First Description of the Hyperpnea–Hypopnea Periodic Breathing in Patients with Interstitial Lung Disease-Obstructive Sleep Apnea: Treatment Implications in a Real-Life Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. OSA Assessment
2.3. Statistical Analysis
3. Results
3.1. OSA Is Highly Prevalent in ILDs
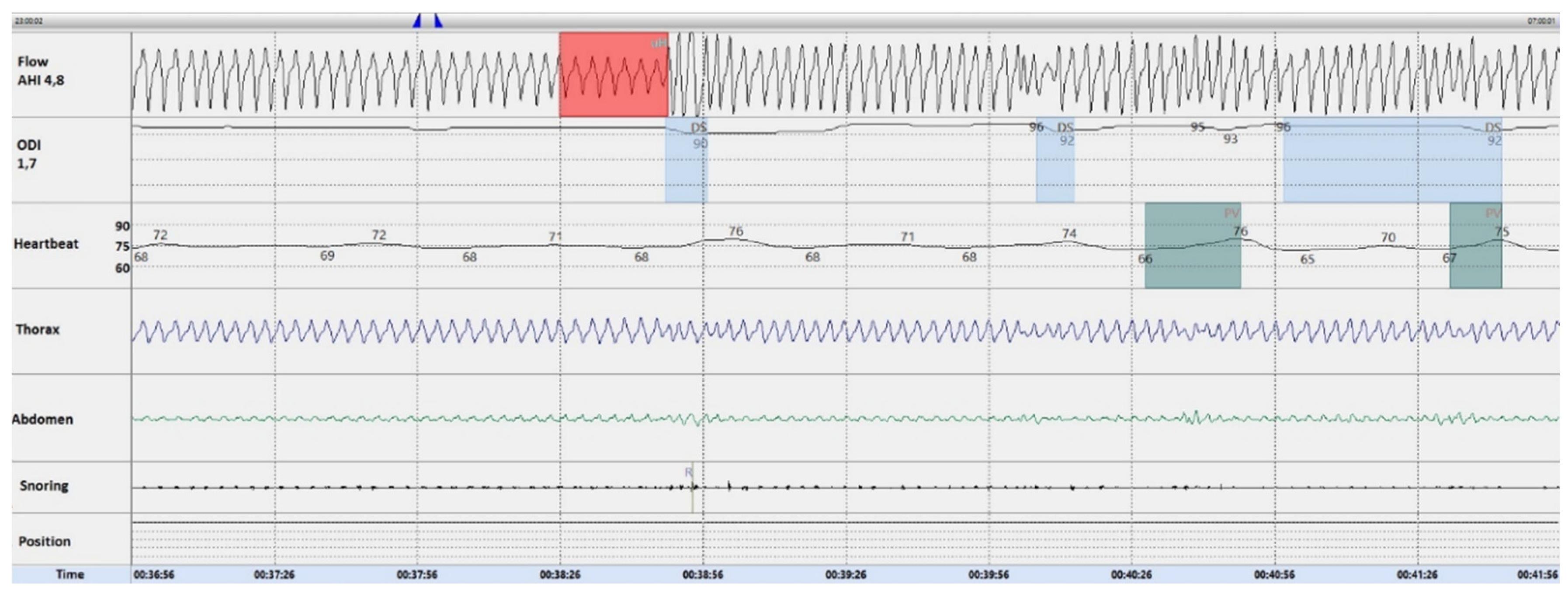
3.2. Description of the Hyperpnea–Hypopnea Periodic Breathing in ILD-OSA
3.3. Oxygen Resolves the Hyperpnea–Hypopnea Periodic Breathing in ILD-OSA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mikolasch, T.A.; Garthwaite, H.S.; Porter, J.C. Update in diagnosis and management of interstitial lung disease. Clin. Med. 2016, 16, 71–78. [Google Scholar] [CrossRef]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Garcia, C.A.C.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: Treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Weycker, D.; Edelsberg, J.; Bradford, W.Z.; Oster, G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Amatto, V.C.; Behr, J.; Stowasser, S. Comorbidities in idiopathic pulmonary fibrosis patients: A systematic literature review. Eur. Respir. J. 2015, 46, 1113–1130. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.; Ehlers-Tenenbaum, S.; Palmowski, K.; Bruhwyler, J.; Oltmanns, U.; Muley, T.; Heussel, C.P.; Warth, A.; Kolb, M.; Herth, F.J. Impact of comorbidities on mortality in patients with idiopathic pulmonary fibrosis. PLoS ONE 2016, 11, e0151425. [Google Scholar] [CrossRef] [PubMed]
- Pihtili, A.; Bingol, Z.; Kiyan, E.; Cuhadaroglu, C.; Issever, H.; Gulbaran, Z. Obstructive sleep apnea is common in patients with interstitial lung disease. Sleep Breath. 2013, 17, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Mermigkis, C.; Chapman, J.; Golish, J.; Mermigkis, D.; Budur, K.; Kopanakis, A.; Polychronopoulos, V.; Burgess, R.; Foldvary-Schaefer, N. Sleep related breathing disorders in patients with idiopathic pulmonary fibrosis. Lung 2007, 185, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, L.H.; Mason, W.R.; Parnell, J.A.; Rice, T.W.; Loyd, J.E.; Milstone, A.P.; Collard, H.R.; Malow, B.A. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest 2009, 136, 772–778. [Google Scholar] [CrossRef]
- Rasche, K.; Orth, M. Sleep and breathing in idiopathic pulmonary fibrosis. J. Physiol. Pharm. 2009, 60, 13–14. [Google Scholar]
- Mermigkis, C.; Stagaki, E.; Tryfon, S.; Schiza, S.; Amfilochiou, A.; Polychronopoulos, V.; Panagou, P.; Galanis, N.; Kallianos, A.; Mermigkis, D.; et al. How common is sleep-disordered breathing in patients with idiopathic pulmonary fibrosis? Sleep Breath. 2010, 14, 387–390. [Google Scholar] [CrossRef]
