Evaluation of Qualitative Dietary Protocol (Diet4Hashi) Application in Dietary Counseling in Hashimoto Thyroiditis: Study Protocol of a Randomized Controlled Trial
Abstract
1. Introduction
1.1. Research Objective and Hypothesis
- Dietetic counseling with the use of a qualitative dietary protocol can facilitate patient understanding and long-term adherence to dietary recommendations compared to conventional dietetic counseling.
- A well-composed diet supported with a simple, qualitative dietary protocol, by improving food selection and diet quality, can improve the quality of life of HT subjects, reduce adiposity, and improve metabolic parameters. Furthermore, a better effect of a qualitative dietary protocol than conventional dietetic counseling is possible.
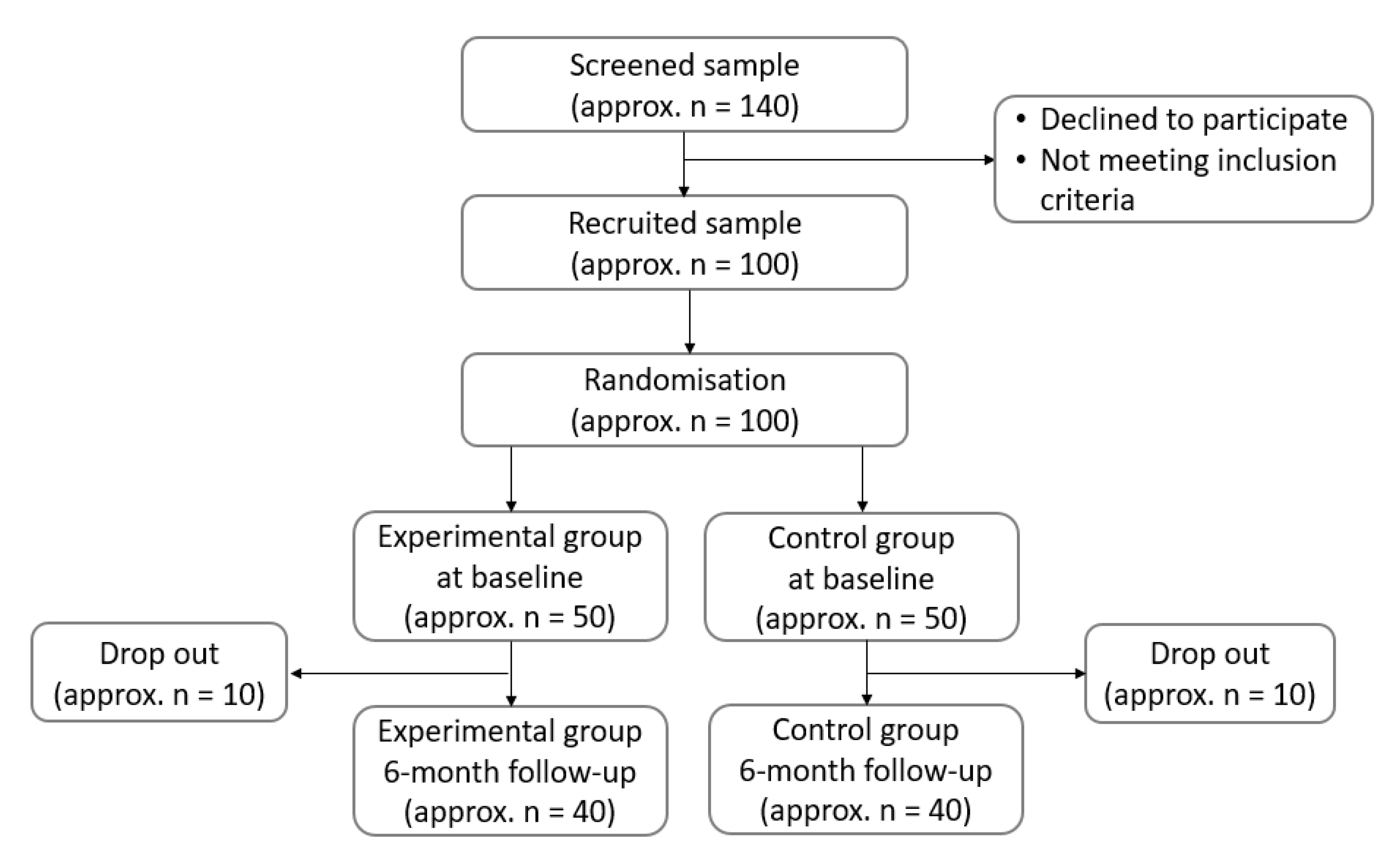
1.2. Study Design
2. Materials and Methods
2.1. Ethical Approval
2.2. Participants Selections
2.3. Randomization and Blinding
2.4. Dietary Intervention with QDP and CDC
2.5. Diet Quality
2.6. Other Lifestyle Factors
2.7. Quality of Life (QoL)
2.8. Nutrition Knowledge and Sociodemographic Factors
2.9. Adiposity Markers
2.10. Thyroid Function Tests (TFTs) and Metabolic Parameters
2.11. Sample Size
2.12. Statistical Analysis
3. Discussion
3.1. Basis for Development of a Self-Monitoring Diary for Recommended Foods (A)
3.1.1. Vegetables
3.1.2. Foods Rich in Calcium
3.1.3. Fruits
3.1.4. Whole Grains
3.1.5. Animal Foods Rich in Zinc
3.1.6. Animal Foods Rich in Selenium
3.1.7. Nuts and Seeds
3.2. Basis for the Development of a Self-Monitoring Diary for Foods with Limited Consumption (B)
3.2.1. Raw Cruciferous Vegetables
3.2.2. Sweets, Sugar, and Honey
3.2.3. Sweetened Beverages and Energy Drinks
3.2.4. Fast Food
3.2.5. Soybean and Millet
3.2.6. Alcohols
3.3. Strengths and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 95%CI | confidence interval |
| AdhS | Adherence Score to the QDP |
| BF | body fat |
| BIA | bioelectrical impedance analysis |
| CDC | conventional dietetic counseling |
| DBP | diastolic blood pressure |
| FBG | serum fasting glucose |
| FFQs | food frequency questionnaires |
| FT3 | free triiodothyronine |
| FT4 | free thyroxine |
| HT | Hashimoto thyroiditis |
