Fragility Fracture Prevention—Implementing a Fracture Liaison Service in a High Volume Orthopedic Hospital
Abstract
1. Introduction
2. Materials and Methods
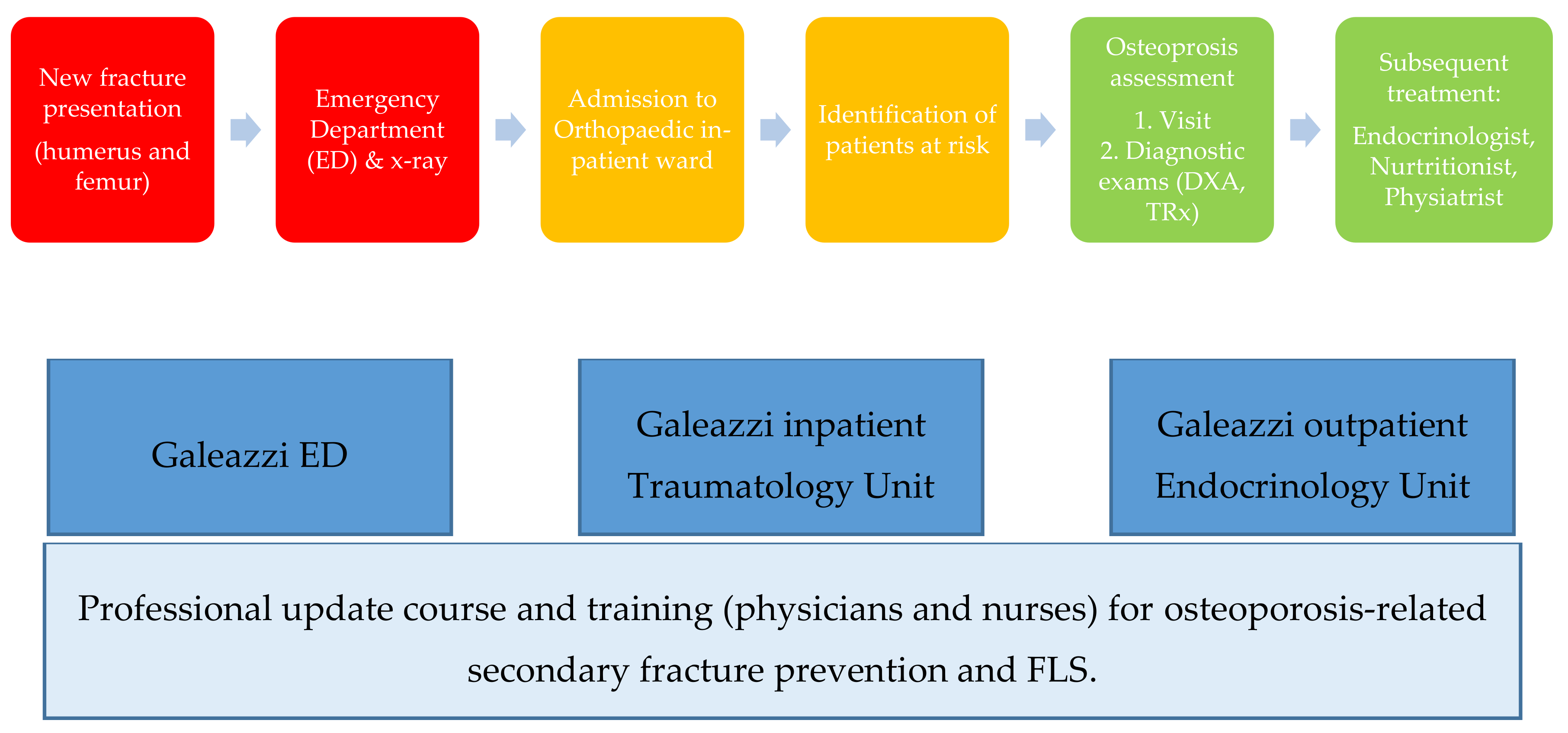
- Identifying patients at risk of osteoporosis-related secondary fracture, both of the upper limb (humerus) and lower limb (femur).
