Derivative | C9H8O4 |
| 3. | 522.9860 | 2.828 | Caffeoyl Glucose Derivative | C15H18O9 |
| 4. | 503.0967 | 2.379 | Caffeoyl Hexoside Derivative | C15H17O9 |
| 5. | 162.6028 | 2.773 | Coumaric Acid | C9H8O3 |
| 6. | 195.3490 | 2.941 | Gluconic Acid | C6H12O7 |
| 7. | 143.6920 | 3.390 | Kynurenic Acid Derivative | C10H7NO3 |
| 8. | 271.4930 | 1.708 | Pinobanksin | C15H12O5 |
| 9. | 191.0423 | 1.875 | Quinic Acid | C7H12O6 |
| 10. | 357.1258 | 6.689 | Niazimicin | C16H23NO6S |
| 11. | 488.1492 | 4.064 | Bisosthenon B | C28H24O8 |
| 12. | 384.1932 | 4.727 | (6β,7α,12β,13β)-7-Hydroxy-11,16-dioxo-8,14-Apianadien-22,6-Olide | C23H28O5 |
| 13. | 365.2007 | 8.411 | Aegle Marmelos Alkaloid C | C23H27NO3 |
| 14. | 341.0460 | 11.911 | 7-chloro-6-Demethylcepharadione B | C18H12ClNO4 |
| 15. | 117.0415 | 9.870 | N-Acetylglycine | C4H7NO3 |
| 16. | 426.3835 | 10.650 | Lanosterol | C30H50O |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, N.Z.; Chin, K.-Y.; Zarkasi, K.A.; Ahmad, F. The Beneficial Effects of Stingless Bee Honey from Heterotrigona itama against Metabolic Changes in Rats Fed with High-Carbohydrate and High-Fat Diet. Int. J. Environ. Res. Public Health 2019, 16, 4987. https://doi.org/10.3390/ijerph16244987
Ramli NZ, Chin K-Y, Zarkasi KA, Ahmad F. The Beneficial Effects of Stingless Bee Honey from Heterotrigona itama against Metabolic Changes in Rats Fed with High-Carbohydrate and High-Fat Diet. International Journal of Environmental Research and Public Health. 2019; 16(24):4987. https://doi.org/10.3390/ijerph16244987
Chicago/Turabian StyleRamli, Nur Zuliani, Kok-Yong Chin, Khairul Anwar Zarkasi, and Fairus Ahmad. 2019. "The Beneficial Effects of Stingless Bee Honey from Heterotrigona itama against Metabolic Changes in Rats Fed with High-Carbohydrate and High-Fat Diet" International Journal of Environmental Research and Public Health 16, no. 24: 4987. https://doi.org/10.3390/ijerph16244987
APA StyleRamli, N. Z., Chin, K.-Y., Zarkasi, K. A., & Ahmad, F. (2019). The Beneficial Effects of Stingless Bee Honey from Heterotrigona itama against Metabolic Changes in Rats Fed with High-Carbohydrate and High-Fat Diet. International Journal of Environmental Research and Public Health, 16(24), 4987. https://doi.org/10.3390/ijerph16244987