| IQR | interquartile range |
| ISAK | Iinternational Standards for Anthropometric Assessment |
| NHANES | the National Health and Nutrition Examination Survey |
| nHDI | non-Healthy Diet Index |
| pHDI | pro-Healthy Diet Index |
| QDP | qualitative dietary protocol |
| ORs | odds ratios |
| RDA | recommended dietary allowance |
| SBP | systolic blood pressure |
| SMI | skeletal muscle index |
| TC | total cholesterol |
| TG | triglycerides |
| TgAb | serum autoantibodies against thyroglobulin |
| ThyPRO | thyroid-specific questionnaire |
| TPOAb | serum autoantibodies against thyroid peroxidase |
| TSH | thyrotropin |
| WC | waist circumference |
| WHtR | waist-to-height ratio |
References
- Ahmed, R.; Al-Shaikh, S.; Akhtar, M. Hashimoto thyroiditis: A century later. Adv. Anat. Pathol. 2012, 19, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Latina, A.; Gullo, D.; Trimarchi, F.; Benvenga, S. Hashimoto’s thyroiditis: Similar and dissimilar characteristics in neighboring areas: Possible implications for the epidemiology of thyroid cancer. PLoS ONE 2013, 8, e55450. [Google Scholar] [CrossRef] [PubMed]
- Zaletel, K.; Gaberscek, S. Hashimoto’s Thyroiditis: From Genes to the Disease. Curr. Genom. 2011, 12, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.R.; Cobin, R.H.; Gharib, H.; Hennessey, J.V.; Klein, I.; Mechanick, J.I.; Pessah-Pollack, R.; Singer, P.A.; Woeber, K.A. American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pract. 2012, 18, 988–1028. [Google Scholar] [CrossRef] [PubMed]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillen-Grima, F.; Galofre, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Szwajkosz, K.; Wawryniuk, A.; Sawicka, K.; Luczyk, R.; Tomaszewski, A. Hypothyroidism being caused by chronic autoimmune inflammation of the thyroid gland. J. Educ. Health Sport 2017, 7, 41–54. [Google Scholar] [CrossRef]
- GUS. Health Status of the Population in Poland in 2019. Available online: http://stat.gov.pl/cps/rde/xbcr/gus/ZO_stan_zdrowia_2009.pdf (accessed on 10 April 2019).
- GUS. Health of Women in Poland in 2004–2009. Available online: http://stat.gov.pl/cps/rde/xbcr/gus/ZO_Zdrowie_kobiet_w_Polsce_w_latach_2004-2009.pdf (accessed on 10 April 2019).