- Investigating biochemical indicators of bone metabolism associated to Bone Mass Density (BMD), to evaluate the degree of osteoporosis-fracture risk to which the patient is exposed, through specific diagnostic exams (Vertebral and Femoral Dual-Energy X-ray Absorptiometry: DXA; and/or Toracolumbar Spine X-ray: TSRx), and eventually.
- Initiating multidisciplinary treatment under the same provider to which the patient was admitted for surgery.
3. Results
4. Discussion
4.1. Benefits of FLS as Reported in Literature
4.2. FLS Challenges and Room for Improvement as Reported in Literature
- Higher rates of BMD testing are performed in hospitals rather than in outpatient clinics.
- Higher rates of BMD testing are performed in outpatient clinics rather than in communities, with a high variation according to the program.
4.3. Implications for Galeazzi Hospital Protocol
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Osteoporosis Foundation. What is Osteoporosis? Available online: https://www.iofbonehealth.org/what-is-osteoporosis (accessed on 17 July 2019).
- World Health Organization. WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Level. Available online: https://www.who.int/chp/topics/Osteoporosis.pdf (accessed on 17 July 2019).
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.N.; Lake, A.F.; Emory, C.L. Establishing a fracture liaison service: An orthopaedic approach. J. Bone Jt. Surg. Am. 2015, 97, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Tarride, J.E.; Hopkins, R.B.; Leslie, W.D.; Morin, S.; Adachi, J.D.; Papaioannou, A.; Bessette, L.; Brown, J.P.; Goeree, R. The burden of illness of osteoporosis in Canada. Osteoporos. Int. 2012, 23, 2591–2600. [Google Scholar] [CrossRef] [PubMed]
- Eccles, E.; Thompson, J.D.; Roddam, H. An evaluation of Fracture Liaison Services in the detection and management of osteoporotic fragility fractures: A narrative review. Radiography (Lond) 2018, 24, 392–395. [Google Scholar] [CrossRef]
- Chang, L.Y.; Tsai, K.S.; Peng, J.K.; Chen, C.H.; Lin, G.T.; Lin, C.H.; Tu, S.T.; Mao, I.C.; Gau, Y.L.; Liu, H.C.; et al. The development of Taiwan Fracture Liaison Service network. Osteoporos. Sarcopenia 2018, 4, 47–52. [Google Scholar] [CrossRef]
- Piscitelli, P.; Iolascon, G.; Argentiero, A.; Chitano, G.; Neglia, C.; Marcucci, G.; Pulimeno, M.; Benvenuto, M.; Mundi, S.; Marzo, V.; et al. Incidence and costs of hip fractures vs strokes and acute myocardial infarction in Italy: Comparative analysis based on national hospitalization records. Clin. Interv. Aging 2012, 7, 575–583. [Google Scholar] [CrossRef]
- Leibson, C.L.; Tosteson, A.N.; Gabriel, S.E.; Ransom, J.E.; Melton, L.J. Mortality, disability, and nursing home use for persons with and without hip fracture: A population-based study. J. Am. Geriatr. Soc. 2002, 50, 1644–1650. [Google Scholar] [CrossRef]
- Noordin, S.; Allana, S.; Masri, B.A. Establishing a hospital based fracture liaison service to prevent secondary insufficiency fractures. Int. J. Surg. 2018, 54, 328–332. [Google Scholar] [CrossRef]