- Reid, T.; Vennelle, M.; McKinley, M.; Macfarlane, P.A.; Hirani, N.; Simpson, A.J.; Riha, R.L. Sleep-disordered breathing and idiopathic pulmonary fibrosis—Is there an association? Sleep Breath. 2015, 19, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Kolilekas, L.; Manali, E.; Vlami, K.A.; Lyberopoulos, P.; Triantafillidou, C.; Kagouridis, K.; Baou, K.; Gyftopoulos, S.; Vougas, K.N.; Karakatsani, A.; et al. Sleep oxygen desaturation predicts survival in idiopathic pulmonary fibrosis. J. Clin. Sleep Med. 2013, 9, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Mavroudi, M.; Papakosta, D.; Kontakiotis, T.; Domvri, K.; Kalamaras, G.; Zarogoulidou, V.; Zarogoulidis, P.; Latka, P.; Huang, H.; Hohenforst-Schmidt, W. Sleep disorders and health-related quality of life in patients with interstitial lung disease. Sleep Breath. 2017, 22, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Bosi, M.; Milioli, G.; Fanfulla, F.; Tomassetti, S.; Ryu, J.H.; Parrino, L.; Riccardi, S.; Melpignano, A.; Vaudano, A.E.; Ravaglia, C.; et al. OSA and prolonged oxygen desaturation during sleep are strong predictors of poor outcome in IPF. Lung 2017, 195, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population—A review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar] [PubMed]
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia-revisited-the bad ugly and good: Implications to the heart and brain. Sleep Med. Rev. 2015, 20, 27–45. [Google Scholar] [CrossRef]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbe, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pepin, J.-L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef]
- Rosenzweig, I.; Glasser, M.; Polsek, D.; Leschziner, G.D.; Williams, S.C.; Morrell, M.J. Sleep apnoea and the brain: A complex relationship. Lancet Respir. Med. 2015, 3, 404–414. [Google Scholar] [CrossRef]
- Mermigkis, C.; Stagaki, E.; Amfilochiou, A.; Polychronopoulos, V.; Korkonikitas, P.; Mermigkis, D.; Bregou, M.; Kouris, N.; Bouros, D. Sleep quality and associated daytime consequences in patients with idiopathic pulmonary fibrosis. Med. Princ. Pract. 2009, 18, 10–15. [Google Scholar] [CrossRef]
- Aydoğdu, M.; Ciftçi, B.; Güven, S.F.; Ciftçi, T.U.; Erdoğan, Y. Assessment of sleep with polysomnography in patients with interstitial lung disease. Tuberk. Toraks 2006, 54, 213–221. [Google Scholar]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Johns, M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Khoo, M.C.; Gottschalk, A.; Pack, A.I. Sleep-induced periodic breathing and apnea: A theoretical study. J. Appl. Physiol. 1991, 70, 2014–2024. [Google Scholar] [CrossRef]