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T4 and thyroid antibodies in the United States population (1988–1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Tamer, G.; Mert, M.; Tamer, I.; Mesci, B.; Kilic, D.; Arik, S. Effects of thyroid autoimmunity on abdominal obesity and hyperlipidaemia. Endokrynol. Pol. 2011, 62, 421–428. [Google Scholar]
- Herwig, A.; Ross, A.W.; Nilaweera, K.N.; Morgan, P.J.; Barrett, P. Hypothalamic thyroid hormone in energy balance regulation. Obes. Facts 2008, 1, 71–79. [Google Scholar] [CrossRef]
- Tuchendler, D.; Bolanowski, M. Rola osteoprotegeryny i witaminy D w patologii tarczycy. Endokrynol. Pol. 2009, 60, 470–475. [Google Scholar]
- Gierach, M.; Gierach, J.; Skowronska, A.; Rutkowska, E.; Spychalska, M.; Pujanek, M.; Junik, R. Hashimoto’s thyroiditis and carbohydrate metabolism disorders in patients hospitalised in the Department of Endocrinology and Diabetology of Ludwik Rydygier Collegium Medicum in Bydgoszcz between 2001 and 2010. Endokrynol. Pol. 2012, 63, 14–17. [Google Scholar] [PubMed]
- Ott, J.; Promberger, R.; Kober, F.; Neuhold, N.; Tea, M.; Huber, J.C.; Hermann, M. Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: A prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid 2011, 2, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Cooper, D.S. The clinical significance of subclinical thyroid dysfunction. Endocr. Rev. 2008, 29, 76–131. [Google Scholar] [CrossRef] [PubMed]
- Ruchala, M. Choroba Hashimoto i Niedoczynność Tarczycy; Leczenie, Medycyna Praktyczna: Kraków, Poland, 2015. [Google Scholar]
- Krysiak, R.; Szkrobka, W.; Okopien, B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp. Clin. Endocrinol. Diabetes 2018, 127, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Liontiris, M.I.; Mazokopakis, E.E. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients. Points that need more investigation. Hell J. Nucl. Med. 2017, 20, 51–56. [Google Scholar] [CrossRef] [PubMed]
- American Thyroid Association. Low Iodine Diet. Available online: https://www.thyroid.org/low-iodine-diet/ (accessed on 10 June 2019).
- American Dietetic Association. Iodine, a Critically Important Nutrient and 7 Cancer Prevention Tips for Your Diet. Available online: https://www.eatright.org/search-results?keyword=thyroid (accessed on 15 June 2019).
- Szostak-Węgierek, D.; Bednarczuk, T.; Respondek, W.; Traczyk, I.; Cukrowska, B.; Ostrowska, L.; Wlodarek, D.; Anna, J.; Joanna, B.; Ewa, L.; et al. Zasadność stosowania diety bezglutenowej w chorobie Hashimoto: Stanowisko Grupy Ekspertów Sekcji Dietetyki Medycznej Polskiego Towarzystwa Żywienia Pozajelitowego, Dojelitowego i Metabolizmu (POLSPEN) (The rationale for using a gluten-free diet in Hashimoto’s disease: The position of the Expert Group of the Medical Dietetics Section of the Polish Society of Parenteral Nutrition, Enteral Nutrition and Metabolism (POLSPEN)). Postępy Żywienia Klinicznego 2018, 2, 47. [Google Scholar]
- Abbott, R.D.; Sadowski, A.; Alt, A.G. Efficacy of the Autoimmune Protocol Diet as Part of a Multi-disciplinary, Supported Lifestyle Intervention for Hashimoto’s Thyroiditis. Cureus 2019, 11, 4556. [Google Scholar] [CrossRef]
- Matana, A.; Torlak, V.; Brdar, D.; Popovic, M.; Lozic, B.; Barbalic, M.; Perica, V.B.; Punda, A.; Polašek, O.; Hayward, C.; et al. Dietary factors associated with plasma thyroid peroxidase and thyroglobulin antibodies. Nutrients 2017, 9, 1186. [Google Scholar] [CrossRef]
- Mitchell, L.J.; Ball, L.E.; Ross, L.J.; Barnes, K.A.; Williams, L.T. Effectiveness of Dietetic Consultations in Primary Health Care: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr Diet. 2017, 12, 1941–1962. [Google Scholar] [CrossRef]
- Crichton, G.E.; Howe, P.R.; Buckley, J.D.; Coates, A.M.; Murphy, K.J.; Bryan, J. Long-term dietary intervention trials: Critical issues and challenges. Trials 2012, 13, 111. [Google Scholar] [CrossRef]
- American Thyroid Association. Guidelines for the Treatment of Hypothyroidism: Prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Available online: https://www.thyroid.org/professionals/ata-professional-guidelines/ (accessed on 15 March 2019).