- Rossini, M.; Piscitelli, P.; Fitto, F.; Camboa, P.; Angeli, A.; Guida, G.; Adami, S. Incidence and socioeconomic burden of hip fractures in Italy. Reumatismo 2005, 57, 97–102. [Google Scholar] [CrossRef]
- Yates, C.J.; Chauchard, M.A.; Liew, D.; Bucknill, A.; Wark, J.D. Bridging the osteoporosis treatment gap: Performance and cost-effectiveness of a fracture liaison service. J. Clin. Densitom. 2015, 18, 150–156. [Google Scholar] [CrossRef]
- Hopkins, R.B.; Burke, N.; Von Keyserlingk, C.; Leslie, W.D.; Morin, S.N.; Adachi, J.D.; Papaioannou, A.; Bessette, L.; Brown, J.P.; Pericleous, L.; et al. The current economic burden of illness of osteoporosis in Canada. Osteoporos. Int. 2016, 27, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Major, G.; Holliday, E.; Attia, J.; Bogduk, N. Evidence of effectiveness of a fracture liaison service to reduce the re-fracture rate. Osteoporos. Int. 2016, 27, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Den Teuling, J.W.A.M.; Pauwels, B.S.; Janssen, L.; Wyers, C.E.; Janzing, H.M.J.; van den Bergh, J.P.W.; Morrenhof, J.W. The influence of bone mineral density and cortical index on the complexity of fractures of the proximal humerus. Bone Jt. Res. 2014, 6, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, L.; Papaioannou, A.; Cranney, A.; Zytaruk, N.; Adachi, J.D. Fragility fractures and the osteoporosis care gap: An international phenomenon. Semin. Arthritis Rheum. 2006, 35, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Akesson, K.; Marsh, D.; Mitchell, P.J.; McLellan, A.R.; Stenmark, J.; Pierroz, D.D.; Kyer, C.; Cooper, C.; IOF Fracture Working Group. Capture the fracture: A best practice framework and global campaign to break the fragility fracture cycle. Osteoporos. Int. 2013, 24, 2135–2152. [Google Scholar] [CrossRef] [PubMed]
- International Osteoporosis Foundation. Fracture Liaison Service. Available online: https://capturethefracture.org/fracture-liaison-services (accessed on 26 September 2019).
- Nayak, S.; Greenspan, S.L. How can we improve osteoporosis care? A systematic review and meta-analysis of the efficacy of quality improvement strategies for osteoporosis. J. Bone Min. Res. 2018, 33, 1585–1594. [Google Scholar] [CrossRef]
- Ministero della Salute, Agenzia Nazionale per i Servizi Sanitari Regionali. Piano Nazionale Esiti. 2016. Available online: http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=2905&area=programmazioneSanitariaLea&menu=vuoto (accessed on 30 September 2019).
- Moja, L.; Piatti, A.; Pecoraro, V.; Ricci, C.; Virgili, G.; Salanti, G.; Germagnoli, L.; Liberati, A.; Banfi, G. Timing matters in hip fracture surgery: Patients operated within 48 hours have better outcomes. A meta-analysis and meta-regression of over 190,000 patients. PLoS ONE 2012, 7, e46175. [Google Scholar] [CrossRef]
- International Osteoporosis Foundation. IRCCS Istituto Ortopedico Galeazzi. Available online: https://capturethefracture.org/irccs-istituto-ortopedico-galeazzi (accessed on 27 September 2019).