- Bye, P.T.P.; Issa, F.; Berthon-Jones, M.; Sullivan, C.E. Studies of oxygenation during sleep in patients with interstitial lung disease. Am. Rev. Respir. Dis. 1984, 129, 27–32. [Google Scholar] [PubMed]
- Perez-Padilla, R.; West, P.; Lertzman, M.; Kryger, M.H. Breathing during sleep in patients with interstitial lung disease. Am. Rev. Respir. Dis. 1985, 132, 224–229. [Google Scholar] [PubMed]
- McNicholas, W.T.; Coffey, M.; Fitzgerald, M.X. Ventilation and gas exchange during sleep in patients with interstitial lung disease. Thorax 1986, 41, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Midgren, B.; Hansson, L.; Eriksson, L.; Arikkala, P.; Elmqvist, D. Oxygen desaturation during sleep and exercise in patients with interstitial lung disease. Thorax 1987, 42, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; Ward, A.J.; Lanes, S.; Hayflinger, D.C.; Rosenberg, D.M.; Hunsche, E. Burden of illness in idiopathic pulmonary fibrosis. J. Med. Econ. 2012, 15, 829835. [Google Scholar] [CrossRef]
- Gille, T.; Didier, M.; Boubaya, M.; Moya, L.; Sutton, A.; Carton, Z.; Baran-Marszak, F.; Sadoun-Danino, D.; Israël-Biet, D.; Cottin, V.; et al. Obstructive sleep apnoea and related comorbidities in incident idiopathic pulmonary fibrosis. Eur. Respir. J. 2017, 49, 1601934. [Google Scholar] [CrossRef]
- Wellman, A.; Malhotra, A.; Jordan, A.S.; Stevenson, K.E.; Gautam, S.; White, D.P. Effect of oxygen in obstructive sleep apnea: Role of loop gain. Respir. Physiol. Neurobiol. 2008, 162, 144–151. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M.; Mehra, R.; Patel, S.R.; Quan, S.F.; Babineau, D.C.; Tracy, R.P.; Rueschman, M.; Blumenthal, R.S.; Lewis, E.F.; et al. CPAP versus oxygen in obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 2276–2285. [Google Scholar] [CrossRef]
- Loredo, J.S.; Ancoli-Israel, S.; Kim, E.-J.; Lim, W.J.; Dimsdale, J.E. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: A placebo-CPAP-controlled study. Sleep 2006, 29, 564–571. [Google Scholar] [CrossRef]
- Sands, S.A.; Edwards, B.A.; Terrill, P.I.; Butler, J.P.; Owens, R.L.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.; Hess, L.B.; Smales, E.T.; et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur. Respir. J. 2018, 52, 1800674. [Google Scholar] [CrossRef]

| Variables | Controls (n = 50) | IPF (n = 54) | No-IPF (n = 46) | Overall p |
|---|---|---|---|---|
| Gender, female (n) | 17 (34) | 14 (25.9) | 17 (37) | 0.54 |