- IZZ. Piramida Zdrowego Zywienia i Aktywnosci Fizycznej [Pyramid of Healthy Eating and Physical Activity]. Available online: http://www.izz.waw.pl/zasady-prawidowego-ywienia (accessed on 23 March 2019).
- Jarosz, M. Normy Zywienia dla Populacji Polskiej—Nowelizacja; Nutrition Standards for the Polish Population—Amendment; Wyd. IZZ: Warszawa, Poland, 2017; ISBN 978-83-86060-89-4. [Google Scholar]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Składu i Wartości Odżywczej Żywności [Tables of food composition and nutritional values], 2nd ed.; PZWL: Warszawa, Poland, 2019. [Google Scholar]
- Jezewska-Zychowicz, M.; Gawecki, J.; Wadolowska, L.; Czarnocinska, J.; Galinski, G.; Kollajtis-Dolowy, A.; Roszkowski, W.; Wawrzyniak, A.; Przybylowicz, K.; Krusinska, B.; et al. Dietary Habits and Nutrition Beliefs Questionnaire and the Manual for Developing of Nutritional Data; The Committee of Human Nutrition, Polish Academy of Sciences, 2017; Available online: http://www.knozc.pan.pl/images/stories/MLonnie/EN_Kwestionariusz_KomPAN_i_PROCEDURA_versja_2_znak_tow_2019_2.pdf (accessed on 10 June 2019).
- Kowalkowska, J.; Wadolowska, L.; Czarnocinska, J.; Czlapka-Matyasik, M.; Galinski, G.; Jezewska-Zychowicz, M.; Bronkowska, M.; Dlugosz, A.; Laboda, D.; Wyka, J. Reproducibility of a Questionnaire for Dietary Habits, Lifestyle and Nutrition Knowledge Assessment (KomPAN) in Polish Adolescents and Adults. Nutrients 2018, 10, 1845. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Gutaj, N.; Watt, T.; Sowinski, J.; Gutaj, P.; Waligorska-Stachura, J.; Ruchala, M. ThyPROpl—The Polish version of the thyroid-specific quality of life questionnaire ThyPRo. Endokrynol. Pol. 2015, 66, 367–380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watt, T.; Hegedus, L.; Groenvold, M.; Bjorner, J.B.; Rasmussen, A.K.; Bonnema, S.J.; Feldt-Rasmussen, U. Validity and reliability of the novel thyroid-specific quality of life questionnaire, ThyPRO. Eur. J. Endocrinol. 2010, 162, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Watt, T.; Cramon, P.; Hegedus, L.; Bjorner, J.B.; Bonnema, S.J.; Rasmussen, A.K.; Feldt-Rasmussen, U.; Groenvold, M. The thyroid-related quality of life measure ThyPRO has good responsiveness and ability to detect relevant treatment effects. J. Clin. Endocrinol. Metab. 2014, 99, 3708–3717. [Google Scholar] [CrossRef]
- ISAK. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Potchefstroom, South Africa, 2001; Available online: http://www.ceap.br/material/MAT17032011184632.pdf (accessed on 10 July 2018).
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 266, 1059–1062. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity; WHO/NUT/NCD/98.1.1998; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- National Institutes of Health, National Heart, Lung, and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. NO. 98-4083. Available online: https://www.ncbi.nlm.nih.gov/books/NBK2003/ (accessed on 28 October 2019).