- Ganda, K.; Puech, M.; Chen, J.S.; Speerin, R.; Bleasel, J.; Center, J.R.; Eisman, J.A.; March, L.; Seibel, M.J. Models of care for the secondary prevention of osteoporotic fractures: A systematic review and meta-analysis. Osteoporos. Int. 2013, 24, 393–406. [Google Scholar] [CrossRef]
- Ruggiero, C.; Zampi, E.; Rinonapoli, G.; Baroni, M.; Serra, R.; Zengarini, E.; Baglioni, G.; Duranti, G.; Ercolani, S.; Conti, F.; et al. Fracture prevention service to bridge the osteoporosis care gap. Clin. Interv. Aging 2015, 10, 1035–1042. [Google Scholar]
- Harvey, N.C.; McCloskey, E.V.; Mitchell, P.J.; Dawson-Hughes, B.; Pierroz, D.D.; Reginster, J.Y.; Rizzoli, R.; Cooper, C.; Kanis, J.A. Mind the (treatment) gap: A global perspective on current and future strategies for prevention of fragility fractures. Osteoporos. Int. 2017, 28, 1507–1529. [Google Scholar] [CrossRef]
- Mitchell, P.; Akesson, K.; Chandran, M.; Cooper, C.; Ganda, K.; Schneider, M. Implementation of models of Care for secondary osteoporotic fracture prevention and orthogeriatric models of care for osteoporotic hip fracture. Best Pract. Res. Clin. Rheumatol. 2016, 30, 536–558. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D.; van der Langerijt, L.; Watts, N.B.; Eastell, R.; Genant, H.; Grauer, A.; Cahall, D.L.; IMPACT Study Group. Underdiagnosis of vertebral fractures is a worldwide problem: The IMPACT study. J. Bone Min. Res. 2005, 20, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.G.; Dunkow, P.; Lang, D.M. Treatment of osteoporosis: Missed opportunities in the hospital fracture clinic. Ann. R. Coll. Surg. Engl. 2004, 86, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Department of Health. Fracture Prevention Services: An Economic Evaluation. Available online: https://webarchive.nationalarchives.gov.uk/20130123201008/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_110098 (accessed on 11 November 2019).
- Porter, M.E. What is Value in Health Care? N. Engl. J. Med. 2010, 363, 2477–2481. [Google Scholar] [CrossRef]
- Nwachukwu, B.U.; Hamid, K.S.; Bozic, K.J. Measuring value in orthopaedic surgery. JBJS Rev. 2013, 19, 1. [Google Scholar] [CrossRef]
- New England Journal of Medicine Catalyst. What is Value Based-Healthcare? Available online: https://catalyst.nejm.org/what-is-value-based-healthcare/ (accessed on 29 September 2019).
- Pennestrì, F.; Banfi, G. Value-based healthcare: The role of laboratory medicine. Clin. Chem. Lab. Med. 2019, 57, 798–801. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, C.H.; Chen, P.H.; Yang, J.J.; Chang, P.C.; Huang, T.C.; Bagga, S.; Sharma, Y.; Lin, R.M.; Chan, D.C. Identifying characteristics of an effective fracture liaison service: Systematic literature review. Osteoporos. Int. 2018, 29, 1023–1047. [Google Scholar] [CrossRef]
- Wu, C.H.; Tu, S.T.; Chang, Y.F.; Chan, D.C.; Chien, J.T.; Lin, C.H.; Singh, S.; Dasari, M.; Chen, J.F.; Tsai, K.S. Fracture liaison services improve outcomes of patients with osteoporosis-related fractures: A systematic literature review and meta-analysis. Bone 2018, 111, 92–100. [Google Scholar] [CrossRef]
- Walters, S.; Khan, T.; Ong, T.; Sahota, O. Fracture liaison services: Improving outcomes for patients with osteoporosis. Clin. Interv. Aging 2017, 12, 117–127. [Google Scholar] [CrossRef]
- Alswat, K.A. Gender disparities in osteoporosis. J. Clin. Med. Res. 2017, 9, 382–387. [Google Scholar] [CrossRef]
- Newman, E.D.; Ayoub, W.T.; Starkey, R.H.; Diehl, J.M.; Wood, G.C. Osteoporosis disease management in a rural health care population: Hip fracture reduction and reduced costs in postmenopausal women after 5 years. Osteoporos. Int. 2003, 14, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Jaglal, S.B.; Donescu, O.S.; Bansod, V.; Laprade, J.; Thorpe, K.; Hawker, G.; Majumdar, S.R.; Meadows, L.; Cadarette, S.M.; Papaioannou, A.; et al. Impact of a centralized osteoporosis coordinator on post-fracture osteoporosis management: A cluster randomized trial. Osteoporos. Int. 2012, 23, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Shipman, K.E.; Doyle, A.; Arden, H. Development of fracture liaison services: What have we learned? Injury 2017, 48, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Cabitza, F.; Dui, L.G.; Banfi, G. PROS in the wild: Assessing the validity of patient reported outcomes in an electronic registry. Comput. Methods Programs Biomed. 2019, 181, 104837. [Google Scholar] [CrossRef] [PubMed]
- International Society of Orthopedic Centers. Available online: https://www.isocweb.org/ (accessed on 3 October 2019).