| Age (years) | 66.5 ± 7.9 | 69.9 ± 7.1 | 66.8 ± 8.8 | 0.52 |
| Smoking habit (n) | <0.001 | |||
| No smokers | 19 (38) | 15 (27.8) | 17 (37.8) | |
| Former smokers | 22 (44) | 39 (72.2) | 19 (42.2) | |
| Smokers | 9 (18) | 0 (0) | 9 (20) | |
| Pack/yr | 25 (0; 30) | 36 (20; 60) | 30 (11.5; 37.5) | 0.315 |
| BMI (Kg/m2) | 31 (26.5; 35) | 28.7 (25.1; 30.7) | 28 (25.7; 31.1) | 0.034 |
| Co-morbidities (n) | ||||
| Systemic arterial hypertension | 29 (58) | 32 (59.3) | 26 (56.5) | 0.978 |
| Gastro-esophageal reflux | 6 (12) | 24 (44.4) | 14 (30.4) | 0.001 |
| Type II diabetes | 9 (18) | 14 (25.9) | 4 (8.7) | 0.082 |
| Cardiovascular diseases (CVD) * | 10 (20) | 13 (24.1) | 10 (21.7) | 0.883 |
| Thyroid disease | 4 (8) | 3 (5.68) | 3 (6.5) | 0.92 |
| Pulmonary hypertension | 0 (0) | 5 (9.3) | 3 (6.5) | 0.076 |
| Epworth sleepiness scale score | 10 (7.8; 12) | 6 (4; 8) | 6 (4; 8) | <0.001 |
| Parameter | Controls (n = 50) | IPF (n = 54) | No-IPF (n = 46) | Overall p |
|---|---|---|---|---|
| Arterial pO2 (mmHg), at rest at 21% FiO2 | 69.7 ± 12 | 70.2 ± 10.8 | 69.1 ± 13.3 | <0.001 |
| SpO2 (%), at rest at 21% FiO2 | 96 (94; 97) | 96 (93.6; 97.4) | 96 (93.8; 97) | <0.001 |
| FVC (% pred) | 69.6 ± 22.1 | 71.5 ± 23.2 | 67.4 ± 20.8 | <0.001 |
| TLC (% pred) | 60.5 ± 19 | 60 ± 19.2 | 61.3 ± 19 | 0.003 |
| RV (% pred) | 49.5 (37; 60.5) | 48 (30.5; 58.5) | 50 (40; 68) | <0.001 |
| DLCOsb (% pred) | 48.6 ± 19 | 47.5 ± 19.4 | 50 ± 18.7 | <0.001 |
| 6-MWT distance (meters) | 410 (318; 528) | 443 (308; 535) | 401 (346; 507) | 0.01 |
| TST (minutes) | 390 (325; 426) | 438 (391; 466) | 446 (402; 458) | <0.001 |
| Supine time (%) | 52 (21; 81) | 12 (3; 26) | 46 (19; 65) | 0.001 |
| AHI (events/hour) | 28.6 (20.6; 68.3) | 10.8 (5.3; 26.9) | 10.8 (7.4; 15.8) | <0.001 |
| OAI (events/hour) | 23.9 (15.5; 65.8) | 2.7 (0.98; 6.7) | 3 (1; 7.8) | <0.001 |
| CAI (events/hour) | 0.1 (0; 0.8) | 0.05 (0; 0.4) | 0 (0; 0.1) | 0.066 |
| Supine AHI (events/hour) | 37.6 (12; 60) | 17 (4.8; 30.8) | 13.5 (2.7; 27.5) | 0.012 |
| HI (events/hour) | 7.7 (3.7; 13.1) | 7.2 (3.3; 11.5) | 7 (3.2; 12.4) | 0.892 |
| ODI (events/hour) | 33.3 (14; 66.5) | 15.9 (7.5; 29) | 12 (7.4; 19.1) | <0.001 |
| t90 (%) | 15.5 (2.8; 37.9) | 6 (1; 26.2) | 4.5 (0; 20.5) | 0.139 |
| Nadir SpO2 (%) | 72.5 (60.2; 79) | 77.5 (67; 82) | 77.5 (70; 82.2) | 0.055 |
| Parameter | Controls | IPF | No-IPF | Overall p |
|---|---|---|---|---|
| OSA | 50 (100) | 42 (77.8) | 37 (80.4) | <0.001 |
| OSA severity | <0.001 | |||
| Mild | 8 (16) | 21 (50) | 25 (67.6) | |