- Sun, G.; French, C.R.; Martin, G.R.; Younghusband, B.; Green, R.C.; Xie, Y.G.; Mathews, M.; Barron, J.R.; Fitzpatrick, D.G.; Gulliver, W.; et al. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am. J. Clin. Nutr. 2005, 81, 74–78. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 2014, 32, 1359–1366. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M. Harmonizing the metabolic syndrome. A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Jialal, I.; Rockwood, J.; Zak, D. Effect of orange juice and beverages with phytosterols on cytokines and PAI-1 activity. Clin. Nutr. 2011, 30, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Wadolowska, L.; Sobas, K.; Szczepanska, J.W.; Slowinska, M.A.; Czlapka-Matyasik, M.; Niedzwiedzka, E. Dairy products, dietary calcium and bone health: Possibility of prevention of osteoporosis in women: The Polish experience. Nutrients 2013, 5, 2684–2707. [Google Scholar] [CrossRef] [PubMed]
- Asik, M.; Gunes, F.; Binnetoglu, E.; Eroglu, M.; Bozkurt, N.; Sen, H.; Akbal, E.; Bakar, C.; Beyazit, Y.; Ukinc, K. Decrease in TSH levels after lactose restriction in Hashimoto’s thyroiditis patients with lactose intolerance. Endocrine 2014, 46, 279–284. [Google Scholar] [CrossRef]
- Xin, J.; Feinstein, D.L.; Hejna, M.J.; Lorens, S.A.; McGuire, S.O. Beneficial effects of blueberries in experimental autoimmune encephalomyelitis. J. Agric. Food Chem. 2012, 60, 5743–5748. [Google Scholar] [CrossRef]
- Yoon, C.H.; Chung, S.J.; Lee, S.W.; Park, Y.B.; Lee, S.K.; Park, M.C. Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Joint Bone Spine 2013, 80, 274–279. [Google Scholar] [CrossRef]
- Tonstad, S.; Nathan, E.; Oda, K.; Fraser, G. Vegan diets and hypothyroidism. Nutrients 2013, 5, 4642–4652. [Google Scholar] [CrossRef]
- Liu, Z.T.; Lin, A.H. Dietary factors and thyroid cancer risk: A meta-analysis of observational studies. Nutr. Cancer 2014, 66, 1165–1178. [Google Scholar] [CrossRef]
- Ch’ng, C.L.; Jones, M.; Kingham, J.G.C. Celiac disease and autoimmune thyroid disease. Clin. Med. Res. 2007, 5, 184–192. [Google Scholar] [CrossRef]
- Ventura, A.; Ronsoni, M.F.; Shiozawa, M.B.; Dantas-Corrêa, E.B.; Canalli, M.H.; Schiavon, L.D.; Narciso-Schiavon, J.L. Prevalence and clinical features of celiac disease in patients with autoimune thyroiditis: Cross-sectional study. Med. J. 2014, 132, 364–371. [Google Scholar] [CrossRef]
- Mehrdad, M.; Mansour-Ghanaei, F.; Mohammadi, F.; Joukar, F.; Dodangeh, S.; Mansour-Ghanaei, R. Frequency of celiac disease in patients with hypothyroidism. J. Thyroid Res. 2012, 2012, 201538. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Laszkowska, M.; Sundstrom, J.; Lebwohl, B.; Green, P.H.; Kampe, O.; Ludvigsson, J.F. Prevalence of Celiac Disease in Patients with Autoimmune Thyroid Disease: A Meta-Analysis. Thyroid 2016, 26, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Sategna, G.; Solerio, E.; Scaglione, N.; Aimo, G.; Mengozzi, G. Duration of gluten exposure in adult coeliac disease does not correlate with the risk for autoimmune disorders. Gut 2001, 49, 502–505. [Google Scholar] [CrossRef]
- Cosnes, J.; Cellier, C.; Viola, S.; Colombel, J.F.; Michaud, L.; Sarles, J.; Hugot, J.P.; Ginies, J.L.; Dabadie, A.; Mouterde, O. Incidence of autoimmune diseases in celiac disease: Protective effect of the gluten-free diet. Clin. Gastroenterol. Hepatol. 2008, 6, 753–758. [Google Scholar] [CrossRef]
- Rybicka, J.; Krawczyk, M.; Stanisz, E.; Gliszczynska-Swiglo, A. Selenium in Gluten-free Products. Plant Foods Hum. Nutr. 2015, 70, 128–134. [Google Scholar] [CrossRef]