| Database | Indicators | Outcome | Relevance | Potential Biases and Limitations |
|---|---|---|---|---|
| 1. Clinical (medical history of patients enrolled to FLS) | 1.1 Regular intake of vitamin D before primary fracture, reported in medical history. | 1.1.1 Absolute number of patients. 1.1.2 Percentage out of FLs patients. | To estimate the spread, knowledge and actual implementation of pharmacological and nutritional prevention of osteoporosis-related primary fractures. | Estimations are limited to the experimental sample size. |
| 1.2 Previous fragility fracture, regardless of the bone involved, reported in medical history. | 1.2.1 Absolute number of patients. 1.2.2 Percentage out of FLs patients. | To estimate the general incidence of secondary fractures. | ||
| 1.3 Pharmacological treatment between primary and secondary fracture (among patients reporting previous fragility fractures, 1.2). | 1.3.1 Absolute number of patients. 1.3.2 Percentage out of FLs patients. | To estimate the spread, knowledge and actual implementation of pharmacological prevention of osteoporosis-related secondary fractures. | ||
| 1.4 Regular consumption of more than three drugs regardless to osteoporotic prevention. | 1.4.1 Absolute number of patients. 1.4.2 Percentage out of FLs patients. | To estimate the degree of comorbidity among the population of patients affected by fragility fractures. | ||
| 1.5 Presence of multiple morbidities. | 1.5.1 Absolute number of patients. 1.5.2 Percentage out of FLs patients. | |||
| 2. Administrative (hospital internal database) | 2.1 Return to hospital for full outpatient osteoporosis care (osteoporosis assessment and subsequent treatment). | 2.1.1 Absolute number of patients. 2.1.2 Percentage out of FLs patients. | To estimate the degree of patients’ sensitivity towards the risk of a further fragility fracture, and/or the effectiveness of the education provided by care-givers. | Patients may have undergone follow-up (full or partial) at other healthcare providers. |
| 2.2 No return to hospital for follow-up outpatient osteoporosis visit, but return to hospital for one diagnostic exam (Vertebral and Femoral Dual-Energy X-ray Absorptiometry: DXA). | 2.2.1 Absolute number of patients. 2.2.2 Percentage out of FLs patients. | |||
| 2.3 No return to hospital for follow-up outpatient osteoporotic visit, bur return to hospital for both diagnostic exams (DXA, Toracolumbar Spine X-ray: TSRx). | 2.3.1 Absolute number of patients. 2.3.2 Percentage out of FLs patients. | |||
| 2.4 No return to hospital for any outpatient osteoporotic treatment but readmitted for another fragility fracture within one year. | 2.4.1 Absolute number of patients with relative sites of fracture. 2.4.2 Percentage out of FLs patients. | To estimate the effectiveness of secondary fracture prevention. | ||
| 2.5 Return to hospital for any outpatient osteoporotic treatment but readmitted for another fragility fracture within one year. | 2.5.1 Absolute number of patients with relative sites of fracture. 2.5.2 Percentage out of FLs patients. |
| Indicator | Absolute # Patients | % out of FLS Patients (403) |
|---|---|---|
| 1.1 Vitamin-D therapy before primary fracture. | 117 | 29.3 |
| 1.2 Previous fragility fracture. | 159 | 39.4 |
| 1.3 Pharmacological treatment between primary or secondary fracture (subgroup of domain 1.2). | 76 | 47.7 (out of 159) |
| 1.4 Patients affected by multiple comorbidities. | 121 | 30 |
| 1.5 Patients regularly assuming more than three drugs. | 203 | 50.3 |