| Moderate | 18 (36) | 10 (23.8) | 6 (16.2) | |
| Severe | 24 (48) | 11 (26.2) | 6 (16.2) | |
| NRF | 16 (32) | 12 (22.2) | 9 (19.6) | 0.319 |
| OSA + NRF | 32 (21.3) | 10 (18.5) | 6 (13) | 0.069 |
| OSA severity + NRF | 0.01 | |||
| Mild | 0 (0) | 4 (40) | 3 (50) | |
| Moderate | 2 (12.5) | 1 (10) | 1 (16.7) | |
| Severe | 14 (87.5) | 5 (50) | 2 (33.3) |
| Parameter | HHPB (with an Hypopnea/Apnea Ratio < 3) | HHPB (with an Hypopnea/Apnea Ratio ≥ 3) | p |
|---|---|---|---|
| Diagnosis | 1 | ||
| IPF | 25 (53.7) | 18 (54.5) | |
| No-IPF | 21 (46.3) | 15 (45.5) | |
| OSA severity | 0.027 | ||
| Mild | 32 (69.6) | 14 (42.4) | |
| Moderate | 5 (10.9) | 11 (33.3) | |
| Severe | 9 (19.6) | 8 (24.2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canora, A.; Nicoletta, C.; Ghinassi, G.; Bruzzese, D.; Rea, G.; Capaccio, A.; Castaldo, S.; Coppola, A.; Polistina, G.E.; Sanduzzi, A.; et al. First Description of the Hyperpnea–Hypopnea Periodic Breathing in Patients with Interstitial Lung Disease-Obstructive Sleep Apnea: Treatment Implications in a Real-Life Setting. Int. J. Environ. Res. Public Health 2019, 16, 4712. https://doi.org/10.3390/ijerph16234712
Canora A, Nicoletta C, Ghinassi G, Bruzzese D, Rea G, Capaccio A, Castaldo S, Coppola A, Polistina GE, Sanduzzi A, et al. First Description of the Hyperpnea–Hypopnea Periodic Breathing in Patients with Interstitial Lung Disease-Obstructive Sleep Apnea: Treatment Implications in a Real-Life Setting. International Journal of Environmental Research and Public Health. 2019; 16(23):4712. https://doi.org/10.3390/ijerph16234712
Chicago/Turabian StyleCanora, Angelo, Carmine Nicoletta, Giacomo Ghinassi, Dario Bruzzese, Gaetano Rea, Annalisa Capaccio, Sabrina Castaldo, Antonietta Coppola, Giorgio Emanuele Polistina, Alessandro Sanduzzi, and et al. 2019. "First Description of the Hyperpnea–Hypopnea Periodic Breathing in Patients with Interstitial Lung Disease-Obstructive Sleep Apnea: Treatment Implications in a Real-Life Setting" International Journal of Environmental Research and Public Health 16, no. 23: 4712. https://doi.org/10.3390/ijerph16234712
APA StyleCanora, A., Nicoletta, C., Ghinassi, G., Bruzzese, D., Rea, G., Capaccio, A., Castaldo, S., Coppola, A., Polistina, G. E., Sanduzzi, A., & Bocchino, M. (2019). First Description of the Hyperpnea–Hypopnea Periodic Breathing in Patients with Interstitial Lung Disease-Obstructive Sleep Apnea: Treatment Implications in a Real-Life Setting. International Journal of Environmental Research and Public Health, 16(23), 4712. https://doi.org/10.3390/ijerph16234712