- Klusek, J.; Nasierowska-Guttmejer, A.; Kowalik, A.; Wawrzycka, I.; Chrapek, M.; Lewitowicz, P.; Radowicz-Chil, A.; Klusek, J.; Gluszek, S. The Influence of Red Meat on Colorectal Cancer Occurrence Is Dependent on the Genetic Polymorphisms of S-Glutathione Transferase Genes. Nutrients 2019, 11, 1682. [Google Scholar] [CrossRef]
- Laskowski, W.; Gorska-Warsewicz, H.; Kulykovets, O. Meat, Meat Products and Seafood as Sources of Energy and Nutrients in the Average Polish Diet. Nutrients 2018, 10, 1412. [Google Scholar] [CrossRef]
- Betsy, A.; Binitha, M.; Sarita, S. Zinc deficiency associated with hypothyroidism: An overlooked cause of severe alopecia. Int. J. Trichology 2013, 5, 40–42. [Google Scholar] [CrossRef]
- Ertek, S.; Cicero, A.F.; Caglar, O.; Erdogan, G. Relationship between serum zinc levels, thyroid hormones and thyroid volume following successful iodine supplementation. Hormones (Athens) 2010, 9, 263–268. [Google Scholar] [CrossRef]
- Mahmoodianfard, S.; Vafa, M.; Golgirl, F.; Knoshniat, M.; Gohari, M.; Solati, Z.; Djali, M. Effects of Zinc and Selenium Supplementation on Thyroid Function in Overweight and Obese Hypothyroid Female Patients: A Randomized Double-Blind Controlled Trial. J. Am. Coll. Nutr. 2015, 34, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, C.; Volpe, S.L. Effect of zinc supplementation on thyroid hormone function. A case study of two college females. Ann. Nutr. Metab. 2007, 51, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Dhawan, D.; Pathak, A. Effect of zinc supplementation on the status of thyroid hormones and Na, K, And Ca levels in blood following ethanol feeding. Biol. Trace Elem. Res. 2011, 140, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kohrle, J.; Jakob, F.; Contempre, B.; Dumont, J.E. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005, 26, 944–984. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Stoffaneller, R.; Morse, N.L. A Review of Dietary Selenium Intake and Selenium Status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2014, 31, 142–147. [Google Scholar] [CrossRef]
- Blazewicz, A.; Klatka, M.; Astel, A.; Korona-Glowniak, I.; Dolliver, W.; Szwerc, W.; Kocjan, R. Serum and urinary selenium levels in obese children: A cross-sectional study. J. Trace Elem. Med. Biol. 2015, 29, 116–122. [Google Scholar] [CrossRef]
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedus, L. Selenium supplementation significantly reduces thyroid autoantibody levels in patients with chronic autoimmune thyroiditis: A systematic review and meta-analysis. Thyroid 2016, 26, 1681–1692. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef] [PubMed]
- Mazokopakis, E.E.; Liontiris, M.I. Commentary: Health Concerns of Brazil Nut Consumption. J. Altern. Complement. Med. 2018, 24, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Dehghan, P.; Tajmiri, S.; Abbasi, M.M. The effects of Nigella sativa on thyroid function, serum Vascular Endothelial Growth Factor (VEGF)–1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: A randomized controlled trial. BMC Complement. Altern. Med. 2016, 16, 471. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, T.; Baranowski, J.C.; Watson, K.B.; Jago, R.; Islam, N.; Beltran, A.; Martin, S.J.; Nguyen, N.; Tepper, B. PROP taster status not related to reported cruciferous vegetable intake among ethnically diverse children. Nutr. Res. 2011, 31, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Lebda, M.A.; Sadek, K.M.; El-Sayed, Y.S. Aspartame and soft drink-mediated neurotoxicity in rats: Implication of oxidative stress, apoptotic signaling pathways, electrolytes and hormonal levels. Metab. Brain Dis. 2017, 32, 1639–1647. [Google Scholar] [CrossRef]
- Sachmechi, I.; Khalid, A.; Awan, S.I.; Malik, Z.R.; Sharifzadeh, M. Autoimmune Thyroiditis with Hypothyroidism Induced by Sugar Substitutes. Cureus 2018, 10, 3268. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Fast Food Pattern and Cardiometabolic Disorders: A Review of Current Studies. Health Promot. Perspect. 2015, 5, 231–240. [Google Scholar] [CrossRef]