| Indicator | Absolute # Patients | % Out of FLS Patients (403) |
|---|---|---|
| 2.1 Patients who came back to outpatient osteoporosis care (visit, exam, treatment). | 132 | 32.70% |
| 2.2 Patients who came back for DXA. | 8 | 1.98% |
| 2.3 Patients who came back for DXA and TRx. | 2 | 0.49% |
| 2.4 Patients who did not come back for osteoporosis care readmitted for another suspected fracture within one year. | 7 (5 Spine, 1 Rotula, 1 Wrist fracture) | 1.73% |
| 2.5 Patients who came back for osteoporosis care but still were readmitted for another fracture within one year. | 1 (Wrist fracture) | 0.24% |
| Model | Identifies Patient | Investigates Osteoporosis | Initiates Treatment(s) | ± Effective |
|---|---|---|---|---|
| Type A (3-I) | Provided under the same setting (hospital, integrated providers: i.e., managed care) in which primary fracture is treated (i.e., operating surgeon or his/her collaborators). | Provided under the same setting in which primary fracture is treated (i.e., surgeon collaborators, other specialists). | Provided under the same setting in which the fracture is treated, in a multidisciplinary and coordinated manner (i.e., rheumatologist, endocrinologist, physiatrist, nutritionist). |  |
| Type B (2-I) | Provided as above. | Provided as above. | Referred or recommended to primary care or other providers | |
| Type C (1-I) | Provided as above. | Referred or recommended to primary care or other providers. | Referred or recommended as above. | |
| Type D (0-I) | The patient is educated about osteoporosis and given lifestyle advice, included falls prevention, regardless of any relation between fracture and osteoporosis. | No recommendations or referral to primary care or other providers. | No recommendations or referral to primary care or other providers. |
| Indicator | Before FLS (2015–2017) | After FLS (2017–2019) |
|---|---|---|
| Number of patients admitted to traumatology for the same site of fracture | 1220 | 1278 |
| Number and percentage of patients evaluated by FLS | 0 (0%) | 403 (31.5%) |
| Number and percentage of secondary fractures | 23 (1.8%) (general fractures) | 159 (39.4%) (only fragility fractures) |
| Number and percentage of secondary fractures within one year | 21 (91.3%) | 1 (0.62%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennestrì, F.; Corbetta, S.; Favero, V.; Banfi, G. Fragility Fracture Prevention—Implementing a Fracture Liaison Service in a High Volume Orthopedic Hospital. Int. J. Environ. Res. Public Health 2019, 16, 4902. https://doi.org/10.3390/ijerph16244902
Pennestrì F, Corbetta S, Favero V, Banfi G. Fragility Fracture Prevention—Implementing a Fracture Liaison Service in a High Volume Orthopedic Hospital. International Journal of Environmental Research and Public Health. 2019; 16(24):4902. https://doi.org/10.3390/ijerph16244902
Chicago/Turabian StylePennestrì, Federico, Sabrina Corbetta, Vittoria Favero, and Giuseppe Banfi. 2019. "Fragility Fracture Prevention—Implementing a Fracture Liaison Service in a High Volume Orthopedic Hospital" International Journal of Environmental Research and Public Health 16, no. 24: 4902. https://doi.org/10.3390/ijerph16244902
APA StylePennestrì, F., Corbetta, S., Favero, V., & Banfi, G. (2019). Fragility Fracture Prevention—Implementing a Fracture Liaison Service in a High Volume Orthopedic Hospital. International Journal of Environmental Research and Public Health, 16(24), 4902. https://doi.org/10.3390/ijerph16244902