- Pereira, M.A.; Kartashov, A.I.; Ebbeling, C.B.; Van Horn, L.; Slattery, M.L.; Jacobs, D.R., Jr.; Ludwig, D.S. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet 2005, 365, 36–42. [Google Scholar] [CrossRef]
- Bhutani, S.; Schoeller, D.A.; Walsh, M.C.; McWilliams, C. Frequency of eating out at both fast-food and sit-down restaurants was associated with high body mass index in non-large metropolitan communities in Midwest. Am. J. Health. Promot. 2018, 32, 75–83. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological Effect of Soy Isoflavones in the Prevention of Civilization Diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Tran, L.; Hammuda, M.; Wood, C.; Xiao, C.W. Soy extracts suppressed iodine uptake and stimulated the production of autoimmunogen in rat thyrocytes. Exp. Biol. Med. 2013, 238, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Redmond, G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: A review of the relevant literature. Thyroid 2006, 16, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Effraimidis, G.; Wiersinga, W.M. Mechanisms in endocrinology autoimmune thyroid disease: Old and new players. Eur. J. Endocrinol. 2014, 170, R241–R252. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M. Clinical relevance of environmental factors in the pathogenesis of autoimmune thyroid disease. Endocrinol. Metab. 2016, 31, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Carle, A.; Pedersen, I.B.; Knudsen, N.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Jorgensen, T.; Laurberg, P. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: A population-based case-control study. Eur. J. Endocrinol. 2012, 167, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Ryterska, K.; Maciejewska, D.; Banaszczak, M.; Milkiewicz, P.; Milkiewicz, M.; Gutowska, I.; Ossowski, P.; Kaczorowska, M.; Gutowska, I.; et al. Nutritional Strategies for the Individualized Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD) Based on the Nutrient-Induced Insulin Output Ratio (NIOR). Int. J. Mol. Sci. 2016, 17, 1192. [Google Scholar] [CrossRef]
- Drabinska, N.; Krupa-Kozak, U.; Ciska, E.; Jarocka-Cyrta, E. Plasma profile and urine excretion of amino acids in children with celiac disease on gluten-free diet after oligofructose-enriched inulin intervention: Results of a randomised placebo-controlled pilot study. Amino Acids 2018, 50, 1451–1460. [Google Scholar] [CrossRef]
- Osadnik, T.; Osadnik, K.; Pawlas, N.; Strzelczyk, J.; Kasperczyk, J.; Polonski, L.; Gasior, M. Metabolic and genetic profiling of young adults with and without a family history of premature coronary heart disease (MAGNETIC). Study design and methodology. Arch. Med. Sci. 2019, 15, 590–597. [Google Scholar] [CrossRef]
- Krusinska, B.; Hawrysz, I.; Wadolowska, L.; Slowinska, M.A.; Biernacki, M.; Czerwinska, A.; Golota, J.J. Associations of Mediterranean diet and a posteriori derived dietary patterns with breast and lung cancer risk: A case-control study. Nutrients 2018, 10, 470. [Google Scholar] [CrossRef]
- Krusinska, B.; Wadolowska, L.; Slowinska, M.A.; Biernacki, M.; Drozdowski, M.; Chadzynski, T. Associations of dietary patterns and metabolic-hormone profiles with breast cancer risk: A case-control study. Nutrients 2018, 10, 2013. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Dietary Assessment: A resource Guide to Method Selection and Application in Low Resource Settings; Food and Agriculture Organization: Rome, Italy, 2018; Available online: http://www.fao.org/3/i9940en/I9940EN (accessed on 20 June 2019).
- Verbestel, V.; De Henauw, S.; Bammann, K.; Barba, G.; Hadjigeorgiou, C.; Eiben, G.; Konstabel, K.; Kovács, E.; Pitsiladis, Y.; Reisch, L.; et al. Are context-specific measures of parental-reported physical activity and sedentary behaviour associated with accelerometer data in 2-9-year-old European children? Public Health Nutr. 2015, 18, 860–868. [Google Scholar] [CrossRef] [PubMed]
| Timing | Activities | Group | |
|---|---|---|---|
| Experimental | Control | ||
| T0 visit (enrolment) |
| ✕ | |
| T1 visit (baseline) |
| ✕ | ✕ |
| ✕ | ✕ | |
| QDP | CDC | |
| Between visits |
| ✕ | |
| T2 visit (1-month follow-up) |
| ✕ | ✕ |
| ✕ | ✕ | |
| ✕ | ✕ | |
| Between visits |
| ✕ | |
| T3 visit (6-month follow-up) |
| ✕ | ✕ |
| ✕ | ✕ | |
Inclusion Criteria:
|
Exclusion Criteria:
|
| Foods Recommended for Consumption Control the Frequency of the Food Consumption Every Meal/Eating Episode (or at least Every Day) and Check Adherence to Recommendations. Minimum Frequencies Are Indicated. | |||||||
|---|---|---|---|---|---|---|---|
| Day | Vegetables (see diary B) | Foods rich in calcium e.g., milk, fermented milk drinks, curd cheese, cheese | Fruit | Whole grains e.g., buckwheat grain, wholemeal wheat and rye bread | Animal foods rich in zinc e.g., meats: beef, lamb, pork; eggs | Animal foods rich in selenium e.g., fish, seafood | Nuts and seeds e.g., nuts, pumpkin seeds, sunflower seeds |
| Several times a day | Several times a day | Once a day | Once a day | Several times a week | Several times a week | Several times a week | |
| Month: Mark ✕ each time after food consumption | |||||||
| 1 | |||||||
| 2 | |||||||
| 3 | |||||||
| … | |||||||
| 31 | |||||||
| Month: June 2019 Sample record of the Patient | |||||||
| 1 | ✕✕ | ✕ | ✕✕ | ✕ | ✕ | ||
| 2 | ✕✕✕ | ✕ | ✕ | ✕ | ✕ | ✕ | |
| 3 | ✕ | ✕✕✕ | ✕ | ✕ | ✕ | ||
| 4 | ✕ | ✕✕ | ✕ | ✕ | ✕ | ||
| 5 | ✕✕✕ | ✕ | ✕ | ✕ | ✕ | ✕ | |
| 6 | ✕✕ | ✕✕ | ✕ | ||||
| 7 | ✕✕✕ | ✕✕ | ✕ | ✕ | |||
| Foods with Limited Consumption Control the Frequency of the Food Consumption Every Meal/Eating Episode (or at least Every Day) and Check Adherence to Recommendations. Maximum Frequencies Are Indicated. | ||||||
|---|---|---|---|---|---|---|
| Day | Raw cruciferous vegetables e.g., kale, bok-choy, white cabbage, red cabbage, broccoli, brussels sprouts, cauliflower | Sweets, sugar and honey including high-sweetened jam, fruits candied, etc. | Sweetened beverages and energy drinks e.g., with sugar, glucose-fructose syrup, artificially sweetened | Fast foods e.g., hot-dogs, hamburgers, chips, French fries, pizza, tortilla, deep-fried foods | Soybean and millet e.g., soya: seeds, sprouts, tofu, milk; millet groats | Alcohols e.g., wine, beer, alcohol drinks, vodka, brandy |
| Once a week | Once a week | Once a week | Once a week | Twice a month | Once a month | |
| Month: Mark ✕ each time after food consumption | ||||||
| 1 | ||||||
| 2 | ||||||
| 3 | ||||||
| … | ||||||
| 31 | ||||||
| Month: June 2019 Sample record of a Patient | ||||||
| 1 | ✕ | |||||
| 2 | ✕ | |||||
| 3 | ✕ | |||||
| 4 | ||||||
| 5 | ✕ | |||||
| 6 | ||||||
| 7 | ✕ | ✕ | ✕ | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtas, N.; Wadolowska, L.; Bandurska-Stankiewicz, E. Evaluation of Qualitative Dietary Protocol (Diet4Hashi) Application in Dietary Counseling in Hashimoto Thyroiditis: Study Protocol of a Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16, 4841. https://doi.org/10.3390/ijerph16234841
Wojtas N, Wadolowska L, Bandurska-Stankiewicz E. Evaluation of Qualitative Dietary Protocol (Diet4Hashi) Application in Dietary Counseling in Hashimoto Thyroiditis: Study Protocol of a Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2019; 16(23):4841. https://doi.org/10.3390/ijerph16234841
Chicago/Turabian StyleWojtas, Natalia, Lidia Wadolowska, and Elżbieta Bandurska-Stankiewicz. 2019. "Evaluation of Qualitative Dietary Protocol (Diet4Hashi) Application in Dietary Counseling in Hashimoto Thyroiditis: Study Protocol of a Randomized Controlled Trial" International Journal of Environmental Research and Public Health 16, no. 23: 4841. https://doi.org/10.3390/ijerph16234841
APA StyleWojtas, N., Wadolowska, L., & Bandurska-Stankiewicz, E. (2019). Evaluation of Qualitative Dietary Protocol (Diet4Hashi) Application in Dietary Counseling in Hashimoto Thyroiditis: Study Protocol of a Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 16(23), 4841. https://doi.org/10.3390/ijerph16234841






